Abstract
The COHORT trial was conducted to compare the efficacy of androgen deprivation therapy (ADT) alone versus combined with radiation therapy (ADT + RT) for clinically node-positive prostate cancer. We reported adverse events and quality of life between the two treatment groups. Fifty-nine patients were randomized to receive ADT alone or ADT + RT and analyzed as per-protocol. Patients allocated to the ADT alone arm received ADT for at least 2 years. Patients in the ADT + RT arm received additional pelvic RT. Higher rates of grade ≥ 2 acute genitourinary (0% vs. 7.1%) and late gastrointestinal adverse events (0% vs. 14.3%) were reported in the ADT + RT arm compared with the ADT alone. However, grade ≥ 2 late genitourinary toxicity was more common in the ADT alone than the ADT + RT arm (9.7% vs. 3.6%). No grade ≥ 3 adverse events were reported. There was no statistically significant difference in EPIC scores between two treatment arms. However, the urinary and bowel domains tended to decrease and recover in the ADT + RT arm. In conclusion, ADT + RT demonstrated higher rates of adverse events compared to ADT alone. However, the addition of RT did not significantly impact the quality of life.
Similar content being viewed by others
Introduction
Prostate cancer is the second most frequently diagnosed cancer in men worldwide after lung cancer1. Although most cases of prostate cancer are diagnosed while localized, approximately 10% of patients present with regional lymph node metastasis2. Prostate cancer is one of the most common cancers treated by radiation therapy (RT)3. The role of RT in organ-confined high-risk prostate cancer is well-established4,5. However, the role of RT in node-positive prostate cancer is controversial, as high-level evidence is lacking. Retrospective population studies comparing local therapy, including RT, versus no local therapy have demonstrated a survival benefit of local therapy6,7. An analysis of data from the control arm of the STAMPEDE trial reported a failure-free survival benefit of RT in the N + M0 subcohort8. Although RT may be the preferred treatment option for clinically node-positive prostate cancer based on these retrospective analyses, confirmatory randomized evidence is required. To address this question, we designed the COHORT trial, a multi-institutional randomized phase III trial aimed at determining the potential benefits of ADT plus RT (ADT + RT) for clinically pelvic lymph node-positive prostate cancer.
Although the efficacy of the treatment modalities is the most important, adverse events and their impact on the quality of life cannot be disregarded, as the risks and benefits of the treatment need to be thoroughly considered in clinical settings. In previous randomized trials, adding RT to ADT increased treatment-related adverse events, especially urinary and gastrointestinal symptoms4,9. For node-positive prostate cancer, the RT field often extends to the pelvic regional lymph node area, and the negative impact of RT may be more severe. Therefore, adverse events of RT and their impact on the quality of life in this subgroup of patients need to be addressed. The purpose of this analysis is to compare genitourinary and gastrointestinal adverse events and the quality of life of patients with node-positive prostate cancer who underwent ADT alone and those who underwent ADT + RT.
Materials and methods
Patients
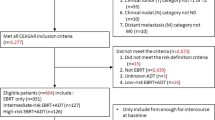
The inclusion criteria for this trial were as follows: pathological confirmation of prostate cancer within 6 months before enrollment; radiological evidence (computed tomography [CT] and magnetic resonance imaging [MRI]) of suspected metastatic pelvic lymph node (short axis ≥ 0.5 cm) that decreased in size after 2–3 months of ADT, indicating at least partial response according to Response Evaluation Criteria in Solid Tumors version 1.110; age ≥ 20 years; Eastern Cooperative Oncology Group performance status of 0–1; satisfactory complete blood count test result within 6 months (absolute neutrophil count ≥ 1500 cells/mm3, platelets ≥ 50,000 cells/mm3, hemoglobin ≥ 8.0 g/dL); satisfactory renal function test within 6 months (creatinine < 2.0 ng/dL); and satisfactory liver function test within 6 months (total bilirubin < 1.5 times the upper reference value of the participating institution; alanine aminotransferase or aspartate aminotransferase < 2.5 times the upper reference value of the participating institution).
The exclusion criteria were as follows: distant metastasis; receipt of hormone therapy more than 6 months before enrollment; history of other definitive treatments for prostate cancer, such as radical prostatectomy; history of prior pelvic RT; and history of cancer other than skin or thyroid cancer. The accrual was conducted from July 2016 until July 2021.
Study design and treatments
The trial was registered at ClinicalTrials.gov on 07/08/2017 (NCT03241537). Patients were screened for enrollment by treating radiation oncologists when they received the first ADT. If the lymph node size reduction was confirmed to be more than partial response after 2–3 months of ADT, patients were enrolled in the trial and were randomly assigned to either the ADT alone arm or the ADT + RT arm in a 1:1 ratio. The block randomization method was used to prepare a randomization table. Patients in the ADT alone arm continued to receive ADT alone, whereas those in the ADT + RT arm underwent both ADT and pelvic external beam RT. ADT was administered using a combination of a gonadotropin-releasing hormone (GnRH) agonist and anti-androgen. At least 2 years of ADT was required unless it was deemed intolerable by the treating clinicians due to adverse events. The extended use of ADT was allowed.
Patients allocated to the ADT + RT arm underwent simulation CT for RT planning. Intensity-modulated RT is required for RT planning. The high-risk clinical target volume (HR-CTV) included the entire prostate, the involved extraprostatic tissue, seminal vesicles, and metastatic pelvic lymph nodes. For cT3a disease, proximal seminal vesicles were included in the HR-CTV. For cT3b and cT4 disease, the entire seminal vesicles were included in the HR-CTV. The low-risk (LR) CTV included the obturator, external iliac, internal iliac, and presacral lymphatic areas according to Radiation Therapy Oncology Group (RTOG) consensus11 published in 2009, which was available at the time of protocol establishment. The CTV-to-planning target volume (PTV) expansion was 3–5 mm for the prostate and seminal vesicle region and 5–7 mm for the nodal area. The prescribed dose to the HR-PTV was no less than the biologically effective dose (BED) of 170 Gy with an alpha–beta ratio of 1.5, except for the pelvic metastatic lymph nodes. A BED of no less than 120 Gy was required for the lymph nodes. The dose prescribed for the LR-PTV was not less than 100 Gy. Institutional dose fractionation regimens were permitted if the study requirements were met. In practice, most of the patients received 70 Gy to the prostate with or without seminal vesicles, 50.4 Gy to the pelvic nodal basin, and 59.92–61.6 Gy to suspected lymph nodes in 28 fractions. The plan was optimized to cover 95% of the PTV with 100% of the prescribed dose. Organs-at-risk (OARs) included the bladder, rectum, and penile bulb and were delineated according to RTOG guidelines12. The dose constraints for the OARs are summarized in Supplementary Table 1. RT was initiated within 10 days of the simulation CT scans. The fractions were delivered 5 days per week. RT should be completed within 10 weeks of its initiation. Pelvic RT was permitted as a salvage treatment in the ADT alone arm if needed.
The patients were followed up at 6-months intervals for 3 years from enrollment and once a year thereafter. The required follow-up period for oncologic outcomes was 5 years. Adverse event evaluations, and quality of life assessments were performed at every follow-up visit.
Endpoints and statistical analysis
Genitourinary and gastrointestinal adverse events were classified as acute (occurring within < 6 months) or late (occurring ≥ 6 months) based on the onset from the start of the treatment and graded according to Common Terminology Criteria for Adverse Events version 4.0. Quality of life was assessed using the Expanded Prostate Index Composite (EPIC)13. The rates of acute and late gastrointestinal and genitourinary adverse events were compared between the two arms using the chi-squared test. The EPIC was scored for each domain (urinary, bowel, sexual, and hormonal) and subscale. Changes in each domain and subscale score from enrollment were compared using a linear mixed model with the allocated arm and time as fixed effects and each patient as a random effect. The scores for each domain and subscale at 12, 24, and 36 months were compared with the baseline scores measured at enrollment using the Wilcoxon rank sum test. These analyses were performed as per-protocol.
The sample size for this trial was calculated based on the assumption that the 5-years recurrence-free survival rate would be 40% in the ADT alone arm and 80% in the ADT + RT arm. Sixty participants were required, using a log-rank test with a two-sided significance level of 5%, 80% statistical power, and a 10% dropout rate. Statistical significance was defined as a p-value < 0.05. All statistical analyses were conducted using R software (version 4.2.1; The R Foundation for Statistical Computing, Vienna, Austria).
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Samsung Medical Center (no. 2015-11-139) before trial initiation. Written informed consent was obtained from each participant before enrollment. All procedures performed in this study were in accordance with the ethical standards of the institutional and national regulations and with the 1964 Helsinki Declaration and its later amendments.
Results
Patient characteristics and treatment
This study enrolled 60 patients: 31 allocated to the ADT alone arm and 29 allocated to the ADT + RT arm. One patient in the ADT + RT arm refused to undergo RT and was consequently excluded from the per-protocol analysis, leaving 59 patients in the analysis. A CONSORT diagram is shown in Fig. 1. The median follow-up duration was 3.31 years (range: 0.24–6.87 years). Seven (22.6%) patients in the ADT alone arm were either lost to follow-up (N = 2) or withdrew their consent (N = 5), while no patients in the ADT + RT arm were removed from the follow-up. However, no statistical difference in follow-up duration was reported (ADT alone arm: median 2.99 years; ADT + RT arm: median 3.57 years; p = 0.106). The baseline characteristics of the patients and their treatment specifics are summarized in Table 1. The patient characteristics were well balanced between the two arms. The median age of all patients was 70 years (range: 49–83 years). The median initial PSA was 35.9 ng/mL (range: 3.2–1032 ng/mL). Most patients (84.7%) had a Gleason score of ≥ 8, and all patients had T3 or T4 disease. The median number of metastatic pelvic lymph nodes was two (range: 1–7), and the median lymph node size was 12 mm (range: 5–41 mm).
Leuprolide (59.3%), triptorelin (35.6%), and goserelin (5.1%) were initially used as the GnRH agonists in ADT. All patients were administered bicalutamide as an anti-androgen. The median duration of ADT for all patients was 2.69 years (range: 0.24–6.58 years). A total of six (10.2%) stopped taking the ADT during follow-up. All six patients who stopped taking ADT were in the ADT + RT arm. Among them, five stopped ADT after 2 years at the discretion of the clinicians. One patient discontinued ADT because of a suspected drug rash. For RT, 26 of 28 patients underwent treatment with a dose fractionation of 70 Gy in 28 fractions. One patient received 67.2 Gy in 28 fractions due to a high rectal dose, and another patient received 77 Gy in 33 fractions.
Adverse events
The acute and late gastrointestinal and genitourinary adverse events are summarized in Table 2. All reported gastrointestinal and genitourinary adverse events were grade 1 or 2. There was no grade ≥ 3 in acute and late adverse events. A statistically significant difference in the rates of acute genitourinary and late gastrointestinal adverse events between the two treatment arms were reported (p < 0.001). The incidence of acute grade 2 genitourinary adverse events was 0% in the ADT alone and and 7.1% in the ADT + RT arm. For the late grade 2 gastrointestinal adverse events, the rates were 0% in the ADT alone arm and 14.3% in the ADT + RT arm.
For acute genitourinary toxicities, two patients reported grade 2 adverse events. Both patients experienced frequency, and one patient experienced urgency. These symptoms spontaneously subsided to grade 1, and at the 18-months visit, both patients no longer reported any genitourinary adverse events. Regarding late gastrointestinal toxicities, a total of 4 patients reported grade 2 adverse events. Three patients experienced moderate rectal hemorrhage; however, only one patient required elective argon plasma coagulation, and the rectal hemorrhage regressed to intermittent grade 1 events for all three patients. Two patients reported proctitis, and one patient reported dyspepsia. The proctitis and dyspepsia events were temporary. As for late genitourinary toxicities, four patients reported grade 2 adverse events. The symptoms included frequency, urgency, pain, incontinence, and obstructive symptoms. However, these symptoms were not persistent.
Quality of life
An analysis of the quality of life based on EPIC scores was conducted in 54 patients. All 59 patients responded to the EPIC during enrollment, but five declined to respond to the EPIC during follow-up. Additionally, two patients refused to respond to the sexual domain questions during enrollment. The median baseline EPIC scores for the urinary, bowel, sexual, and hormonal domains during enrollment were 87.5 (range: 52.1–100.0), 98.2 (range: 75.0–100.0), 27.5 (range: 1.9–56.4), and 88.6 (range: 45.5–100.0), respectively. The baseline EPIC scores for each domain, as well as each subscale, did not show statistically significant differences between the two groups.
The change in the EPIC score from enrollment in each domain is shown in Fig. 2. As not all patients responded to the EPIC during follow-up, the number of observations is shown in each panel of the figure. In the linear mixed model analysis, no statistically significant association was observed between the allocated arms and the EPIC score for each domain (urinary, p = 0.202; bowel, p = 0.050; sexual, p = 0.270; hormonal, p = 0.069). When analyzed with the 10 subscales, all subscales did not show a statistically significant difference between the two treatment arms (Supplementary Fig. 1).
Domain and subscale scores at 12, 24, and 36 months for both treatment arms are summarized in Table 3. When compared with the baseline scores, the scores at 12, 24, and 36 months in the ADT alone arm did not show a statistically significant. In the ADT + RT arm, the urinary function (p = 0.013) and incontinence (p = 0.004) subscales were significantly lower than the baseline at 12 months, and the bowel bother subscale was significantly lower at 24 months (p = 0.031). However, these scores did not show a significant difference from the baseline at 36 months. On the other hand, the overall sexual domain score (p = 0.026) and sexual bother subscale (p = 0.004) were significantly higher than the baseline at 36 months.
Discussion
The COHORT trial was designed to evaluate the differences in treatment outcomes, adverse events, and quality of life between ADT alone and ADT + RT. Currently available clinical guidelines prefer definitive RT with ADT in node-positive prostate cancer14,15,16. However, no randomized evidence was available for patients with node-positive prostate cancer, and reports of adverse events and quality of life in these patients were scarce. In this analysis, statistically higher crude rates of acute genitourinary and late gastrointestinal adverse events in the ADT + RT arm were shown. However, most of the reported adverse events were mild, and there were no grade ≥ 3 events. Furthermore, the impact of adding pelvic RT on the quality of life appears to be temporary and limited. This report adds valuable reference for evaluating risk and benefit when making clinical decisions for node-positive prostate cancer, as the patient cohort in this study had well-balanced characteristics between the two arms, and patients in the same arm were generally treated homogeneously.
The crude rates of adverse events were higher in the ADT + RT arm in this trial, as expected based on previous trials comparing ADT alone versus ADT + RT in high-risk localized prostate cancer settings4,9. Crude rates only show the number of patients who experienced certain adverse events during the follow-up period, without considering recovery. In this trial, changes in the EPIC score for each domain from enrollment were analyzed, which may show the temporal trend of the functional changes evaluated by the patient. The changes in the urinary and bowel domains of the EPIC score were not significantly different between the two arms. Although not statistically significant, the urinary domain of the EPIC was lower in the ADT + RT arm until 24 months of treatment, with a similar trend in the bowel domain at the 18- and 24-months evaluations. However, these scores recovered afterward. Similar trends were observed in the temporal analysis of the subscales. At 12 months, the function and incontinence subscales of the urinary domain were lower than the baseline, and the bother subscale of the bowel domain was lower than the baseline in the ADT + RT arm. However, these subscales at 36 months were not different from the baseline, suggesting possible recovery. Although adding RT to ADT can increase the occurrence of adverse events, these effects may not persist in the long term, and the impact on the quality of life would be limited. Previous randomized trials that investigated adding pelvic RT in various clinical settings also reported that although pelvic RT was associated with more adverse events, the effect of adding pelvic RT on the quality of life was limited17,18,19, and these results are in concordance with the results of this analysis. Clinicians may not have to hesitate to apply RT to clinically node-positive prostate cancer due to concerns about genitourinary and gastrointestinal toxicities.
The baseline sexual domain of the EPIC was low in both arms, with a median of 27.5 out of 100. Although not statistically significant, the sexual domain in the ADT alone arm decreased further in 30–36 months, which may be associated with the extended use of ADT in this arm. The 36-months sexual domain score and sexual bother subscale were significantly higher than the baseline in the ADT + RT arm, which may be attributed to a higher rate of ADT discontinuation in this arm. Although the role of ADT prolongation for > 3 years in definitive treatment settings has not yet been established and remains under debate20,21, some clinicians in this trial preferred extending ADT, especially when other definitive treatment modalities were not applied. Both ADT and RT can have a detrimental effect on sexual function22,23. Based on the observations of this trial, continuous usage of ADT may have a more significant impact on decreasing sexual function than the long-term effects of RT. However, only a small number of patients were available for long-term sexual function evaluation in this trial, and more patients would be needed to obtain confirmative results.
This study has several limitations that must be acknowledged. Firstly, protocol adherence in the ADT alone arm was low, with seven patients withdrawing or being lost to follow-up. The follow-up required visits to the radiation oncology department for questionnaire completion, but some patients who were only followed up clinically at the urology department did not comply with the protocol and chose to withdraw. This may have led to the underreporting of late adverse events in the ADT alone arm. Secondly, the duration of ADT was not strictly determined in the trial protocol, and the rate of ADT cessation during follow-up differed between the two arms. The protocol specified a minimum ADT duration of 2 years, and the decisions of treating clinicians regarding the ADT duration could differ, given that patients in the two groups did not undergo the same treatment. These varying patterns of ADT usage may have influenced adverse events and quality of life outcomes. Finally, patient adherence to the EPIC questionnaire request was low, even though it was planned for every patient at every follow-up visit. This was often due to patients refusing to visit the radiation oncology department for the questionnaire while attending the urology department for clinical follow-up. Limited adherence to the EPIC questionnaire can hinder the representativeness of the quality of life results in this trial. Despite these limitations, this trial provides new high-level evidence for the treatment of patients with clinically node-positive prostate cancer.
In conclusion, the current analysis of the COHORT trial has reported a higher incidence of acute genitourinary and late gastrointestinal adverse events in the ADT + RT arm compared to the ADT alone arm. Nonetheless, no severe adverse events were reported, and the grade 2 adverse events that did occur were not persistent. In the quality of life analysis using the EPIC, the deterioration of urinary and bowel domain scores eventually started to recover after 2 years from the treatment. We look forward to the maturation of the COHORT trial for the long-term clinical outcomes.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due the potential disclosure of personal information of the participants but are available from the corresponding author on reasonable request.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Siegel, D. A., O’Neil, M. E., Richards, T. B., Dowling, N. F. & Weir, H. K. Prostate cancer incidence and survival, by stage and race/ethnicity—United States, 2001–2017. MMWR Morb. Mortal Wkly Rep. 69, 1473–1480 (2020).
Kim, E. et al. Clinical utilization of radiation therapy in Korea between 2017 and 2019. Radiat. Oncol. J. 40, 251–259 (2022).
Mason, M. D. et al. Final report of the intergroup randomized study of combined androgen-deprivation therapy plus radiotherapy versus androgen-deprivation therapy alone in locally advanced prostate cancer. J. Clin. Oncol. 33, 2143–2150 (2015).
Fosså, S. D. et al. Ten- and 15-yr prostate cancer-specific mortality in patients with nonmetastatic locally advanced or aggressive intermediate prostate cancer, randomized to lifelong endocrine treatment alone or combined with radiotherapy: Final results of the scandinavian prostate cancer group-7. Eur. Urol. 70, 684–691 (2016).
Tward, J. D., Kokeny, K. E. & Shrieve, D. C. Radiation therapy for clinically node-positive prostate adenocarcinoma is correlated with improved overall and prostate cancer-specific survival. Pract. Radiat. Oncol. 3, 234–240 (2013).
Rusthoven, C. G. et al. The impact of definitive local therapy for lymph node-positive prostate cancer: A population-based study. Int. J. Radiat. Oncol. Biol. Phys. 88, 1064–1073 (2014).
James, N. D. et al. Failure-free survival and radiotherapy in patients with newly diagnosed nonmetastatic prostate cancer: Data from patients in the control arm of the STAMPEDE trial. JAMA Oncol. 2, 348 (2016).
Widmark, A. et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): An open randomised phase III trial. Lancet 373, 301–308 (2009).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Lawton, C. A. F. et al. RTOG GU radiation oncology specialists reach consensus on pelvic lymph node volumes for high-risk prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 74, 383–387 (2009).
Gay, H. A. et al. Pelvic normal tissue contouring guidelines for radiation therapy: A radiation therapy oncology group consensus panel atlas. Int. J. Radiat. Oncol. Biol. Phys. 83, e353–e362 (2012).
Wei, J. T., Dunn, R. L., Litwin, M. S., Sandler, H. M. & Sanda, M. G. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology 56, 899–905 (2000).
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology - Prostate Cancer, Version 1.2023. (2023).
Lieng, H. et al. Radiotherapy for node-positive prostate cancer: 2019 Recommendations of the Australian and New Zealand Radiation Oncology Genito-Urinary group. Radiother. Oncol. 140, 68–75 (2019).
Parker, C. et al. Prostate cancer: ESMO clinical practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 31, 1119–1134 (2020).
Fransson, P. et al. Quality of life in patients with locally advanced prostate cancer given endocrine treatment with or without radiotherapy: 4-year follow-up of SPCG-7/SFUO-3, an open-label, randomised, phase III trial. Lancet Oncol. 10, 370–380 (2009).
Boevé, L. et al. Patient-reported quality of life in patients with primary metastatic prostate cancer treated with androgen deprivation therapy with and without concurrent radiation therapy to the prostate in a prospective randomised clinical trial; Data from the HORRAD trial. Eur. Urol. 79, 188–197 (2021).
Brundage, M. et al. Impact of radiotherapy when added to androgen-deprivation therapy for locally advanced prostate cancer: Long-term quality-of-life outcomes from the NCIC CTG PR3/MRC PR07 randomized trial. JCO 33, 2151–2157 (2015).
Souhami, L., Bae, K., Pilepich, M. & Sandler, H. Impact of the duration of adjuvant hormonal therapy in patients with locally advanced prostate cancer treated with radiotherapy: A secondary analysis of RTOG 85–31. J. Clin. Oncol. 27, 2137–2143 (2009).
Giobbie-Hurder, A., Gelber, R. D. & Regan, M. M. Challenges of guarantee-time bias. J. Clin. Oncol. 31, 2963–2969 (2013).
Nguyen, P. L. et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur. Urol. 67, 825–836 (2015).
Nguyen, D.-D., Berlin, A., Matthew, A. G., Perlis, N. & Elterman, D. S. Sexual function and rehabilitation after radiation therapy for prostate cancer: A review. Int. J. Impot. Res. 33, 410–417 (2021).
Author information
Authors and Affiliations
Contributions
T.H.L. was responsible for data analysis and manuscript writing. H.P., G.S.Y., J.H.K., and S.S.J. were responsible for data acquisition and project administration. S.I.S., B.C.J., H.G.J., H.H.S., M.K., W.S., and J.H.C. were responsible for data acquisition. W.P. was responsible for study designing, data acquisition, and project administration. All authors contributed to the revision of the manuscript and approval of the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, T.H., Pyo, H., Yoo, G.S. et al. Androgen deprivation alone versus combined with pelvic radiation for adverse events and quality of life in clinically node-positive prostate cancer. Sci Rep 14, 8207 (2024). https://doi.org/10.1038/s41598-024-54976-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-54976-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.