Abstract
Odor identification ability may serve as an important diagnostic biomarker in Alzheimer’s disease (AD). The aim of the study is to investigate the contribution of A/T/N neuroimaging biomarkers to impaired odor identification ability in the Alzheimer’s disease spectrum. In 127 participants, we compared A/T/N neuroimaging biomarkers between normosmia and hyposmia groups, and performed correlation analysis between the biomarkers and Cross-Cultural Smell Identification Test (CCSIT) scores. Additionally, path analysis for odor identification ability was performed using cognitive function as a mediator. In between-group comparison, individuals with hyposmia showed higher frequency of amyloid-β (Aβ) positivity, and lower neuropsychological test performance than those with normosmia. After correction for covariates including total cognition scores, there was no difference in the Aβ or tau burden between the normosmia and hyposmia groups, and no correlation between CCSIT scores and Aβ or tau burden. Meanwhile, cortical volumes in the lateral and medial temporal cortices were smaller in the hyposmia group and decreased with the worsening of CCSIT scores. Path analysis showed that only neurodegeneration had a direct effect on odor identification, while Aβ and tau burden contributed to odor identification with the mediation of cognition. In the Alzheimer’s disease spectrum, impaired odor identification ability may be attributable to neurodegeneration rather than the direct effect of Aβ or tau burden.
Similar content being viewed by others
Introduction
Odor identification ability is impaired in patients with Alzheimer’s disease (AD)1. A step-wise deterioration of odor identification ability with the advancement of cognitive status enabled discrimination of cognitively unimpaired individuals, mild cognitive impairment (MCI), and AD patients2,3. Longitudinal studies on MCI patients also showed that poor odor identification ability was associated with cognitive decline4,5, and thereby predicted conversion from MCI to AD4,6. In postmortem studies, impaired antemortem odor identification ability was associated with the amyloid-β (Aβ) burden in the global cortex7, and tau in the entorhinal cortex and hippocampus7,8, and was therefore helpful for predicting Aβ-positivity by in vivo positron emission tomography (PET)9,10. Additionally, impaired odor identification has been associated with the CSF biomarker for neurodegeneration11 and volume atrophy in the medial temporal cortex12,13 where tau pathology appears early in AD14. However, in these A/T/N biomarkers, there is no information regarding which biomarker has the greatest effect on odor identification.
Olfactory function not only requires the physical activation of diverse olfactory receptor cells by chemical substances, but also includes adaptation process to provide sensitivity and amplification and inhibition to enhance the odor detection. Subsequently, olfactory information is transferred to the cerebral cortex to interact with emotional response and higher olfactory function such as odor identification15,16. This process requires memory and naming functions, suggesting that cognitive impairment may directly affect the odor identification ability17,18. Moreover, the brain regions related to the central olfactory pathway, such as the entorhinal cortex, amygdala, and hippocampus overlap with the regions most vulnerable to pathological changes in AD19,20,21. Therefore, it remains unclear whether AD pathology directly effects odor identification ability, or if it is mediated by cognitive dysfunction.
In this study, we sought to investigate the relationship between odor identification ability and each A/T/N imaging biomarker. We additionally investigated whether cognitive dysfunction mediates the association between the biomarkers and impaired odor identification.
Results
Demographic characteristics
Detailed demographic characteristics of the study participants are summarized in Table 1. Out of 127 participants, 72 participants showed normosmia and 55 showed hyposmia. The ApoE ε4 allele was more frequently found in the hyposmia group than the normosmia group. The hyposmia group was older and more likely to be Aβ-positive and dementia status than the normosmia group. Likewise, the hyposmia group showed worse global cognition as measured by MMSE score, CDR-SB, and total cognition score and worse cognitive domain functions including memory, language and related, visuospatial, and frontal/executive functions.
In 59 Aβ-positive individuals, the hyposmia group was still older than the normosmia group, and showed worse global cognition and worse cognitive domain functions including language and related and frontal/executive functions. In addition, Cross-Cultural Smell Identification Test (CCSIT) scores correlated with total cognition scores (Fig. 1).
Correlation between the total cognition scores and CCSIT scores. Correlation analysis was performed after adjusting for age, sex, presence of ApoE ε4 and years of education in all 127 participants (a), and 59 amyloid-β positive participants (b). Blue dots represent CU, green dots MCI patients, and red dots DEM. Abbreviations: Aβ+ /− = Aβ-positivity, SUVR = standardized uptake value ratio, CU = cognitively unimpaired; MCI = mild cognitive impairment; DEM = dementia, ApoE = apolipoprotein E, SR = Standardized residual, CCSIT = Cross-Cultural Smell Identification Test.
Comparison of A/T/N biomarkers between the normosmia and hyposmia groups
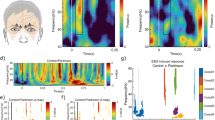
After correction for age, sex, years of education, presence of ApoE ε4, and total cognition score in all participants, the normosmia and hyposmia group did not show difference in the regional Aβ and tau burden. Meanwhile, the hyposmia group exhibited smaller volumes in the entorhinal cortex (Fig. 2a). In Aβ-positive individuals, the hyposmia group showed smaller volumes in the global cortex, superior and middle temporal, and entorhinal cortices, amygdala, and hippocampus (Fig. 2b).
Comparison of 18F-florbetaben, 18F-flortaucipir SUVR and cortical volume between normosmia and hyposmia groups in all participants (a), and amyloid-β positive participants (b). Regional SUVR values were compared between the two groups after adjusting for age, sex, years of education, presence of ApoE ε4 and total cognition scores. For the comparison of regional cortical volumes between the two groups, total intracranial volume was added to the list of covariates. Data are presented as means (horizontal bars) and standard deviations (error bars) of normosmia (red) and hyposmia (blue) groups. P-values for the difference between two groups are expressed as − log10 P. P-values presented with red bars indicate the regions that survived correcting for region-wise multiple comparisons (false discovery rate-corrected P < 0.05), and blue dotted lines represent uncorrected P < 0.05. Abbreviations: Aβ+ /− = Aβ-positivity, SUVR = standardized uptake value ratio, A = 18F-florbetaben SUVR, T = 18F-flortaucipir SUVR, N = cortical volume.
Association between odor identification ability and A/T/N biomarkers
In contrast to Aβ and tau burden which did not correlate with CCSIT score, low CCSIT score was associated with the atrophy in the global cortex, lateral temporal, entorhinal, and parahippocampal cortices, and amygdala (Fig. 3a). In Aβ-positive individuals, low CCSIT score was related with the atrophy in the global cortex, inferior parietal, superior and middle temporal, and entorhinal cortices, amygdala, and hippocampus (Fig. 3b).
Correlation of CCSIT score with 18F-florbetaben, 18F-flortaucipir SUVR and cortical volume in all participants (a), and amyloid-β positive participants (b). We used Pearson’s correlation between the standardized residuals of CCSIT scores and regional SUVR values obtained with multiple linear regression model after adjusting for age, sex, years of education, presence of ApoE ε4 and total cognition scores. A covariate of intracranial volume was additionally adjusted for cortical volume. Horizontal bars represent P-values as − log10 P. Red bars represent the regions that survived correction for region-wise multiple comparisons (false discovery rate-corrected P < 0.05), and blue dotted lines represent uncorrected P < 0.05. In the right side panel, brain regions showing highest significance in correlation with 18F-florbetaben, 18F-flortaucipir, and cortical volume are presented respectively. Blue dots represent CU participants, green dots represent MCI patients, and red dots represent DEM. Abbreviations: Aβ+ /− = Aβ-positivity, SUVR = standardized uptake value ratio, A = 18F-florbetaben SUVR, T = 18F-flortaucipir SUVR, N = cortical volume, SR = Standardized residual, CCSIT = Cross-Cultural Smell Identification Test, CU = cognitively unimpaired; MCI = mild cognitive impairment; DEM = dementia, ApoE = apolipoprotein E.
Because there was a correlation between the CCSIT and total cognition scores, we primarily included total cognition score as a covariate to investigate the pure effect of tau and Aβ burden on deterioration of odor identification function independent of worsening global cognition. However, because the tau and Aβ burden also increase with the worsening of cognition, there remains a concern that the correlation between the CCSIT scores and tau and Aβ burden might not be detected. Therefore, we repeated the analysis without controlling for total cognition score and found that CCSIT score correlated with Aβ and tau burden in the widespread cortical regions in all participants (see Supplementary Fig. S1a) and with tau burden in the global cortex, frontal, parietal, occipital, and posterior cingulate cortices, and amygdala in Aβ-positive individuals (see Supplementary Fig. S1b).
Mediation of cognitive function between A/T/N neuroimaging biomarkers and odor identification ability
Path analysis was used to investigate whether the effect of A/T/N biomarkers on odor identification ability was directly or indirectly mediated by cognitive function. Volumes of the amygdala and entorhinal cortex showed direct effects on the CCSIT score, and an indirect effect mediated by total cognition score was also noted (Fig. 4c,d). In contrast to volume, regional Aβ and tau burden did not show direct effects on the CCSIT score in the regions that showed high significance in correlation analyses (Fig. 4a,b). The effect sizes of indirect effects mediated by total cognition score were relatively small for the regional cortical volumes, compared to the regional Aβ and tau burden.
Schematic diagram of the path analyses for effect of A/T/N imaging biomarkers on odor identification ability. Path analysis for odor identification ability was performed using 18F-florbetaben, 18F-flortaucipir SUVR and cortical volume as predictors and the total cognition score as a mediator. In the upper part, total effects (direct effect + indirect effect) of the A/T/N imaging biomarkers on odor identification are shown. In (c, d), the lower side of the triangle represents the direct effects of the cortical volume on odor identification ability. Indirect effects of the A/T/N imaging biomarkers on odor identification ability are obtained by multiplying two β-coefficients between A/T/N imaging biomarkers and cognition, and between cognition and odor identification ability. Only significant β-coefficients are shown. **P < 0.001, *P < 0.05.
Discussion
In this study, we found that the hyposmia group exhibited smaller volume in the entorhinal cortex than the normosmia group. In Aβ-positive individuals, the hyposmia group exhibited more prominent volume atrophy in the medial and lateral temporal cortices than the normosmia group. In addition, deterioration of odor identification ability correlated with the volume atrophy in the medial and lateral temporal cortices. Meanwhile, there were no group differences in Aβ or tau burden, which did not correlate with the odor identification ability. Finally, of the three imaging biomarkers, only regional volume was directly related to the odor identification ability, while Aβ and tau burden were indirectly related to odor identification ability with cognitive dysfunction as a mediator.
Previous PET imaging studies have shown a higher negative predictive value of odor identification ability on Aβ burden in the elderly, suggesting that individuals with preserved odor identification ability are less likely to have Aβ deposit9,10. In contrast, another PET study showed a correlation between odor identification ability and regional Aβ burden in the posterior cingulate, temporoparietal, and lingual cortices in MCI and AD patients22. In a postmortem study, antemortem odor identification ability was negatively correlated with the pathological Aβ burden7. In contrast to these studies which did not control for the effect of cognitive function, our study demonstrated a lack of correlation between the burden and deterioration of odor identification ability, after controlling for total cognition score. Likewise, there was an indirect effect of Aβ burden on the odor identification ability mediated by cognition in the path analysis. These results indicate that Aβ burden has little direct effect on the odor identification ability.
Olfactory information is transmitted from the olfactory bulb to the primary olfactory cortex encompassing the anterior olfactory nucleus, piriform cortex, anterior cortical nucleus of the amygdala, and entorhinal cortices. The cortical regions such as insula, hippocampus, and hypothalamus have neuronal connections with the primary olfactory cortex for the processing of olfactory information23. Neurofibrillary tangle (NFT) pathology is found in the olfactory bulbs of AD patients8,24 and the spatial distribution of NFT pathology in the early stage of AD overlaps the key regions for olfactory information processing such as the entorhinal cortex, hippocampus, and amygdala25. We therefore may expect a contribution of tau pathology to impaired olfactory function in AD. However, a CSF tau biomarker study26, and postmortem studies, did not find a correlation between tau burden and deterioration of olfactory function27,28. One postmortem study including cognitively unimpaired elderly and AD patients showed an association between odor identification ability and NFT pathology in the entorhinal cortex and hippocampus7. However, this correlation might be driven by AD patients with high NFT burden and worse cognition, because the study did not for control cognitive function. In our study, tau burden in the widespread cerebral cortex correlated with the deterioration of odor identification score, without adjusting for cognitive function. However, this correlation disappeared after the inclusion of cognitive function in the list of covariates. Similar to the results for Aβ, we found an indirect effect of tau burden on odor identification ability mediated by cognition in the path analysis.
Atrophy in the primary olfactory cortex and hippocampus is consistently found in individuals with impaired olfactory function in cognitively unimpaired9, amnestic MCI12, and AD groups29. In accordance with previous studies, we found that impaired odor identification ability was associated with volume atrophy in the medial and lateral temporal cortices even after controlling for cognitive function. In contrast to our expectation that AD pathology, especially tau burden, might explain the volume atrophy and impaired odor identification ability, we found no direct effect of Aβ or tau burden on odor identification. Therefore, impaired olfactory function in AD may be attributable to neurodegeneration in the regions related to olfactory processing rather than the Aβ or tau burden. However, we must consider the contribution of other types of pathologies in AD. Coexisting Lewy body-related pathology in up to 50% of AD patients is a potential candidate30. Lewy body-related pathology in the olfactory bulb and amygdala, which receives the olfactory projection is reported as a potential cause of olfactory dysfunction in neurodegenerative diseases27,28. Another potential candidate is TDP-43 pathology found in 30–70% of AD31. TDP-43 preferentially accumulates in the medial temporal cortex and even in the olfactory bulb in AD, and may cause deterioration of odor identification function32.
Our study was limited first by a relatively small sample size. Additionally, we used a brief odor identification test, with only 12 items, instead of using the more complex University of Pennsylvania Smell Identification Test (UPSIT) with 40 items. Even though these two tests were cross-validated33, and the brief odor identification test is being widely used across studies, UPSIT may provide more precise cut-off for dichotomizing normosmia and hyposmia groups. In addition, we did not test for odor detection. Although this is highly correlated with odor identification test1, additional information about the olfactory function at receptor level may enable more accurate localization of the origin of olfactory dysfunction in AD. Lastly, with the cross-sectional study design, we could not examine changes in biomarkers throughout disease progression.
In conclusion, we identified the effects of A/T/N imaging biomarkers of AD on the odor identification ability. While Aβ and tau burden may have an indirect effect on odor identification ability mediated by cognition, neurodegeneration in the cortical regions related to olfactory processes may have a direct effect on odor identification in AD. Therefore, odor identification ability may be a useful biomarker for the neurodegeneration in AD.
Methods
Participants
For this study, we included 129 individuals who completed the Cross-Cultural Smell Identification Test (CCSIT), neuropsychological test battery, genotyping for apolipoprotein E (ApoE), brain magnetic resonance (MR) imaging, and two PET scans (18F-florbetaben for Aβ and 18F-flortaucipir for tau) at the Memory Disorder Clinic in Gangnam Severance Hospital during the period from January, 2015 to September, 2018. All patients with dementia (DEM) first presented with memory impairment and were clinically diagnosed with probable AD, as proposed by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer Disease and Related Disorders Association34. MCI patients were diagnosed according to the Petersen’s criteria based on their neuropsychological test performances35. No patient showed clinical features atypical of AD. Cognitively unimpaired individuals were healthy volunteers fulfilling Christensen’s diagnostic criteria36, and showing normal cognition in neuropsychological test. We excluded two participants who had a rhinological problem at the time of CCSIT or a history of rhinological surgery. Finally, 127 participants (40 cognitively unimpaired, 51 MCI, and 36 DEM) were included in this study. This study was approved by the institutional review board of Gangnam Severance Hospital (Ref# 3-2014-0286) and written informed consent was obtained from all participants and/or their legal guardians. All research was performed in accordance with the relevant guidelines and regulations.
Olfactory assessment
Odor identification ability was assessed using the CCSIT33. The CCSIT includes 12 microencapsulated odorants attached to test cards. Participants were instructed to scratch, sniff, and select one of the 4 examples. The final score was calculated as the number of correct answers. Based on the resulting CCSIT scores, all participants were classified into either the normosmia (score ≥ 8) or hyposmia (score < 8) group, as previously reported37.
Neuropsychological tests
All participants underwent the Seoul Neuropsychological Screening Battery38,39. We calculated subdomain scores for memory, language and related, visuospatial, frontal/executive, and attention functions with the items in the battery using the method described in a previous study38. Total cognition score was calculated as the sum of all subdomain scores. Mini-Mental State Examination (MMSE) and Clinical Dementia Rating sum-of-boxes (CDR-SB) scores were also assessed for global cognitive function.
Acquisition of PET and MR images
Using a Biograph mCT PET/CT scanner (Siemens Medical Solutions, Malvern, PA, USA), we acquired PET images for 20 min at 80 min after the injection of 18F-flortaucipir, and at 90 min after the injection of 18F-florbetaben. The two PET scans were done in separate days. Computed tomography (CT) images were acquired for attenuation correction prior to the PET scan. Finally, 3D PET images were reconstructed in a 256 × 256 × 223 matrix with 1.591 × 1.591 × 1 mm voxel size using the ordered-subsets expectation maximization algorithm. Aβ-positivity was determined by the agreement of two nuclear medicine specialists using a validated visual assessment method40,41. Axial T1-weighted brain MR images were acquired by 3.0 T MR scanner (Discovery MR750; GE Medical Systems, Milwaukee, WI, USA) with 3D-spoiled gradient-recalled sequences (512 × 512 matrix with voxel spacing 0.43 × 0.43 × 1 mm voxel size).
Image processing steps
T1-weighted MR images were processed with FreeSurfer 5.3 (Massachusetts General Hospital, Harvard Medical School; https://surfer.nmr.mgh.harvard.edu) software for creating participant-specific volume-of-interests (VOIs) mask images as described in our previous study42. MR images were first resliced to FreeSurfer space (256 × 256 × 256 matrix with 1 mm isovoxel) 25 segmented into gray and white matter, and then 3D-surfaces for gray and white matter were reconstructed. Cortical regions were parcellated using curvature information, and subcortical regions were segmented with the probabilistic registration method. By merging anatomically-related regions, participant-specific composite VOI images for 20 cortical and subcortical regions were created. Voxel counts for each region were considered to be regional volume.
Statistical parametric mapping 12 (Wellcome Trust Centre for Neuroimaging, London, UK) and in-house software implemented in MATLAB R2015b (MathWorks, Natick, MA, USA) were used to process the PET images and measure the regional uptake values. PET images were coregistered to individual MR images within FreeSurfer space, and then partial volume effect (PVE) was corrected with the region-based voxel-wise method by using the participant-specific VOI images43. PVE-corrected SUVR images were then created with the cerebellar crus median obtained by overlaying the template cerebellar crus VOI on the spatially normalized PET images. Finally, by overlaying the participant-specific composite VOI, we measured regional PVE-corrected SUVR values.
Statistical analysis
SPSS 23 (IBM Corp., Armonk, NY, USA) was primarily used for the statistical analysis. For between-group comparisons, two-sample t-tests, and chi-square tests were used for continuous and categorical demographic data, respectively. Using the general linear model, regional SUVR values were compared between the normosmia and hyposmia groups after adjusting for age, sex, years of education, presence of ApoE ε4, and total cognition score. For the comparison of regional cortical volumes between the two groups, total intracranial volume was added to the list of covariates. For the correlation analysis between CCSIT scores and regional SUVR values, we first obtained standardized residuals of CCSIT scores and regional SUVR values after adjusting for age, sex, years of education, presence of ApoE ε4, and total cognition score by using the multiple linear regression model. For correlation analysis between CCSIT scores and regional cortical volumes, we used total intracranial volume as additional covariate for obtaining the standardized residuals. Pearson’s correlation was then used to test for correlation between the standardized residuals of CCSIT scores and those of regional SUVR and cortical volume. We repeated all tests without controlling for total cognition score. Only the regions that survived after correcting for multiple comparisons with the Benjamini-Hochberg’s false-discovery rate (FDR) method were considered to be significant44. To evaluate whether cognition mediates the effects of A/T/N imaging biomarkers on CCSIT scores, path analysis was performed using R Statistical Package (Version 3.6.1; Institute for Statistics and Mathematics, Vienna, Austria) using the regional SUVR and cortical volume as predictors and total cognition score as a mediator. Brain regions showing the highe significance for correlation were included in the analysis. Bootstrapping mediation analysis with 1,000 repetitions was used in pathway analyses45.
Data availability
Data generated by this study are available from the corresponding author on reasonable request. The data are not publicly available due to privacy restriction.
References
Mesholam, R. I., Moberg, P. J., Mahr, R. N. & Doty, R. L. Olfaction in neurodegenerative disease: A meta-analysis of olfactory functioning in Alzheimer’s and Parkinson’s diseases. Arch. Neurol. 55, 84–90 (1998).
Quarmley, M. et al. Odor identification screening improves diagnostic classification in incipient Alzheimer’s disease. J. Alzheimer’s Dis. 55, 1497–1507 (2017).
Djordjevic, J., Jones-Gotman, M., De Sousa, K. & Chertkow, H. Olfaction in patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 29, 693–706 (2008).
Conti, M. Z. et al. Odor identification deficit predicts clinical conversion from mild cognitive impairment to dementia due to Alzheimer’s disease. Arch. Clin. Neuropsychol. 28, 391–399 (2013).
Roberts, R. O. et al. Association between olfactory dysfunction and amnestic mild cognitive impairment and Alzheimer disease dementia. JAMA Neurol. 73, 93–101 (2016).
Devanand, D. P. et al. Olfactory deficits predict cognitive decline and Alzheimer dementia in an urban community. Neurology 84, 182–189 (2015).
Wilson, R. S. et al. The relationship between cerebral Alzheimer’s disease pathology and odour identification in old age. J. Neurol. Neurosurg. Psychiatry 78, 30–35 (2007).
Attems, J., Lintner, F. & Jellinger, K. A. Olfactory involvement in aging and Alzheimer’s disease: an autopsy study. J Alzheimers Dis 7, 149–157 (2005) (discussion 173–180).
Vassilaki, M. et al. Neuroimaging biomarkers and impaired olfaction in cognitively normal individuals. Ann. Neurol. 81, 871–882 (2017).
Kreisl, W. C. et al. Odor identification ability predicts PET amyloid status and memory decline in older adults. J. Alzheimers Dis. 62, 1759–1766 (2018).
Reijs, B. L. R. et al. Relation of odor identification with Alzheimer’s disease markers in cerebrospinal fluid and cognition. J. Alzheimers Dis. 60, 1025–1034 (2017).
Hagemeier, J. et al. Odor identification deficit in mild cognitive impairment and Alzheimer’s disease is associated with hippocampal and deep gray matter atrophy. Psychiatry Res. Neuroimaging 255, 87–93 (2016).
Dintica, C. S. et al. Impaired olfaction is associated with cognitive decline and neurodegeneration in the brain. Neurology 92, e700–e709 (2019).
Braak, H. & Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82, 239–259 (1991).
Tortora, G. J. & Derrickson, B. H. Principles of Anatomy and Physiology (Wiley, Hoboken, 2018).
Su, C.-Y. & Menuz, K. Carlson JRJC (2009) Olfactory perception: Receptors, cells, and circuits. Cell 139, 45–59 (2009).
Murphy, C. Olfactory and other sensory impairments in Alzheimer disease. Nat. Rev. Neurol. 15, 11–24 (2019).
Masurkar, A. V. & Devanand, D. J. C. G. R. Olfactory dysfunction in the elderly: basic circuitry and alterations with normal aging and Alzheimer’s disease. Curr. Geriatr. Rep. 3, 91–100 (2014).
Wilson, R. S., Arnold, S. E., Tang, Y. & Bennett, D. A. Odor identification and decline in different cognitive domains in old age. Neuroepidemiology 26, 61–67 (2006).
Ohm, T. G. & Braak, H. Olfactory bulb changes in Alzheimer’s disease. Acta Neuropathol. 73, 365–369 (1987).
Tsuboi, Y. et al. Tau pathology in the olfactory bulb correlates with Braak stage, Lewy body pathology and apolipoprotein ɛ4. Neuropathol. Appl. Neurobiol. 29, 503–510 (2003).
Bahar-Fuchs, A. et al. Olfactory deficits and amyloid-β burden in Alzheimer’s disease, mild cognitive impairment, and healthy aging: A PiB PET study. J. Alzheimers Dis. 22, 1081–1087 (2010).
Benarroch, E. E. Olfactory system: Functional organization and involvement in neurodegenerative disease. Neurology 75, 1104–1109 (2010).
Hyman, B. T., Arriagada, P. V. & Van Hoesen, G. W. Pathologic changes in the olfactory system in aging and Alzheimer’s disease. Ann N Y Acad Sci 640, 14–19 (1991).
Braak, H. & Braak, E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol. Aging 18, 351–357 (1997).
Lafaille-Magnan, M. E. et al. Odor identification as a biomarker of preclinical AD in older adults at risk. Neurology 89, 327–335 (2017).
McShane, R. H. et al. Anosmia in dementia is associated with Lewy bodies rather than Alzheimer’s pathology. J. Neurol. Neurosurg. Psychiatry 70, 739–743 (2001).
Olichney, J. M. et al. Anosmia is very common in the Lewy body variant of Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 76, 1342–1347 (2005).
Murphy, C., Jernigan, T. L. & Fennema-Notestine, C. Left hippocampal volume loss in Alzheimer’s disease is reflected in performance on odor identification: A structural MRI study. J. Int. Neuropsychol. Soc. 9, 459–471 (2003).
Twohig, D. & Nielsen, H. M. Alpha-synuclein in the pathophysiology of Alzheimer’s disease. Mol. Neurodegener. 14, 23 (2019).
Josephs, K. A. et al. Updated TDP-43 in Alzheimer’s disease staging scheme. Acta Neuropathol. 131, 571–585 (2016).
Josephs, K. A. & Dickson, D. W. TDP-43 in the olfactory bulb in Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 42, 390–393 (2016).
Doty, R. L., Marcus, A. & Lee, W. W. Development of the 12-item Cross-Cultural Smell Identification Test (CC-SIT). Laryngoscope 106, 353–356 (1996).
McKhann, G. et al. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–944 (1984).
Petersen, R. C. et al. Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol. 56, 303–308 (1999).
Christensen, K. J., Multhaup, K. S., Nordstrom, S. & Voss, K. A cognitive battery for dementia: development and measurement characteristics. Psychol. Assess. J. Consult. Clin. Psychol. 3, 168 (1991).
Park, S. J., Lee, J. E., Lee, K. S. & Kim, J. S. Comparison of odor identification among amnestic and non-amnestic mild cognitive impairment, subjective cognitive decline, and early Alzheimer’s dementia. Neurol. Sci. 39, 557–564 (2018).
Ahn, H. J. et al. Seoul Neuropsychological Screening Battery-dementia version (SNSB-D): a useful tool for assessing and monitoring cognitive impairments in dementia patients. J. Korean Med. Sci. 25, 1071–1076 (2010).
Kang, Y. & Na, D. L. Seoul Neuropsychological Screening Battery (SNSB) (Human Brain Research & Consulting Co., Seoul, 2003).
Sabri, O. et al. Florbetaben PET imaging to detect amyloid beta plaques in Alzheimer’s disease: Phase 3 study. Alzheimers Dement. 11, 964–974 (2015).
Villemagne, V. L. et al. Amyloid imaging with (18)F-florbetaben in Alzheimer disease and other dementias. J. Nucl. Med. 52, 1210–1217 (2011).
Cho, H. et al. In vivo cortical spreading pattern of tau and amyloid in the Alzheimer disease spectrum. Ann. Neurol. 80, 247–258 (2016).
Thomas, B. A. et al. The importance of appropriate partial volume correction for PET quantification in Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 38, 1104–1119 (2011).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995).
Efron, B. & Tibshirani, R. J. An Introduction to the Bootstrap (CRC Press, Boca Raton, 1994).
Acknowledgements
This research was supported by a grant from Korean Neurological Association (KNA-19-MI-12), faculty research grant of Yonsei University College of Medicine for (6-2018-0068), Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2017R1A2B2006694) and the Ministry of Education (NRF-2018R1D1A1B07049386), and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (Grant number : HI18C1159).
Author information
Authors and Affiliations
Contributions
M.S.B. contributed with: conception and design, collection and assembly of data, data analysis and interpretation, and manuscript writing. H.C. contributed with: conception and design, collection and assembly of data, data analysis and interpretation. H.S.L. contributed with: data analysis and interpretation. J.H.L. and Y.H.R. contributed with: collection and assembly of data, data analysis and interpretation. C.H.L. contributed with: conception and design, administrative support, collection and assembly of data, data analysis and interpretation, manuscript writing, and final approval of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baek, M.S., Cho, H., Lee, H.S. et al. Effect of A/T/N imaging biomarkers on impaired odor identification in Alzheimer's disease. Sci Rep 10, 11556 (2020). https://doi.org/10.1038/s41598-020-68504-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-68504-2
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.