Abstract
A simple screening test to identify the early stages of Alzheimer’s disease (AD) is urgently needed. We investigated whether odor identification impairment can be used to differentiate between stages of the A/T/N classification (amyloid, tau, neurodegeneration) in individuals with amnestic mild cognitive impairment or AD and in healthy controls. We collected data from 132 Japanese participants visiting the Toranomon Hospital dementia outpatient clinic. The odor identification scores correlated significantly with major neuropsychological scores, regardless of apolipoprotein E4 status, and with effective cerebrospinal fluid (CSF) biomarkers [amyloid β 42 (Aβ42) and the Aβ42/40 and phosphorylated Tau (p-Tau)/Aβ42 ratios] but not with ineffective biomarkers [Aβ40 and the p-Tau/total Tau ratio]. A weak positive correlation was observed between the corrected odor identification score (adjusted for age, sex, ApoE4 and MMSE), CSF Aβ42, and the Aβ42/40 ratio. The odor identification score demonstrated excellent discriminative power for the amyloidogenesis stage , according to the A/T/N classification, but was unsuitable for differentiating between the p-Tau accumulation and the neurodegeneration stages. After twelve odor species were analyzed, a version of the score comprising only four odors—India ink, wood, curry, and sweaty socks—proved highly effective in identifying AD amyloidogenesis, showing promise for the screening of preclinical AD.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) is the most prevalent cause of dementia and a substantial challenge to healthcare, medical, and socioeconomic systems worldwide. AD accounts for 60–80% of dementia cases. Approximately 6.7 million older patients in the United States are affected by AD1. Without improvement in available therapies, this number could increase to 13.8 million by 20601. The economic value of unpaid caregiving for patients with dementia in the US was approximately $339.5 billion in 20221.
AD pathology is characterized by accumulation of amyloid β (Aβ) protein in extracellular senile plaques and of intracellular neurofibrillary tangles (NFTs) composed of phosphorylated Tau (p-Tau)2. Aβ accumulation begins approximately 25 years before the onset of symptoms, whereas p-Tau accumulation starts approximately 10 years before symptom onset3. The sequence of events from Aβ accumulation to subsequent p-Tau-induced neurotoxicity and neuroinflammation by activated microglia is known as the amyloid cascade hypothesis2,4 and has been the basis for the development of disease-modifying therapies5.
In 2011, the US National Institute on Aging/Alzheimer’s Association (NIA-AA) classified AD into three stages: preclinical stage, mild cognitive impairment (MCI) due to AD, and AD dementia. Preclinical AD can be diagnosed based on Aβ42 levels in the cerebrospinal fluid (CSF)6. The following biochemical biomarkers for AD diagnosis have been established: decreased CSF Aβ42 levels, decreased Aβ42/40 ratio, increased p-Tau/Aβ42 ratio, and increased total Tau (t-Tau)/Aβ42 ratio7,8. Plasma biomarkers such as the Aβ42/amyloid precursor protein (APP) ratio determined using immunoprecipitation/mass spectrometry9 and pTau181 levels quantified using an ultrasensitive automated enzyme-linked immunosorbent assay (ELISA) system10 have also been developed. As a result, AD diagnosis has shifted from being based primarily on clinical assessments to relying on biochemical markers.
In 2016, the NIA-AA proposed the classification system known as A/T/N based on Aβ (A), p-Tau (T), and neurodegeneration (N). This system has been widely adopted to categorize patients with AD according to their biomarker profiles11,12 and has shown promising results in the prediction of disease progression and the identification of intervention targets11,13. However, concerns have been raised about the accuracy of the A/T/N classification due to the variability in the CSF measurements among different facilities14,15. Particularly, Aβ42 is easily adsorbed, and its abundance may therefore be underestimated14. This variability in measurement may lead to misclassification of some healthy individuals as amyloid positive.
Lumbar puncture for CSF collection and positron emission tomography (PET) are commonly performed to identify the biomarker required for the A/T/N classification11. However, these methods have limitations, including invasiveness, high costs, and radiation exposure. Therefore, alternative, less invasive, more cost-effective screening tests are required. One of the most promising is the odor identification score, which is a well-established early marker for AD16. However, there are no available reports on the suitability of the odor identification score for categorization based on the A/T/N classification system. In this study, our objective was to assess the effectiveness of the odor identification score for early AD diagnosis.
Methods
Participants
This single-center prospective study was conducted in Japan in compliance with the Declaration of Helsinki. The Clinical Research Ethics Committee of Toranomon Hospital approved the study protocol (Clinical Research No. 1388). A total of 150 Japanese participants of both sexes were recruited. All participants provided written informed consent before the study and underwent physical and neurological examinations at the outpatient dementia clinic of the Toranomon Hospital in Tokyo between April 2017 and April 2021. Eighteen potential participants were excluded because they met at least one of the exclusion criteria, which were as follows: having sample artifacts [bloody CSF (one participant)] or additional diseases [dementia with Lewy bodies (DLB) (seven participants), frontotemporal dementia (FTD) (one participant), multiple system atrophy (one participant), depression (two participants), bipolar disorder (two participants), alcoholism (one participant), severe paranasal sinusitis (two participants), normal-pressure hydrocephalus (one participant), vascular dementia, epileptic seizures, hepatic encephalopathy, postoperative sinus, hypothyroidism, or any blood diseases], heavy smoking, or treatment with antiplatelet or anticoagulant drugs.
All participants underwent physical and neurological examinations and neurological testing, including the Mini-Mental State Examination (MMSE), Alzheimer-Disease Assessment Scale-Cognitive-Japanese version (ADAS-cog J), Frontal Assessment Battery (FAB), 15-item Geriatric Depression Scale (GDS), and Wechsler Memory Scale-revised (WMS-R). Brain function was evaluated based on scores from the attention and concentration (A/C), delayed recall (DR), and logical memory II (LM-II) subtests of the WMS-R. Additional imaging studies were performed, including magnetic resonance imaging (MRI) and 123I N-isopropyl-p-iodoamphetamine brain perfusion single-photon emission computed tomography (SPECT). Patients with DLB were excluded based on 123I-metaiodobenzylguanidine (MIBG) and dopamine transporter (DAT) scans. These tests were conducted in cases exhibiting symptoms such as olfactory dysfunction, constipation, rapid eye movement sleep behavior disorder, hallucinations, extrapyramidal symptoms, altered consciousness, or posterior cerebral hypoperfusion assessed via SPECT. Participants with MIBG H/M ratios below 2.2 or with DAT striatal or putaminal binding ratios of − 2.0 z-scores or lower were classified as having neurodegenerative parkinsonism and excluded from the study17.
AD was diagnosed based on the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition Text Revision (DSM-IV-TR) and the National Institute of Neurological Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association clinical definition18 of probable AD. Participants with AD diagnosed by these diagnostic criteria had MMSE scores ranging from 9 to 30 and ADAS-cog J scores of 7 or higher. In contrast, aMCI was diagnosed according to the clinical criteria of the Alzheimer’s Association Workgroup of the National Institute on Aging and classified as amnesia19. Participants with memory complaints but no significant impairment in daily functioning were classified as having aMCI if they scored between 23 and 30 on the MMSE and 12 or lower on the ADAS-cog J and were > 1.0 standard deviations below the age- and education-adjusted cutoff scores on the DR portion of one LM-II of the WMS-R. Healthy controls without cognitive impairments had normal daily functioning, scored between 25 and 30 on the MMSE and 8 or lower on the ADAS-cog J, and were within 1.0 standard deviations of the age- and education-adjusted cutoff scores on the DR portion of one LM-II story of the WMS-R.
All neuropsychological tests and imaging diagnoses mentioned above were used as clinical aids by an experienced neurologist and geriatrician, who strictly adhered to the diagnostic criteria.
Odor identification test
The Open Essence (OE) test (Fujifilm Wako Pure Chemicals Corporation, Osaka, Japan) was employed to measure odor identification ability. The OE test was specifically developed to assess the odor identification ability in the Japanese population. This card-based odor identification test involves the microencapsulation of the same 12 odors featured in the Smell Stick Identification Test (OSIT-J) in a solid cream form. It comprises a total of 12 odors, including Indian ink (borneol), wood (essential oil), perfume, menthol (menthol), Japanese orange (artificial flavor), curry (natural flavor), cooking gas (tetrahydrothiophene), rose, hinoki (Japanese cypress essential oil), sweaty socks (isovaleric acid), condensed milk (artificial flavor), and roasted garlic (natural flavor). Each card folded in two contains microcapsules containing one of the 12 odors on the inner adhesive surface. The microcapsulated odor is released upon unfolding of the card. The participant was then required to select one of the six printed answers (four different odors, “unknown,” and “odorless”) in the manner of a choice task with six alternatives. Its user-friendly nature makes this test suitable for outpatient clinics, and it has been widely used in recent olfaction studies carried out in Japan20.
Collection of CSF and plasma samples
CSF and plasma samples were obtained from participants. These samples were not collected from patients who had been treated with antiplatelet or anticoagulant drugs or had any blood diseases. The participants were instructed to have their last meal at 9:00 p.m. on the night before the lumbar puncture, refrain from consuming alcohol, and sleep for at least 6 h. Lumbar punctures were performed between 9:00 and 10:00 a.m. A 23-25G top spinal needle with stainless steel and a polypropylene base was used to collect CSF into a sterile 10ml polypropylene spit tube. All procedures were performed by an experienced physician using the same technique and equipment. The collected CSF was centrifuged at 2200 × g for 7 min at 20 °C to precipitate the cells and other insoluble materials. The resulting supernatant was separated in 500 µl aliquots into 1.5 ml polypropylene microtubes. Following CSF collection, whole blood was collected from the left upper arm in a vacuum tube containing EDTA 2Na. The plasma was centrifuged at 2200 × g for 15 min at 4 °C and stored in 500 µl aliquots. All samples were stored at − 80 °C within 1 h of collection until analysis. Only one freeze–thaw cycle was performed.
ELISA
The concentrations of Aβ1-40, Aβ1-42, Tau phosphorylated at threonine 181 (p-Tau181), and t-Tau in CSF were determined using commercially available ELISA kits. Specifically, Human β Amyloid (1–40) and Human β Amyloid (1–42) ELISA Kits (Fujifilm Wako, Osaka, Japan) were used to measure Aβ1-40 and Aβ1-42 levels, respectively. TAU (pT181) Human ELISA Kit and TAU (Total) Human ELISA Kit (Thermo Fisher Scientific, Waltham, MA, USA) were used to measure p-Tau181 and t-Tau levels, respectively. These ELISA kits employ enzyme antibody labeling for the colorimetric detection of the target molecules.
Identification of the apolipoprotein E phenotype
The apolipoprotein E (ApoE) phenotype was identified by separating plasma samples using isoelectric electrophoresis, probing with an ApoE polyclonal antibody, and performing western blot analysis using an ApoE isoelectric focusing system (JOKOH, Tokyo, Japan). For sample loading, a pre-treatment solution was prepared by combining 10 μl of the sample with 100 μl of preparing solution. This pre-treatment solution was then applied to 3.5 × 10 mm filter paper for sample application and allowed to absorb at 22 °C for 15 min. Samples of 3/3 and 2/4 phenotypes were concurrently run in each gel as a routine verification measure (Supplementary Fig. S1).
Statistical analyses
To investigate the correlations and associations among the odor identification scores, neuropsychological test scores, and CSF biomarkers, we employed Pearson’s correlation to assess linear relations, Spearman’s correlation to assess nonlinear relations, and chi-squared tests to assess associations between categorical variables. One-way analysis of variance (ANOVA) or Kruskal–Wallis tests were performed to compare three or more groups. If multiple comparisons were necessary as a post-hoc analysis, the Tukey Kramer test was used after ANOVA, and the Scheffe test was employed after the Kruskal–Wallis test.
Receiver operating characteristic (ROC) analysis was performed using R (version 4.1.1; R Foundation for Statistical Computing, Vienna, Austria) to determine the diagnostic cutoff values for CSF markers in patients with AD versus patients with aMCI or healthy individuals with normal cognition (Table 1, Supplementary Fig. S2). The ROC curve in this context is presented in Supplementary Fig. S2. The cutoff value was defined as the value corresponding to the point on the ROC curve that minimizes the Euclidean distance to the coordinates (0, 1). Specificity and sensitivity were calculated based on the coordinates of that point. Based on lower Aβ42 (A+), higher p-Tau (T+), and higher t-Tau (N+) than the respective cutoff values, eight A ± /T ± /N ± groups were defined (Table 2, Supplementary Fig. S3).
The cutoff values for odor identification scores were determined through ROC analysis to distinguish among four disease stages (normal biomarkers, amyloidogenesis, p-Tau accumulation, AD neurodegeneration) consisting of seven distinct pathological conditions (A−/T−/N−, A+/T−/N−, A+/T+/N−, A+/T+/N+, A−/T+/N−, A−/T+/N+, and A−/T−/N+). The pathological interpretation of each category within the ATN classification is as follows: A−T−N− signifies normal biomarkers. A + T−N−, A + T + N−, A + T + N+, and A + T−N + denote the AD continuum, with the potential inclusion of suspected non-AD pathophysiology. (SNAP) in the A + T−N + category. Additionally, A−T + N−, A−T−N+, and A−T + N + may be construed as indicative of SNAP12 (Table 3, Supplementary Fig. S4).
Next, we examined the correlations between the odor identification score and neuropsychological score or CSF biomarkers in four different stages based on the A/T/N classification (A−/T−/N−, A+/T−/N−, A+/T+/N−, and A+/T+/N+) and considering the presence or absence of ApoE4 (Table 4). We analyzed the correlation between odor identification scores and CSF biomarkers, adjusting for age, sex, ApoE4 status, and MMSE scores across three groups: A, B, and C. (Table 5: A: a group of the normal class and three AD continuum classes, B: a group of the normal class and two p-Tau accumulation classes without amyloidogenesis, C: a group of the normal class and three SNAP classes). Differences in the area under the curve (AUC) were analyzed using the bootstrap method with a resampling frequency of 20,000 to determine whether the odor identification score could replace each of the CSF biomarkers considered (Table 6, Supplementary Fig. S5).
Finally, we conducted logistic regression on 12 olfactory species for the odor identification score (Standard Version) and identified the optimal combination with the minimum Akaike Information Criterion (AIC) to detect amyloidogenesis in individuals diagnosed with AD (Table 7. The effectiveness of this combination was compared to that of the standard combination using ROC analysis (Tables 7 and 8, Supplementary Fig. S6).
Results
A total of 132 patients were analyzed in this study, including 49 patients with AD [mean age: 71.7 ± 7.9 years, age range: 50–79 years, 34 men (69%) and 15 women (31%)], 23 patients with amnestic MCI (aMCI) [mean age: 72.7 ± 6.9 years, age range: 56–80 years, 11 men (48%) and 12 women (52%)], and 60 healthy controls [mean age: 70.2 ± 7.3 years, age range: 41–80 years, 35 men (60%) and 25 women (40%)] (Fig. 1, Supplementary Table ST1).The study workflow is illustrated in Fig. 1.
Flowchart of participants from examination to A/T/N classification and evaluation of olfactory discrimination. CSF cerebrospinal fluid; DLB diffuse Lewy body disease; FTD frontotemporal dementia; MCI mild cognitive impairment; AD Alzheimer's disease; ROC receiver operating characteristic; Aβ amyloid-β; p-Tau phosphorylated Tau; t-Tau total Tau; A+ amyloid-positive individuals; A− amyloid-negative individuals; T+ phosphorylated Tau-positive individuals; T− phosphorylated Tau-negative individuals; T+ total Tau-positive individuals; T− total Tau-negative individuals; SNAP suspected non-Alzheimer’s disease pathophysiology; ApoE apolipoprotein E; MMSE Mini-Mental State Examination; AUC area under the curve.
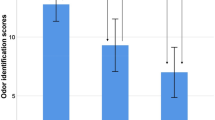
The average age of the 132 participants was 71.2 ± 7.5 years. Sixty-seven were men (50.8%) and 65 were women (49.2%). The years spent in formal education averaged 14.2 ± 2.2, and there were no significant differences observed among the normal, aMCI, and AD groups. The overall number of ApoE4 carriers was 44 individuals (33.6%), with a significantly higher prevalence in the AD group (32 individuals; 65.3%). The MCI group included seven carriers (30.4%), and the healthy control group included five (8.5%). The odor identification score had an average value of 5.3 ± 2.5 points, with the healthy control and MCI groups scoring 6.2 ± 2.1 and 6.1 ± 2.4 points, respectively. In contrast, participants with AD scored 3.8 ± 2.1, indicating a significant decline compared to the other two groups (Supplementary Table ST1).
Participant attributes based on clinical diagnosis classification (Supplementary Table ST1) and A/T/N classification were compared (Table 2) using one-way ANOVA or Kruskal–Wallis tests. Significant differences were observed in MMSE, ADAS-cog J, FAB, WMS-R A/C, DR, LM-II, and odor identification scores, as well as in CSF Aβ1-42, p-Tau181, and t-Tau levels, and the Aβ42/40 ratio (all P < 0.001). Additionally, significant differences between the healthy control and the AD group were noted for all these variables. Correlations between the values from the aMCI and AD groups were found for MMSE, ADAS-cog J, FAB, WMS-R A/C, DR, LM-II, and odor identification scores, as well as for CSF Aβ1-42 and t-Tau levels (Supplementary Table ST1).
ROC analysis was performed to further evaluate the discriminatory power of the odor identification score in differentiating between the three experimental groups. The AUC for the odor identification score (0.785) was similar to that of Aβ42/Aβ40 (0.777; Table 1). The resulting cutoff values were 658.48 pg/ml (AUC: 0.825, sensitivity: 71.4%, specificity: 85.5%) for Aβ42, 58.37 pg/ml (AUC: 0.721, sensitivity: 79.6%, specificity: 60.2%) for p-Tau and 756.6 pg/ml (AUC: 0.721, sensitivity: 63.3%, specificity: 77.1%) for t-Tau (Table 1, Supplementary Fig. S2). We categorized participants based on the A/T/N classification using these specific cutoff values, resulting in the distribution outlined in Table 2. The normal AD biomarker group (A−T−N−) showed significant declines in multiple neuropsychological assessments compared to the AD continuum groups (A+/T−/N−, A+/T+/N−, and A+/T+/N+), worsening with higher ATN stages. Specifically, significant differences were observed in MMSE, ADAS, FAB, DR, LM-II, and odor identification (all P < 0.001). This result indicates that the A/T/N classification system accurately reflects clinical progression, as depicted in Supplementary Fig. S3. Multiple comparisons were conducted across the eight stages of the A/T/N classification, focusing on p-values indicating significance at the 5% and 10% levels (Supplementary Table ST2). In some cases, even though we initially observed differences at a level of significance of 5% in the ANOVA or Kruskal–Wallis test (Table 2), subsequent multiple comparisons did not show any differences at this significance level. A summary of the statistical comparison between different categories of the A/T/N classification is shown in Supplementary Table ST2.
Post-hoc analysis revealed significant differences between the group with normal AD biomarker levels (A−/T−/N−, group 1) and the AD continuum group (A+/T−/N−, group 2; A+/T+/N−, group 3; A+/T+/N+, group 4; and A+/T−/N+, group 5) for MMSE [(1) vs. (3), P = 0.043], ADAS-cog J [(1) vs. (3), P = 0.010; (1) vs. (4), P = 0.010; (1) vs. (5), P = 0.025], delayed recall [(1) vs. (3), P = 0.004; (1) vs. (4), P < 0.001], and logical memory II [(1) vs. (3), P = 0.008; (1) vs. (4), P < 0.001]. However, no statistically significant differences at the 5% significance level were found for the odor identification score [(1) vs. (4), P = 0.060; (1) vs. (5), P = 0.069] or FAB [(1) vs. (3), P = 0.080]. In the comparison between the group with normal biomarker levels and the SNAP group (A−/T + N−, group 6; A−/T+/N+, group 7; and A−/T−/N+, group 8), significant differences were evident for MMSE [(1) vs. (7), P = 0.038] and DR [(1) vs. (7), P = 0.039]. Furthermore, significant differences were observed between the AD continuum and the SNAP groups for ADAS-cog J [(4) vs. (8), P = 0.028], DR [(3) vs. (8), P = 0.035; (4) vs. (8), P = 0.007], and LM-II [(4) vs. (8), P = 0.011] (Supplementary Table ST2).
Furthermore, we assessed the discriminatory ability of the odor identification score at each A/T/N pathological stage (Table 3). The odor identification score had a higher AUC (0.761, sensitivity = 0.659, specificity = 0.676) during amyloidogenesis compared to changes in biomarker levels (Analysis No. 1), irrespective of the presence or absence of neurodegeneration. However, during the p-Tau accumulation (Analysis No. 2, AUC = 0.717, sensitivity = 0.514, specificity = 0.860) and neurodegeneration stages (Analysis No. 3, AUC = 0.716, sensitivity = 0.545, specificity = 0.786), the sensitivity of the odor identification score was low, making the distinction between p-Tau accumulation and neurodegeneration after amyloidogenesis challenging. In addition, the AUC (0.670) and sensitivity (0.486) were low in the comparison of individuals from the normal to the p-Tau accumulation stages with or without neurodegeneration (Analysis No. 4). Therefore, the odor identification score offers superior performance for the identification of individuals in the amyloidogenesis stage, but it is unsuitable for discriminating between the p-Tau accumulation and neurodegeneration stages, or for identifying p-Tau accumulation without amyloidogenesis and SNAP. The details of the findings are presented in Table 3 and Supplementary Fig. S4. A post-hoc power analysis showed that all analyses had a significance level of P < 0.05 and a power above 0.8. The power values for the comparisons described above were 0.996, 0.966, 0.925, 0.837, and 0.809, respectively. As statistically significant ROC curves were obtained for the biomarker of interest, as illustrated in Supplementary Fig. S4, we were able to determine the cutoff value even with our current sample size. However, for a comprehensive evaluation of the estimated cutoff value, it is imperative to apply the same analysis to an independent set of samples. This step will confirm the adequacy of the sample size for the intended purpose.
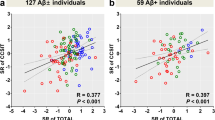
Further analyses were performed to examine the association between the odor identification score, the neuropsychological test scores, and CSF biomarker levels in individuals with and without the ApoE4 isoform (Table 4). The results showed a significant correlation between the odor identification score and the scores of the neuropsychological tests (MMSE, P < 0.0001; ADAS-cog, P < 0.0001; FAB, P < 0.0001; DR, P < 0.0001; and LM-II, P < 0.0001), independently of ApoE4 status. Significant correlations were observed between the odor identification score and the levels of CSF biomarkers that are valid for AD diagnosis (CSF Aβ42, P = 0.0005; Aβ42/40, P = 0.0001; p-Tau/Aβ42 ratio, P < 0.0001; Table 4). Further analysis showed that the odor identification score had a weak correlation with Aβ42 levels in CSF and with the Aβ42/40 ratio, after adjusting for age, sex, ApoE4 status, and performance in the MMSE test in the normal biomarker and selected AD continuum group (Spearman’s rho = 0.230, P = 0.043 and rho = 0.292, P = 0.010, respectively; Table 5A). The odor identification score displayed no correlation with any AD biomarkers in the CSF for either the group classified as the normal biomarkers and p-Tau accumulation without amyloidogenesis group (Table 5B), or in the normal and SNAP group (Table 5C).
Finally, we investigated whether the odor identification score could be used as a substitute for CSF biomarkers in AD diagnosis. The AUC for the odor identification score and for several CSF biomarkers were compared using the bootstrap method. No significant differences were observed in AUC between the odor identification score and any of the CSF biomarkers, including Aβ42 (P = 0.455) and the Aβ42/Aβ40 (P = 0.898) and p-Tau/Aβ42 ratios (P = 0.315). These findings demonstrate that odor identification score has the potential to serve as a surrogate marker for these CSF biomarkers in AD diagnosis and can discriminate amyloid changes in AD. The findings are summarized in Table 6, and ROC curves are presented in Supplementary Fig. S5. Logistic regression on the overall odor identification score and on each of the subscores corresponding to 12 individual odors revealed greater contributions from four odors: India ink, wood, curry, and sweaty socks. Compared to the entire array of 12 odors, the use of this specific combination of four resulted in an improved Akaike's Information Criterion (AIC) value and in a higher sensitivity (0.854 > 0.659) and AUC value (0.809 > 0.761) according to the ROC analysis (Tables 7 and 8, Supplementary Fig. S6).
Discussion
Odor identification tests have gained attention as a potential screening tool for the early stages of AD. Approximately 85–90% of patients with AD exhibit olfactory impairment, with a reported sensitivity and specificity of approximately 85% compared to healthy individuals21. Individuals with severe olfactory impairment, even with normal cognitive function, have a higher risk of developing MCI22. Moreover, individuals with MCI that exhibit olfactory impairment are more likely to progress to advanced cognitive dysfunction and develop AD23. A reduced odor identification score indicates symptom progression even in cases of subjective cognitive decline24,25. Furthermore, the odor identification score has been reported to possess superior performance when distinguishing between AD and healthy participants compared to between stages of AD26. The odor identification score excelled in differentiating between healthy controls and prodromal AD (AUC = 0.908) but was not as effective in distinguishing between prodromal AD and AD dementia (AUC = 0.773)27. These findings show that odor identification scores are highly sensitive in the early stages of AD but may not be as suitable for monitoring disease progression27. High scores on the 40-item University of Pennsylvania Smell Identification Test are typically linked to a negative amyloid PET scan result28. Impaired odor identification was observed in older adults with elevated cortical amyloid levels, suggesting the potential of the odor identification score for detecting preclinical AD in cognitively normal individuals29.
However, meta-analysis reveals minimal associations between odor identification and PET or CSF biomarkers for AD among older adults30. Moreover, impaired odor identification within the AD spectrum may be a result of neurodegeneration rather than a direct impact of Aβ or p-Tau burden31.
Association between the odor identification score and Aβ deposition
In both mouse models and in humans, evidence suggests that Aβ protein deposition in the olfactory system is associated with olfactory dysfunction and neuronal damage. Mouse models such as Tg2576 mice show Aβ deposition in the olfactory bulb (OB) before cognitive impairment32,33,34. Similarly, Amyloid precursor protein/Presenilin1 mice exhibit Aβ deposition throughout the olfactory circuit35. Furthermore, 5 × Familial AD mice, a model for AD pathology, display high levels of Aβ accumulation in the olfactory sensory network36. In humans, amyloid deposition directly affects olfactory regions in the brain, leading to olfactory impairment37. Autopsy studies on 536 cases examining OB neuropathology in AD cases showed Aβ accumulation in the OB starting from stage 0 of Tahl Aβ phase, progressing as they advance to stage 537.
Soluble Aβ has been identified in nasal secretions of patients with AD, and high levels of nasal oligomers have been linked to AD progression within three years38. This suggests that Aβ oligomers may impair the olfactory nerve even before Aβ accumulation is observed in the OB. Nevertheless, odor identification scores in AD may not be directly related to Aβ burden, as indicated by reports of no difference in odor discrimination scores between Pittsburgh compound B (PiB)-positive and PiB-negative patients with MCI39. Aβ pathology reaches a plateau early during the symptomatic phase of the disease and does not correlate well with clinical features or with AD severity40.
Association between odor identification score and p-Tau and other Tau accumulation
Alternatively, olfactory impairment in AD may be linked to p-Tau accumulation rather than the presence of Aβ in the OB. NFTs, a pathological hallmark of AD and neurodegeneration41, are frequently observed in the OB42,43. The distribution of NFTs in AD is classified according to the Braak classification into stages I–VI40. In the preclinical stage of AD, p-Tau accumulation begins in the entorhinal cortex (Braak stages I and II). This p-Tau accumulation leads to olfactory impairment associated with NFT accumulation in the entorhinal cortex and CA1 region of the hippocampus44.
Tau is more likely to accumulate in the OB as AD progresses, increasing with the Braak stage, leading to a worsening in olfactory dysfunction. Tau is believed to be the primary protein involved in the pathogenesis of olfactory impairment in AD. However, it has been reported that while Aβ pathology in the OB aligns with AD manifestation, Tau pathology in the OB is universally present in older adults and does not serve as an indicator of AD severity37. This suggests that Aβ deposition in the OB may serve as an early marker of AD onset. Olfactory impairment is correlated with CSF t-Tau/Aβ1-42 ratio, p-Tau/Aβ1-42 ratio, and t-Tau levels, but not with Aβ1-42 levels alone, except in ApoE ε4 carriers45. However, in the analysis of CSF biomarkers, no significant associations were observed between the odor identification score and CSF p-Tau or Aβ1-42 levels30.
Association between odor identification score and neurodegeneration
Baek et al. analyzed olfactory function according to the A/T/N classification using PET and found a correlation between declining olfactory function and decreased neuropsychological test scores. However, after adjusting for cognitive function, olfactory impairment was not correlated with Aβ or Tau but rather with the volume of the temporal lobe cortex31. These findings suggest that olfactory impairment is primarily influenced by neurodegeneration rather than by amyloid or Tau pathologies.
CSF biomarkers reportedly exhibit a faster rate of change in AD compared to PET imaging46. PET imaging may be more useful to assess a later stage of the disease compared to the A/T/N classification based on CSF biomarkers, likely influenced by neurodegeneration.
Our findings, odor identification impairments, and CSF biomarkers
Our institution has implemented a standardized CSF sample collection method for A/T/N classification to prevent potential misclassification of healthy individuals as patients with AD due to inappropriately measured Aβ42 levels. Additionally, the A/T/N classification system excludes SNAP cases, enhancing AD diagnostic accuracy. By excluding SNAP cases and improving A/T/N classification accuracy, we found that olfactory ability correlated slightly with the Aβ42/40 ratio but not with p-Tau after narrowing down the participant population. The A/T/N classification system based on CSF biomarkers captures pathological brain changes at an earlier stage compared to PET imaging. Therefore, odor identification impairments precede stage 0 of the Tahl Aβ phase, characterized by Aβ42 accumulation in the OB37. Olfactory impairment also occurs shortly after the onset of Aβ42 accumulation in the cerebral cortex.
Our results suggest that olfactory identification tests are effective in distinguishing preclinical AD during the transition from normal to amyloidogenesis. We also confirm the decrease in olfactory identification ability as the disease progresses, from A + to T + and N +. This indicates that the olfactory identification score can serve as a reliable, non-invasive, and cost-effective marker for improving the accuracy of screening for very early stage of AD. Moreover our clinical classification study found a correlation between olfactory impairment and several cognitive test scores, including the ADAS-cog J, DR, LM-II, and FAB. This demonstrates the involvement of the frontal lobe beyond the olfactory cortex and limbic system. Early Aβ42 accumulation in the default mode network (DMN), which includes the orbitofrontal cortex (OFC) and posterior cingulate cortex (PCC)47, likely impairs olfactory function by damaging these areas. The OFC serves as the endpoint of the olfactory pathway, whereas the PCC is functionally connected to the anterior cingulate cortex and hippocampus/intraorbital cortex47. Functional MRI studies have indicated that reduced task-related activation of the olfactory network is associated with decreased task-related inhibition of the DMN, which coincides with Aβ plaque deposition in the frontal lobe48,49. Therefore, Aβ may impair the DMN, gradually decreasing the olfactory identification score before p-Tau accumulates in the entorhinal cortex.
Improved discriminative ability of a 4-item odor identification score for accumulated Aβ42 in the orbitofrontal cortex
As shown in Table 8 and Supplementary Fig. S6, the use of a combination of four odors (India ink, wood, curry, and sweaty socks) identified AD amyloidogenesis with a higher sensitivity, suggesting its potential for adoption in a clinical setting. Recent studies suggest that odor discrimination, particularly that involved in distinguishing between types of smells, primarily occurs in the orbitofrontal cortex (OFC), whereas the piriform cortex (PirC) and amygdala show less intricate discrimination50. The accumulation of Aβ42 in the OFC suggests that variations in the specific regions within the OFC may determine the types of impaired olfactory components.
Limitations, challenges, and potential clinical application of odor identification score to other neurodegenerative disorders
This study has certain limitations. The exclusive focus on a Japanese population raises concerns about generalizability due to potential regional variations influenced by environmental and dietary factors. Excluding SNAP cases (including those of Parkinson's disease [PD]) affects the diagnostic accuracy of AD, posing challenges in real-world clinical settings. While the odor identification score could indicate early AD stages, larger-scale studies encompassing diverse ethnic backgrounds are required. Excluding patients with severe dementia due to challenges in advanced assessments like ADAS-cog J or WMS-R might introduce bias. This exclusion potentially constrains the study's relevance to those with mild to moderate dementia. Nevertheless, it is noteworthy that both odor identification score measurements and CSF biomarker analyses were conducted in patients with severe dementia, aiding in bias mitigation.
A significant limitation is that not all participants had MIBG or DAT scans. Olfactory dysfunction is a common early sign of both DLB/PD and AD, the most prevalent neurodegenerative diseases in older adults51. Out of the 132 participants, only 43.9% underwent these scans, possibly concealing latent DLB or PD cases. Nevertheless, participants without these scans exhibited posterior cerebral hypoperfusion on SPECT, leading to their exclusion from the study. Low odor identification scores can also be observed in frontotemporal dementia FTD51. Thus, the study emphasizes the need for a comprehensive approach, considering clinical symptoms, brain MRI, and specialized tests such as MIBG and DAT scans for accurate diagnosis52.
Our data challenges conventional beliefs, suggesting CSF Aβ42 levels alone have higher specificity than the Aβ42/40 ratio. This ratio is essential for differentiating AD and non-AD cases identified by amyloid PET using CSF biomarkers. Notably, Aβ42 alone exhibits better predictive performance for AD onset in patients with MCI8. Our study also demonstrates that the odor identification score could be used as a substitute for CSF biomarkers, with its associated AUC being almost equivalent to that observed for Aβ42/Aβ40. However, it is not a direct determinant for future anti-amyloid therapy. Positive scores necessitate confirmation through CSF biomarkers or PET scans for a definitive diagnosis.
Nevertheless, the score is a very useful and cost-effective screening tool51. Conversely, in specific conditions such as progressive supranuclear palsy or corticobasal degeneration, low odor identification scores are not typically observed52, making the odor identification score useful in ruling out these conditions.
Conclusion
The odor identification score offered a superior performance in the discrimination of amyloidogenesis with and without neurodegeneration but not in that between p-Tau accumulation and neurodegeneration. These findings indicate that the odor identification score could be useful for differentiating amyloid changes according to the A/T/N classification. Thus, the odor identification score may be a useful diagnostic marker for preclinical AD and may be a suitable screening tool for disease-modifying therapies.
Data availability
The corresponding author will make the data supporting the findings of this study available upon request.
References
Alzheimer’s disease facts and figures. Alzheimers Dement. 19, 1598–1695 (2023).
Hardy, J. A. & Higgins, G. A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 256, 184–185 (1992).
Sperling, R. A. et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 280–292 (2011).
Jack, C. R. Jr. et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 9, 119–128 (2010).
Tolar, M., Abushakra, S. & Sabbagh, M. The path forward in Alzheimer’s disease therapeutics: Reevaluating the amyloid cascade hypothesis. Alzheimers Dement. 16, 1553–1560 (2020).
McKhann, G. M. et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 263–269 (2011).
Fiandaca, M. S., Mapstone, M. E., Cheema, A. K. & Federoff, H. J. The critical need for defining preclinical biomarkers in Alzheimer’s disease. Alzheimers Dement. 10, S196–S212 (2014).
Hansson, O., Lehmann, S., Otto, M., Zetterberg, H. & Lewczuk, P. Advantages and disadvantages of the use of the CSF amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer’s disease. Alzheimers Res. Ther. 11, 34 (2019).
Nakamura, A. et al. High performance plasma amyloid-beta biomarkers for Alzheimer’s disease. Nature 554, 249–254 (2018).
Tatebe, H. et al. Quantification of plasma phosphorylated tau to use as a biomarker for brain Alzheimer pathology: Pilot case-control studies including patients with Alzheimer’s disease and down syndrome. Mol. Neurodegener. 12, 63 (2017).
Hampel, H. et al. Developing the ATX(N) classification for use across the Alzheimer disease continuum. Nat. Rev. Neurol. 17, 580–589 (2021).
Jack, C. R. Jr. et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14, 535–562 (2018).
Tan, M. S. et al. Longitudinal trajectories of Alzheimer’s ATN biomarkers in elderly persons without dementia. Alzheimers Res. Ther. 12, 55 (2020).
Hansson, O. et al. The Alzheimer’s Association international guidelines for handling of cerebrospinal fluid for routine clinical measurements of amyloid β and tau. Alzheimers Dement. 17, 1575–1582 (2021).
Hansson, O. et al. The impact of preanalytical variables on measuring cerebrospinal fluid biomarkers for Alzheimer’s disease diagnosis: A review. Alzheimers Dement. 14, 1313–1333 (2018).
Murphy, C. Olfactory and other sensory impairments in Alzheimer disease. Nat. Rev. Neurol. 15, 11–24 (2019).
Lanfranchi, F. et al. Different z-score cut-offs for striatal binding ratio (SBR) of DaT SPECT are needed to support the diagnosis of Parkinson’s disease (PD) and dementia with Lewy bodies (DLB). Eur. J. Nucl. Med. Mol. Imaging 50, 1090–1102 (2023).
McKhann, G. et al. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–944 (1984).
Morris, J. C. Revised criteria for mild cognitive impairment may compromise the diagnosis of Alzheimer disease dementia. Arch. Neurol. 69, 700–708 (2012).
Sanke, H. et al. Olfactory dysfunction predicts the development of dementia in older patients with type 2 diabetes. Diabetes Res. Clin. Pract. 174, 108740 (2021).
Woodward, M. R. et al. Validation of olfactory deficit as a biomarker of Alzheimer disease. Neurol. Clin. Pract. 7, 5–14 (2017).
Yaffe, K. et al. Olfaction and risk of dementia in a biracial cohort of older adults. Neurology 88, 456–462 (2017).
Albers, M. W. et al. At the interface of sensory and motor dysfunctions and Alzheimer’s disease. Alzheimers Dement. 11, 70–98 (2015).
Jobin, B., Zahal, R., Bussières, E. L., Frasnelli, J. & Boller, B. Olfactory identification in subjective cognitive decline: A meta-analysis. J. Alzheimers Dis. 79, 1497–1507 (2021).
Wang, Q. et al. Olfactory dysfunction is already present with subjective cognitive decline and deepens with disease severity in the Alzheimer’s disease spectrum. J. Alzheimers Dis. 79, 585–595 (2021).
Quarmley, M. et al. Odor identification screening improves diagnostic classification in incipient Alzheimer’s disease. J. Alzheimers Dis. 55, 1497–1507 (2017).
Audronyte, E., Pakulaite-Kazliene, G., Sutnikiene, V. & Kaubrys, G. Properties of odor identification testing in screening for early-stage Alzheimer’s disease. Sci. Rep. 13, 6075 (2023).
Kreisl, W. C. et al. Odor identification ability predicts PET amyloid status and memory decline in older adults. J. Alzheimers Dis. 62, 1759–1766 (2018).
Growdon, M. E. et al. Odor identification and Alzheimer disease biomarkers in clinically normal elderly. Neurology 84, 2153–2160 (2015).
Tu, L. et al. Association of odor identification ability with amyloid-beta and tau burden: A systematic review and meta-analysis. Front. Neurosci. 14, 586330 (2020).
Baek, M. S. et al. Effect of A/T/N imaging biomarkers on impaired odor identification in Alzheimer’s disease. Sci. Rep. 10, 11556 (2020).
Wesson, D. W. et al. Sensory network dysfunction, behavioral impairments, and their reversibility in an Alzheimer’s beta-amyloidosis mouse model. J. Neurosci. 31, 15962–15971 (2011).
Cao, L. et al. Abeta alters the connectivity of olfactory neurons in the absence of amyloid plaques in vivo. Nat. Commun. 3, 1009 (2012).
Yoo, S. J. et al. Differential spatial expression of peripheral olfactory neuron-derived BACE1 induces olfactory impairment by region-specific accumulation of beta-amyloid oligomer. Cell Death Dis. 8, e2977 (2017).
Yao, Z. G., Hua, F., Zhang, H. Z., Li, Y. Y. & Qin, Y. J. Olfactory dysfunction in the APP/PS1 transgenic mouse model of Alzheimer’s disease: Morphological evaluations from the nose to the brain. Neuropathology 37, 485–494 (2017).
Son, G. et al. Region-specific amyloid-beta accumulation in the olfactory system influences olfactory sensory neuronal dysfunction in 5xFAD mice. Alzheimers Res. Ther. 13, 4 (2021).
Tremblay, C. et al. Olfactory bulb amyloid-beta correlates with brain Thal amyloid phase and severity of cognitive impairment. J. Neuropathol. Exp. Neurol. 81, 643–649 (2022).
Yoo, S. J. et al. Longitudinal profiling of oligomeric Abeta in human nasal discharge reflecting cognitive decline in probable Alzheimer’s disease. Sci. Rep. 10, 11234 (2020).
Bahar-Fuchs, A. et al. Olfactory deficits and amyloid-beta burden in Alzheimer’s disease, mild cognitive impairment, and healthy aging: A PiB PET study. J. Alzheimers Dis. 22, 1081–1087 (2010).
Braak, H. & Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259 (1991).
Fukutani, Y. et al. Neuronal loss and neurofibrillary degeneration in the hippocampal cortex in late-onset sporadic Alzheimer’s disease. Psychiatry Clin. Neurosci. 54, 523–529 (2000).
Wen, Y., Yang, S., Liu, R. & Simpkins, J. W. Transient cerebral ischemia induces site-specific hyperphosphorylation of Tau protein. Brain Res. 1022, 30–38 (2004).
Mundiñano, I. C. et al. Increased dopaminergic cells and protein aggregates in the olfactory bulb of patients with neurodegenerative disorders. Acta Neuropathol. 122, 61–74 (2011).
Wilson, R. S., Arnold, S. E., Schneider, J. A., Tang, Y. & Bennett, D. A. The relationship between cerebral Alzheimer’s disease pathology and odour identification in old age. J. Neurol. Neurosurg. Psychiatry 78, 30–35 (2007).
Lafaille-Magnan, M. E. et al. Odor identification as a biomarker of preclinical AD in older adults at risk. Neurology 89, 327–335 (2017).
Hansson, O. Biomarkers for neurodegenerative diseases. Nat. Med. 27, 954–963 (2021).
Yu, M., Sporns, O. & Saykin, A. J. The human connectome in Alzheimer disease — relationship to biomarkers and genetics. Nat. Rev. Neurol. 17, 545–563 (2021).
Lu, J. et al. Disruptions of the olfactory and default mode networks in Alzheimer’s disease. Brain Behav. 9, e01296 (2019).
Sedghizadeh, M. J. et al. Olfactory response as a marker for Alzheimer’s disease: Evidence from perceptual and frontal lobe oscillation coherence deficit. PLOS ONE 15, e0243535 (2020).
Sagar, V., Shanahan, L. K., Zelano, C. M., Gottfried, J. A. & Kahnt, T. High-precision mapping reveals the structure of odor coding in the human brain. Nat. Neurosci. 26, 1595–1602 (2023).
Walker, I. M., Fullard, M. E., Morley, J. F. & Duda, J. E. Olfaction as an early marker of Parkinson’s disease and Alzheimer’s disease. Handb. Clin. Neurol. 182, 317–329 (2021).
Tsuboi, Y., Wszolek, Z. K., Graff-Radford, N. R., Cookson, N. & Dickson, D. W. Tau pathology in the olfactory bulb correlates with Braak stage, Lewy body pathology and apolipoprotein epsilon4. Neuropathol. Appl. Neurobiol. 29, 503–510 (2003).
Acknowledgements
The authors express their sincere gratitude to the collaborators of this study. We express our heartfelt gratitude to Mr. Hirahito Hazama, a clinical psychologist, for his invaluable contributions in conducting the neuropsychological assessments of the participants. We also express our gratitude to Dr. Masamichi Imai and Ms. Kyoko Yamamoto, a registered dietitian, for their assistance in organizing the scores of neuropsychological and odor identification tests. In addition, we acknowledge the dedication of the nurses involved in performing lumbar punctures and that of the laboratory technicians responsible for storing and managing specimens. Furthermore, our deepest appreciation goes to all the participants and their families who generously participated in this study. This research was made possible by the collective efforts and support of these individuals, and we are truly thankful for their invaluable contributions.
Funding
The funding was provided by Okinaka Memorial Foundation (2017, 2018, 2020 and 2021, without grant numbers).
Author information
Authors and Affiliations
Contributions
Y.I. contributed to drafting/revision of the manuscript content, including medical writing; study concept or design; and analysis or interpretation of data. Y.I. accepts responsibility for conducting the research. I.H. had a major role in statistical analysis and data interpretation. K.Y. had a major role in biochemical examination and drafting/revision of the manuscript content, including medical writing. Y.O. contributed to drafting/revision of the manuscript content, including medical writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Igeta, Y., Hemmi, I., Yuyama, K. et al. Odor identification score as an alternative method for early identification of amyloidogenesis in Alzheimer’s disease. Sci Rep 14, 4658 (2024). https://doi.org/10.1038/s41598-024-54322-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-54322-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.