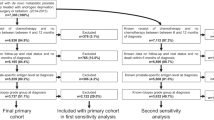
Abstract
Background
While enzalutamide plus androgen deprivation therapy (ADT) significantly reduces the risk of radiographic progression-free survival (rPFS) and improves overall survival in metastatic hormone-sensitive prostate cancer (mHSPC), the efficacy in clinically relevant subgroups of patients based on prior local and systemic therapy, disease volume, and risk has not been analyzed to date. These post hoc analyses of the phase 3 ARCHES trial (NCT02677896) evaluated the efficacy of enzalutamide plus ADT according to prior local and systemic treatment, disease volume, and risk, assessed at trial baseline.
Methods
In ARCHES, a global, double-blind, placebo-controlled, phase 3 study, 1150 patients with mHSPC were randomized 1:1 to receive enzalutamide (160 mg/day) plus ADT or placebo plus ADT, stratified by prior docetaxel therapy and disease volume. Primary endpoint was rPFS. Secondary endpoints included time to prostate-specific antigen progression, symptomatic skeletal events, and prostate-specific antigen and radiographic responses. Analyses of clinical endpoints were completed by prior local therapy, prior docetaxel exposure, CHAARTED (NCT00309985)-defined disease volume, and LATITUDE (NCT01715285)-defined risk groups.
Results
Patients were randomized to enzalutamide plus ADT (n = 574) and placebo plus ADT (n = 576). Enzalutamide plus ADT significantly improved rPFS (hazard ratio: 0.39; p < 0.0001), with similar improvements reported in all subgroups based on prior local and docetaxel treatment, disease volume, and risk. Treatment benefits were observed with enzalutamide plus ADT in multiple secondary clinical endpoints in the overall population and all subgroups.
Conclusions
Enzalutamide plus ADT demonstrated clinical benefit across all patients with mHSPC, irrespective of prior local and systemic treatment, disease volume, and risk.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Researchers may request access to anonymized participant-level data, trial-level data, and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing, see https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx. All authors had full access to all data and the corresponding author had final responsibility for the decision to submit for publication.
References
Fizazi K, Jenkins C, Tannock IF. Should docetaxel be standard of care for patients with metastatic hormone-sensitive prostate cancer? Pro and contra. Ann Oncol. 2015;26:1660–7.
Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–46.
James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–77.
James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338–51.
Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352–60.
Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381:121–31.
Armstrong AJ, Szmulewitz RZ, Petrylak DP, Holzbeierlein J, Villers A, Azad A, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37:2974–86.
Chi KN, Agarwal N, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, Given R, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381:13–24.
Mottet N, Cornford P, van den Bergh RCN, Briers E, De Santis M, Fanti S, et al. EAU guidelines: prostate cancer. EAU Guidelines Office; Arnhem, the Netherlands 2020. https://uroweb.org/guideline/prostate-cancer/#1.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines®). Prostate cancer. Version 2.2021. National Comprehensive Cancer Network; 2021. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
Parker CC, James ND, Brawley CD, Clarke NW, Hoyle AP, Ali A, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392:2353–66.
Kinsey EN, Zhang T, Armstrong AJ. Metastatic hormone-sensitive prostate cancer: a review of the current treatment landscape. Cancer J. 2020;26:64–75.
Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33.
Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97.
Shore N, Chowdhury S, Villers A, Klotz L, Siemens DR, Phung D, et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomised, double-blind, phase 2 study. Lancet Oncol. 2016;17:153–63.
Penson DF, Armstrong AJ, Concepcion R, Agarwal N, Olsson C, Karsh L, et al. Enzalutamide versus bicalutamide in castration-resistant prostate cancer: the STRIVE trial. J Clin Oncol. 2016;34:2098–106.
Hussain M, Fizazi K, Saad F, Rathenborg P, Shore N, Ferreira U, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378:2465–74.
US Food and Drug Administration. XTANDI highlights of prescribing information. US Food and Drug Administration; 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/203415s015lbl.pdf.
Astellas Pharma US Inc., Medivation Inc. XTANDI US prescribing information. Astellas Pharma US Inc.; 2019. https://www.astellas.us/docs/us/12A005-ENZ-WPI.pdf.
U.S. Food & Drug Administration. FDA approves enzalutamide for metastatic castration-sensitive prostate cancer. U.S. Food & Drug Administration; 2019. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-enzalutamide-metastatic-castration-sensitive-prostate-cancer.
Astellas Pharma US Inc. Xtandi summary of product characteristics. Astellas Pharma US Inc.; 2018. https://www.ema.europa.eu/en/documents/product-information/xtandi-epar-product-information_en.pdf.
Gravis G, Fizazi K, Joly F, Oudard S, Priou F, Esterni B, et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG- AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:149–58.
Kyriakopoulos CE, Chen YH, Carducci MA, Liu G, Jarrard DF, Hahn NM, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36:1080–7.
Clarke NW, Ali A, Ingleby FC, Hoyle A, Amos CL, Attard G, et al. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: long-term survival results from the STAMPEDE trial. Ann Oncol. 2019;30:1992–2003.
ClinicalTrials.gov. ODM-201 in addition to standard ADT and docetaxel in metastatic castration sensitive prostate cancer (ARASENS). ClinicalTrials.gov; 2016. https://clinicaltrials.gov/ct2/show/NCT02799602.
ClinicalTrials.gov. Enzalutamide in first line androgen deprivation therapy for metastatic prostate cancer (ENZAMET). ClinicalTrials.gov; 2018. https://clinicaltrials.gov/ct2/show/NCT02446405?term=NCT02446405&rank=1.
ClinicalTrials.gov. The PEACE (Posthumous Evaluation of Advanced Cancer Environment) Study (PEACE). ClinicalTrials.gov; 2016. https://clinicaltrials.gov/ct2/show/NCT03004755.
ClinicalTrials.gov. A study of apalutamide (JNJ-56021927, ARN-509) plus androgen deprivation therapy (ADT) versus ADT in Participants with mHSPC (TITAN). ClinicalTrials.gov; 2015. https://clinicaltrials.gov/ct2/show/NCT02489318.
Hoyle AP, Ali A, James ND, Cook A, Parker CC, de Bono JS, et al. Abiraterone in “high-“ and “low-risk” metastatic hormone-sensitive prostate cancer. Eur Urol. 2019;76:719–28.
Acknowledgements
Medical writing and editorial assistance was provided by Beatrice Vetter-Ceriotti, PhD, Folabomi Oladosu, PhD, and Lauren Smith from Complete HealthVizion, funded by the study sponsors. The authors developed the analysis plan and all stages of the manuscript in collaboration with Astellas and Pfizer.
Funding
The study was funded by Astellas Pharma Inc. and Pfizer Inc., the codevelopers of enzalutamide. The sponsors had a role in study design, data analysis and interpretation, and writing of the report.
Author information
Authors and Affiliations
Contributions
AJA had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: AJA, BA, TI, BR, H-JL, GPH, and AS. Acquisition of data: AAA, AJA, AA, RZS, DPP, JH, AV, BA, TI, NDS, FG-V, and AS. Analysis and interpretation of data: AAA, AJA, AA, RZS, DPP, JH, AV, BA, TI, NDS, FG-V, AS, BR, H-JL, GPH, and AS. Drafting of the manuscript: AAA, AJA, AA, RZS, DPP, JH, AV, BA, TI, NDS, FG-V, AS, BR, H-JL, GPH, and AS. Critical revision of the manuscript for important intellectual content: AAA, AJA, AA, RZS, DPP, JH, AV, BA, TI, NDS, FG-V, AS, BR, H-JL, GPH, and AS. Statistical analysis: H-JL. Administrative, technical, or material support: GPH.
Corresponding author
Ethics declarations
Competing interests
AAA has served in a consultant/advisory role for Amgen, Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Ipsen, Janssen, Merck Serono, MSD, Novartis, Noxopharm, Pfizer, Sanofi, Telix Pharmaceuticals, and Tolmar and on speakers’ bureaus for Astellas, Amgen, Bayer, Ipsen, Janssen, Merck Serono, Novartis, Pfizer, and Sanofi and has received investigator or institutional funding from Aptevo Therapeutics, Astellas, AstraZeneca, Bionomics, Bristol Myers Squibb, GlaxoSmithKline, Ipsen, Novartis, MedImmune, Merck Serono, Pfizer, Sanofi Aventis, and SYNthorx and travel accommodation/expenses from Amgen, Astellas, Janssen, Merck Serono, Novartis, Pfizer, and Tolmar. AJA has served in a consultant/advisory role for Astellas, AstraZeneca, Bayer, Clovis, Dendreon, Janssen, Medivation, Merck, Novartis, Pfizer, and Sanofi and on speakers’ bureau for Bayer and Dendreon and has received research funding from Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Constellation, Dendreon, Janssen, Medivation, Merck, Novartis, Pfizer, Roche/Genentech, and Sanofi, honoraria from Dendreon, Janssen, and Sanofi, and travel accommodation/expenses from Astellas, Bayer, Dendreon, and Janssen. AA has received travel/accommodation/expenses from Astellas, Bayer, Ipsen, Janssen, and Olympus. RZS has served in a consultant/advisory role for AstraZeneca, AbbVie, Amgen, Astellas, Exelixis, Janssen Oncology, Merck, Sanofi, and Pfizer, has received research funding from Astellas, AbbVie, Incyte, Janssen Oncology, and Macrogenics, honoraria from Astellas, and travel accommodation/expenses from Corcept Therapeutics, and is the coinventor to Corcept Therapeutics patent licensed by The University of Chicago for combination AR/GR inhibition in prostate cancer. DPP has served in a consultant/advisory role for Ada Cap (Advanced Accelerator Applications), Amgen, Astellas, AstraZeneca, Bayer, Bicycle Therapeutics, Boehringer Ingelheim, Bristol Myers Squibb, Clovis Oncology, Exelixis, Incyte, Janssen, Lilly, Mirati, Monopteros, Pfizer, Pharmacyclics, Roche, Seattle Genetics, and Urogen, has received research funding from Ada Cap (Advanced Accelerator Applications), Agensys Inc, Astellas, AstraZeneca, Bayer, BioXcel Therapeutics, Bristol Myers Squibb, Clovis Oncology, Eisai, Endocyte, Genentech, Innocrin, Lilly, MedImmune, Medivation, Merck, Mirati, Novartis, Pfizer, Progenics, Replimune, Roche, Sanofi Aventis, and Seattle Genetics, and had stock ownership in Bellicum Pharmaceuticals (sold July 2020) and Tyme (sold October 2019). JH has received research funding from Astellas and has been an investigator for MDxHealth. AV has received research funding from Astellas and Ipsen and personal fees from Astellas. BA has served in a consultant/advisory role for Astellas, AstraZeneca, Bayer, BMS, Eisai, Ferring, Janssen, MSD, Pfizer, Roche, and Sanofi and on speakers’ bureau for Astellas, AstraZeneca, Bayer, BMS, Eisai, Ferring, Janssen, MSD, Pfizer, Roche, and Sanofi and has received research funding from Astellas, AstraZeneca, Bayer, BMS, Eisai, Ferring, Janssen, MSD, Pfizer, Roche, and Sanofi and travel accommodation/expenses from Astellas, AstraZeneca, Bayer, BMS, Eisai, Ferring, Janssen, MSD, Pfizer, Roche, and Sanofi. TI has served in a consultant/advisory role for Astellas, Bayer, and Janssen and on speakers’ bureau for Astellas, Bayer, Janssen, and Sanofi and has received research funding from Astellas and Bayer. NDS has served in a consultant/advisory role for Amgen, Astellas, AstraZeneca, Bayer, Dendreon, Ferring, Genentech/Roche, Janssen Scientific Affairs, Medivation, Myovant Sciences, Pfizer, and Tolmar and on speakers’ bureau for Bayer, Dendreon, and Janssen and has received research funding from Amgen, Astellas, AstraZeneca, Bayer, Dendreon, Ferring, Genentech/Roche, Janssen Scientific Affairs, Medivation, Myovant Sciences, Pfizer, and Tolmar and travel accommodation/expenses from Amgen, Astellas, AstraZeneca, Bayer, Dendreon, Ferring, Genentech/Roche, Janssen Scientific Affairs, Medivation, Myovant Sciences, Pfizer, and Tolmar. FG-V has served in a consultant/advisory role for AbbVie, Astellas, AstraZeneca, Bayer, Ferring, GE Healthcare, GlaxoSmithKline, Ipsen, Janssen, and Sanofi and has received honoraria from AbbVie, Astellas, AstraZeneca, Bayer, Ferring, GE Healthcare, GlaxoSmithKline, Ipsen, Janssen, and Sanofi and research funding from AbbVie, Astellas, and AstraZeneca. BR is an employee and has stock ownership in Pfizer. H-JL and GPH are employees of Astellas. AS has served in a consultant/advisory role for Alere, Astellas, Bristol Myers Squibb, Ferring, Ipsen Pharma, Janssen, Roche, Stebatiotechnology, and Synergo, as an investigator for Amgen, Astellas, AstraZeneca, Cepheid, CureVac, GeneDX Bioscience, Immatics, Janssen, Johnson & Johnson, Karl Storz AG, Medivation, Novartis, and Roche, and on speakers’ bureau for Astellas, CureVac, Ipsen Pharma, Janssen, and Sanofi Aventis, has received travel accommodation/expenses from CureVac, Ferring, Ipsen Pharma, Janssen, and Sanofi Aventis, has provided expert testimony for GBA, and has patents pending—A290/99, AT00/0001:C-Trap, and 2018/6579.
Ethics approval and consent to participate
The study protocol was approved by local independent review boards and conducted according to provisions of the Declaration of Helsinki and Good Clinical Practice Guidelines of the International Conference on Harmonisation. All patients provided written informed consent. An independent data safety monitoring board evaluated unblinded safety data on an ongoing basis.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Azad, A.A., Armstrong, A.J., Alcaraz, A. et al. Efficacy of enzalutamide in subgroups of men with metastatic hormone-sensitive prostate cancer based on prior therapy, disease volume, and risk. Prostate Cancer Prostatic Dis 25, 274–282 (2022). https://doi.org/10.1038/s41391-021-00436-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-021-00436-y
This article is cited by
-
Unravelling oligometastatic disease from the perspective of radiation and medical oncology. Part II: prostate cancer and colorectal cancer
Clinical and Translational Oncology (2022)