Abstract
This study evaluated the biocompatibility of mineral trioxide aggregate (MTA) and Biodentine (BD) as root-end filling materials. Six mongrel dogs were divided into two equal groups according to the evaluation period; group A: one month and group B: three months. Three premolars of the same quadrant in each arch were used, summing up 36 teeth (6 teeth/dog). These teeth were randomly subdivided into three subgroups according to the root-end filling material used: MTA, BD and no root-end filling material (control). Endodontic access cavities were performed for induction of periapical pathosis. After the infection period, root canal instrumentation and obturation were accomplished. One day after root canal procedures, root-end surgery was performed. Surgical access was achieved and the root-end was resected approximately 3 mm above the apex. Root-end cavity was prepared ultrasonically and filled with the tested materials. All samples were evaluated by radiography and histopathology (Inflammation and new hard tissue formation). Data were collected and subjected to statistical analysis. In group A, MTA subgroup exhibited significant higher mean inflammatory score than BD subgroup (P < 0.05) while no significant difference was recorded between MTA and BD subgroups in group B (P > 0.05). Regarding mean mineralization score, there was no significant difference between all subgroups in both groups A and B (P > 0.05). Biodentine exhibited favorable biocompatibility in the initial stage of healing than MTA and comparable biomineralization.
Clinical relevance: Biodentine could be considered as an acceptable alternative to MTA in peri-radicular surgeries.
Similar content being viewed by others
Introduction
Elimination of microorganisms from the root canal system and filling of the intracanal space to avoid bacterial colonization are the main objectives of the root canal treatment (RCT)1. However, many factors like perforations, instrument breakage, calcifications and anatomic anomalies may fail RCT2. In certain circumstances, conventional RCT is not enough to treat the case and a surgical endodontic interference is needed3.
During peri-radicular surgeries, resection of the root-end produces an exposed apical dentin surface covered by cementum with a root canal at its center. After ultrasonic root-end preparation, root-end-filling cement is usually used to seal the root-end cavity preparation4. Placement of a root-end filling material after root-end resection is mandatory step to make an apical closure5. Furthermore, the orthograde gutta-percha filling alone is insufficient to support bone regeneration6.
The ideal root-end filling material must has excellent sealing ability, biocompatibility, antibacterial effect and good manipulation characteristics7. An ideal root-end filling material that fulfills all the required characteristics for endodontic surgery has yet to be found8. In the last decades, several materials like amalgam, intermediate restorative material (IRM), Super ethoxy benzoic acid (Super- EBA), glass-ionomer cement and composite resin have been applied9.
Mineral trioxide aggregate has less cytotoxicity, better biocompatibility and microleakage protection, giving it more clinical success over traditional root-end filling materials10,11. However, MTA has some drawbacks such as difficult handling, long setting time, potential discoloration, lower compressive and flexural strengths compared with those of dentin and high cost10,12.
Biodentine™ was introduced as a substitute to MTA. Biodentine offers similar properties to those of MTA with better consistency and faster setting time. The main components of MTA are present in Biodentine such as tricalcium silicate, calcium carbonate, and dicalcium silicate5,12,13,14.
Many in vitro studies tested MTA and BD as root-end filling materials10. Four studies compared the biocompatibility, two studies revealed that BD is better than MTA15,16 and the other two studies showed comparable results17,18. Nine studies compared the sealing ability, six studies showed BD to be better19,20,21,22,23,24, one study revealed comparable results25 and last two studies showed MTA to be better5,26.
Gray ProRoot MTA plays the leading role in the field of root-end filling27. To the authors’ knowledge, there is a lack of in vivo studies tested MTA and BD as root-end filling materials8. Therefore, this study aimed to evaluate histologically, for the first time, the biocompatibility of Gray ProRoot MTA and BD when used as root-end filling materials in a dog model. We hypothesized that both Gray ProRoot MTA and BD would have the same biocompatibility.
Materials and methods
Ethical approval
This work was approved by the Ethical Committee at Faculty of Dentistry, Ain Shams University, Cairo, Egypt (16‑12‑2012-Endo). All international and institutional guidelines for animal care and use were followed. The study was reported in accordance with ARRIVE guidelines.
Animal model
The sample size was determined based on earlier studies3,8 using the G*power software 3.1.9.2, where a large effect size of 1.38 was detected. The significance level (α-error) was set at 0.05 and the power (1-β error) was set at 0.8 using two-sided hypothesis test. The estimated sample size was 6 for each subgroup at each evaluation period, summing up a total sample size of 36 teeth.
Six healthy 1–2-year-old male mongrel dogs (17–20 kg body weight) were selected. The animals were kept in separate kennels during the period of the study under proper conditions of nutrition, clean water, lighting, clean environment, temperature and ventilation. Dogs were provided dry food and milk before the beginning of the procedures and were shifted to soft food and milk during the procedural period.
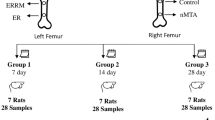
These dogs were classified randomly into two equal groups according to the evaluation period: group A: one month and group B: 3 months. Three premolars of the same quadrant in each arch of each dog were included in this study, summing up the total number of teeth to 36 (6 premolars/ dog and 18 premolars/ group). Each group was randomly subdivided into three subgroups (6 teeth each) according to the root-end filling material used; subgroup 1 (MTA), subgroup 2 (BD) and subgroup 3: no root-end filling material (control, Fig. 1). Coded samples were used throughout the study to avoid possible bias.
Induction of periapical pathosis
All endodontic procedures were performed under general anesthesia. The dogs were pre-medicated with 0.05 mg/kg body weight Atropine sulphate (Atropine sulphate®, ADWIA Co., Egypt) injected subcutaneously and 1mg/kg body weight Xylazine HCl (Xylaject 2%®, ADWIA Co., Egypt) injected intramuscularly. The anesthesia was induced by intravenous Ketamine HCl (Keiran®, EIMC pharmaceuticals Co., Egypt) through intravenous cannula in the cephalic vein at a dose of 5 mg/kg body weight. The anesthesia was maintained with Thiopental sodium (Thiopental sodium®, EIPICO, Egypt) at a dose of 25 mg/kg body weight 2.5% solution injected intravenous (dose to effect).
Endodontic access cavities were prepared (Fig. 2a) with size #2 round bur using high speed handpiece under coolant. The pulp tissues were disrupted with sterile #15–25 Flex-o-File. Supra-gingival plaque contaminated paper points were placed into the canals and sealed with intermediate restorative material (IRM® KinderDent GmbH, Germany).
(a) Representative photograph showing preparation of the access cavities. (b) Representative photo-radiograph showing development of periapical pathosis. (c) Representative photo-radiograph showing determination of the working length. (d) Representative photo-radiograph showing obturated root canals.
Experimental teeth were evaluated by radiography after four weeks to confirm the evidence of development of periapical pathosis (Fig. 2b). Dogs were given soft diet and pain killer, Carprofen 4.4 mg/kg (Rimadyl tab®, Zoetis, USA) orally once daily during this period.
Root canal instrumentation and obturation
After the infection period, the previously infected experimental teeth were re-entered and the paper points were removed. Root canal length was determined by radiography using #15 & #20 Flex-o-Files (Fig. 2c). Root canal instrumentation was accomplished by using step-back technique up to a master apical file #40 Flex-o-File with conjunction with copious irrigation by 5 mL of 2.5% sodium hypochlorite followed by normal saline as final irrigant.
Root canals were subsequently dried with paper points and filled with laterally condensed gutta-percha and AH26 sealer (Fig. 2d). The access cavities were then sealed with intermediate restorative material.
Surgical procedures
One day after root canal procedures, peri-radicular surgery was performed by a single operator (MN). This procedure was performed on two premolars in each quadrant while the third one did not undergo root-end surgery (Control).
Surgical access was achieved by a full muco-periosteal flap with two releasing incisions using a scalpel blade size #15 (BD, São Paulo, São Paulo, Brazil). The flap was reflected and the root apex was approximately localized via pre-determined root canal length. The covering cortical bone of the root ends was removed to expose the required apex of the tooth.
Apical curettage was performed using lucas curette size #85 and #86 (Hu-Friedy, Rio de Janeiro, Rio de Janeiro, Brazil) to remove all necrotic tissues and bone particles from the peri-radicular area. During the surgical phase, 0.5 mL of 2% Xylocaine HCl with 1:50,000 epinephrine was injected into the surgical site to achieve maximum hemostasis.
The root-end was resected approximately 3 mm above the apex using a carbide bur mounted on a high speed handpiece under water coolant at a 90° angle to the long axis of the root. The root-end resection was not performed under microscope magnification. Then, 3 mm in depth from the resected surface was prepared in each sample using an ultrasonic tip (Ultrasonic tip, E32D NSK, Tochigi, Japan) powered by an ultrasonic device (Piezon Master, EMS, Nyon, Switzerland( at a frequency of 32 kHz. Intermittent pressure was employed within-and-out motion to start preparation, then the depth was increased to 3 mm from the resected surface. Finally the tip was moved circumferentially to complete the preparation. A periodontal probe served as measuring device for preparation depth. Then the root-end cavity was irrigated with sterile normal saline.
Root-end cavity preparations were dried with paper points and filled with the tested materials which were mixed according to the previously described manufacturer’s recommendations. Using the carrier (Ultrasonic tip, E32D NSK, Tochigi, Japan), the material was dispensed into the root-end cavity and compacted using a small plugger (Dentsply, York, PA). Excess material was removed and the surface of the root was cleaned with moist gauze. Placement of the tested materials was confirmed by radiography.
The muco-periosteal flap was repositioned and fixed with moderated digital pressure and moist gauze. Suturing with silk thread 4/0 (Ethicon Johnson, São Paulo, São Paulo, Brazil) was done. Sutures were removed 7 days after surgery. Dogs were given soft diet and pain killer as mentioned before during this period.
Methods of evaluation
Radiography evaluation
Periapical radiographs taken after induction of the periapical lesion were compared with follow-up radiographs taken at one month (Group A) and three months (Group B).
Radiography evaluation was done using a modification for the scoring system established by Molven et al.28 as follows:
Score 0: no healing (Increase in size of former radiolucency), Score 1: unsatisfactory healing (No reduction of former radiolucency), Score 2: uncertain healing (Some reduction of former radiolucency), Score 3: incomplete healing and Score 4: complete healing.
The radiographs were evaluated independently by 2 examiners (AMA and AAE). A specific healing category was selected when the two examiners had the same judgment.
Histopathology evaluation
Animals were sacrificed by overdose of general anesthetic (Thiopental sodium rapidly intravenous). Jaws were resected and bone segments including the teeth were cut and prepared for histopathological evaluation. The remnant of the animal body was handled in a proper way (cremated).
Obtained bone blocks were fixed in 10% buffered formalin solution with ratio 1:50. After two weeks of fixation, blocks were decalcified using 17% EDTA solution. The decalcifying solution was renewed on daily basis for about 120 days. After decalcification, samples were dehydrated in ascending concentrations of ethanol and then embedded in paraffin blocks. Blocks were sectioned in bucco-lingual sections at 6µm thickness. Sections were stained with hematoxylin and eosin dye and evaluated by an experienced oral pathologist blinded to the experimental groups. The evaluation included both quantitative and qualitative assessments as follows:
Quantitative evaluation
Inflammatory tissue reaction at the periapical area was evaluated using a scoring system according to Huang et al.29 as follows: Score 0: no inflammatory tissue infiltration (No inflammatory cells or edema detected), Score 1: mild inflammatory tissue infiltration (Sparse infiltration of inflammatory cells with infrequent edema formation), Score 2: moderate inflammatory tissue infiltration (Moderate infiltration of inflammatory cells with frequent edema formation) and Score 3: severe inflammatory tissue infiltration (Dense infiltration of inflammatory cells with intense edema formation).
New hard tissue formation was also assessed using a scoring system according to Huang et al.29 as follows: Score 0: absence of new hard tissue formation, Score 1: partial formation of new hard tissues and Score 2: complete formation of new hard tissue.
Qualitative evaluation
Stained sections were examined under a light microscope at magnification X100, X200 and X400 for assessment of the periapical area, detection of the inflammatory nature and presence of new hard tissue formation.
All measurements were performed by two calibrated and blinded examiners in two different sessions.
Data collection and statistical analysis
Collected data were represented as the mean and standard deviation (SD) values. All data were in form of scores, so non-parametric tests were used for the comparisons. Mann–Whitney U test was used to compare between two groups. Kruskal–Wallis test was used to compare between more than two groups. Wilcoxon signed-rank test was used to study the effect of time in comparisons with two follow up times. Dunn's test was used for pair-wise comparisons when Kruskal–Wallis test had significant results. The significance level was set at P ≤ 0.05. Statistical analysis was performed with SPSS Statistics Version 20 for Windows (SPSS®, Inc., IBM Company, USA).
Results
Radiography findings
Effect of root-end filling materials on radiographic periapical healing score
In group A (After one month), the mean radiography healing scores in subgroup 3 (Control), subgroup 2 (BD) and subgroup 1 (MTA) were 3.17 ± 0.98, 1.50 ± 0.84 and 1.00 ± 0.63, respectively (Figs. 3 and 4). Statistically, there was a significant difference between the three subgroups (P = 0.006). Pair-wise comparisons revealed that subgroup 3 (Control) showed the statistically significant highest mean radiography healing score. There was no statistically significant difference between subgroup 1(MTA) and subgroup 2 (BD); both showed statistically significant lower mean radiography healing score than subgroup 3 (Control).
In group B (After 3 months), the lowest mean radiography healing score was demonstrated in subgroup 2, reaching 2.17 ± 0.41 while the highest mean radiography healing score was demonstrated in subgroup 3, reaching 3.67 ± 0.52 as shown in (Table 1). The mean radiography healing score in subgroup 1 was intermediate (2.83 ± 0.75, Fig. 5). Statistically, there was a significant difference between the three subgroups (P = 0.007). Pair-wise comparisons revealed that subgroup 3 showed the highest mean radiography healing score with non-statistically significant difference from subgroup 1(MTA) but a statistically significant higher mean radiography healing score than subgroup 2 (BD) was recorded. Sub-group 2 (BD) showed the lowest mean radiography healing score with no statistically significant difference from subgroup 1 (P > 0.05) and a statistically significant difference from subgroup 3 (P < 0.05).
Effect of time intervals on radiography periapical healing score for each subgroup
In subgroup 1 (MTA), the mean radiography healing score after one month evaluation period was 1.00 ± 0.63. An increase in this score was recognized after 3 months evaluation period (2.83 ± 0.75). Statistically, this increase was significant (P = 0.020).
In subgroup 2 (BD), the mean radiography healing score after one month evaluation period was 1.50 ± 0.84. An increase in this score was reported after 3 months evaluation period (2.17 ± 0.41). Statistically, this increase was not significant (P = 0.102).
In subgroup 3 (Control), the mean radiography healing score after one month evaluation period was 3.17 ± 0.98. An increase in this score was recorded after 3 months evaluation period (3.67 ± 0.52). Statistically, this increase was not significant (P = 0.180).
Histopathology findings
Quantitative findings
Effect of root-end filling materials on inflammatory tissue reaction at the periapical area in both groups
In group A, the mean inflammatory scores in subgroup 3 (Control), subgroup 2 (BD) and subgroup 1 (MTA) were 1.33 ± 0.52, 2.17 ± 0.41 and 2.83 ± 0.41, respectively (Table 2). Statistically, there was a significant difference between the three subgroups (P = 0.003). Pair-wise comparisons revealed that subgroup 1 showed the statistically significant highest mean inflammatory score. Subgroup 2 showed statistically significant lower mean inflammatory score than subgroup 1. Subgroup 3 showed the statistically significant lowest mean inflammatory score.
In group B, the mean inflammatory scores in subgroup 3 (Control), subgroup 1 (MTA) and subgroup 2 (BD) were 0.33 ± 0.52, 0.83 ± 0.41and 1.33 ± 0.52, respectively as shown in (Table 2). Statistically, there was a statistically significant difference between the three subgroups (P = 0.019). Pair-wise comparisons revealed that subgroup 2 (BD) showed the highest mean inflammatory score with no statistically significant difference from subgroup 1 (P > 0.05) but a statistically significant higher inflammatory score than subgroup 3 (P < 0.05) was noticed. Subgroup 3 showed the lowest mean inflammatory score with no statistically significant difference from subgroup 1 (P > 0.05) and a statistically significant difference from subgroup 2 (P < 0.05).
Effect of time intervals on inflammatory tissue reaction at the periapical area in different subgroups
In subgroup 1 (MTA), the mean inflammatory score after one month evaluation period was 2.83 ± 0.41. A decrease in this score was recognized after 3 months evaluation period (0.83 ± 0.41). Statistically, this decrease was significant (P = 0.024).
In subgroup 2 (BD), the mean inflammatory score after one month evaluation period was 2.17 ± 0.41. A decrease in this score was recorded after 3 months evaluation period (1.33 ± 0.52). Statistically, this decrease was significant (P = 0.025).
In subgroup 3 (Control), the mean inflammatory score after one month evaluation period was 1.33 ± 0.52. A decrease in this score was reported after 3 months evaluation period (0.33 ± 0.52). Statistically, this decrease was significant (P = 0.034).
New hard tissue formation
Effect of root-end filling materials on the rate of new hard tissue formation in both groups
In group A, the mean mineralization scores in subgroup 3 (Control), subgroup 2 (BD) and subgroup 1 (MTA) were 1.17 ± 0.98, 0.83 ± 0.41 and 0.17 ± 0.41, respectively. Statistically, there was no significant difference between the three subgroups (P = 0.067).
In group B, the mean mineralization scores in subgroup 3 (Control), subgroup 1 (MTA) and subgroup 2 (BD) were 2.17 ± 0.98, 1.83 ± 0.75 and 1.17 ± 0.41, respectively as shown in (Table 3). Statistically, there was no significant difference between the three subgroups (P = 0.067).
Effect of time intervals on the rate of new hard tissue formation in different subgroups
In subgroup 1 (MTA), the mean mineralization score after one month evaluation period was 0.17 ± 0.41. An increase in this score was recognized after 3 months evaluation period (1.83 ± 0.75). Statistically, this increase was significant (P = 0.023).
In subgroup 2 (BD), the mean mineralization score after one month evaluation period was 0.83 ± 0.41. An increase in this score was recorded after 3 months evaluation period (1.17 ± 0.41). Statistically, this increase was significant (P = 0.014).
In subgroup 3 (Control), the mean mineralization score after one month evaluation period was 1.17 ± 0.98. An increase in this score was reported after 3 months evaluation period (2.17 ± 0.98). Statistically, this increase was significant (P = 0.014).
Qualitative findings in different subgroups
Subgroup 1 (MTA)
In group A (One month), dense inflammatory cells infiltration (Score 3) was seen with numerous dilated blood vessels and edema spaces engorged by decomposed RBCs. No evidence of newly deposited hard tissue (Fig. 6a).
(a) Representative photomicrograph of subgroup A1 (MTA after one month) showing dense inflammatory cells infiltration, numerous dilated blood vessels and edema spaces (Score 3). (X400, H&E). (b) Representative photomicrograph of subgroup B1 (MTA after three months) showing mild inflammatory cells infiltration (Score 1). (X400, H&E). (c) Representative photomicrograph of subgroup B1showing no inflammatory cells infiltration (Score 0) around MTA in the periapical area (Yellow arrow). Notice the mineralized tissue formed on the surface of MTA (Blue arrows). (X400, H&E).
In group B (Three months), mild chronic inflammatory cells infiltration (Score 1) was observed and separated islands of lymphocytes, macrophages and plasma cells were evident and interlaced with new fibrous ligaments (Fig. 6b). Mineralized tissue was formed on the surface of MTA (Fig. 6c).
Subgroup 2 (BD)
In group A, moderate inflammatory cells infiltration (Score 2) was seen with dilated blood vessels and edema spaces (Fig. 7a). New osseous-like tissue formation could be recognized that was demarcated by dark lines (Fig. 7b).
(a) Representative photomicrograph of subgroup A2 (BD after one month) showing moderate inflammatory cells infiltration (Score 2). (X400, H&E). (b) Representative photomicrograph of the same subgroup showing newly deposited hard tissue (Black arrows) (Score 1). (X400, H&E). (c) Representative photomicrograph of subgroup B2 (BD after three months) showing noticeable increase in blood vessels. (X100, H&E).
In group B, the former appearance of chronic inflammatory cells infiltration decreased but was still evident with numerous dilated blood vessels and edema spaces engorged by decomposed RBCs (Fig. 7c). New osseous-like tissue formation was evident which was demarcated by dark lines.
Subgroup 3 (Control)
In group A, mild inflammatory cells infiltration (Score 1) was seen. New osseous-like tissue formation could be recognized that was demarcated by dark lines.
In group B, no inflammatory cells infiltration (Score 0) was seen (Fig. 8a). New osseous-like tissue formation could be recognized that was demarcated by dark lines. The new osseous-like tissue showed the traditional Howship’s lacunae filled with osteocytes (Fig. 8b).
Discussion
Complete obliteration of the root canal system and induction of a fluid tight seal play a crucial role in successful endodontic therapy. Root-end resection and root-end filling are common surgical procedures for endodontic treatment20,21.
This study evaluated the biocompatibility of two different root-end filling materials, Gray ProRoot MTA and BD. The two tested root-end filling materials were selected because the manufacturer of each material claims its superior qualities in clinical performance. The present results revealed that BD exhibits favorable biocompatibility in the initial stage of healing than MTA and comparable biomineralization. Therefore, the hypothesis of this study is accepted.
The choice of dog as an animal model is based on the fact that dogs have similar apical repair compared with humans in shorter duration (average one sixth of human) due to higher growth rate11,12,30. Double-rooted premolars in one quadrant in each arch were selected summing up a total of 6 teeth in each dog increasing the whole number of samples for a reliable statistical analysis8. Premolars are accessible for endodontic procedures, also having average-sized canals for endodontic manipulation31,32. Age range selected was 1–2 years which was suitable for this study because the premolars are mature at this age range and the animal can withstand general anesthesia procedure at multiple interventions.
Samples were divided into 2 groups according to observation period; one month and three months. One month interval was selected for evaluation of short-term reaction to the committed treatment. Three months interval was selected for evaluation of long-term reaction. This is in agreement with a previous in vivo study in which the periapical healing of both was evaluated by radiography and histopathology after 1 month and 3 months3.
Induction of periapical infection was done in order to simulate clinical conditions. The contaminated paper points were left inside the root canals for four weeks in order to leave sufficient duration for establishment of periapical pathosis as mentioned before by earlier authors32,33.
Although root-end resection is a mandatory step in endodontic surgery, it reduces the total root length and supported root surface. Therefore it changes the biomechanical response of the tooth that may result in unfavorable stress distribution and may increase tooth mobility34. Nevertheless, a 3 mm root-end resection appeared to be mechanically acceptable in order to ensure the long-term prognosis of endodontic surgery35. Moreover, increasing the depth of the retrograde filling significantly decreased apical leakage; there was also a significant increase in leakage as the amount of bevel increased34.
Apical ramifications and laterals canals are very common near root tip36. So, resection at the depth of 3 mm was done. This is in agreement with earlier studies34,35,36. The angle of cutting was 90° to avoid exposing more patent dentinal tubules to bacterial contamination37. The microleakage increases significantly with increased angulations of the resected root-end34,37. On the other hand, the results of an in vitro study showed that when an adequate retrograde cavity depth is prepared, variation in the root-end cutting angle does not necessarily cause any difference in microleakage36.
In group A, most of samples in MTA and BD subgroups showed severe to moderate inflammation. Subgroup 1 exhibited more inflammatory infiltration than subgroup 2 (BD) samples but without significant difference. These results are in accordance with results of other studies that showed comparable healing of BD used in root-end surgery in comparison with MTA38,39,40. These findings are logically attributed to the immediate inflammatory reaction of the peri-radicular tissues to the performed surgical treatment protocol superimposed by the immunological reaction against the previously induced infection. On contrary, the mean inflammatory score (Mild to moderate) in subgroup 3 (Control) was significantly lower than other subgroups. This may be attributed to absence of root-end surgery in subgroup 3.
Regarding new hard tissue formation in group A, the results showed a variable behavior among all the experimental subgroups. The lowest mean mineralization score was demonstrated by samples of MTA while the highest score was demonstrated by the control teeth followed by samples of BD. A possible explanation for superiority of BD is its bioactivity that activates angiogenesis and progenitor periodontal cells, thus promoting healing and remineralization40. Moreover, BD lacks cytotoxicity and stimulates collagen fiber and fibroblast formation41. Biodentine also stimulates osteogenic differentiation of human bone marrow stem cells39. Lee et al. suggested use of BD and MTA as root-end filling materials because in contact with mesenchymal stem cells they induce osteoblast differentiation38.
In group B, the inflammatory scores decreased in all subgroups compared to those at one month evaluation period. This may be attributed to subsiding of inflammation by time.
Regarding new hard tissue formation, healing took place in all surgical sites after three months. The highest mean mineralization score was demonstrated in subgroup 3. There was no significant difference in mean mineralization score between MTA and BD subgroup. These results are in agreement with results of other studies that showed comparable healing of BD and MTA when used in root-end surgery40,42,43. A possible explanation for biocompatibility of MTA after three months evaluation is its unique feature that is osseous-like tissue formation directly on its surface44. It is possible that calcium oxide, in MTA formulation, reacts with water or tissue fluids, forming calcium hydroxide and stimulating hard tissue deposition5,45.
In the present study, mineralized tissue was also seen on the surfaces of MTA and BD and the resected dentin. However, the cementum and bone have very similar characteristics, and there was no specific staining technique to differentiate between the two structures. Hence, the term osseous-like tissue was used, mainly on the basis of the location of this mineralized tissue and some features observed on hematoxylin–eosin stained slides. The difficulty in distinguishing between cementum and bone in the staining is considered a challenge associated with the qualitative assessment. Therefore, the nature of this mineralized tissue and mechanism of its formation need further investigation in the future.
In the present work, the histology findings are in agreement with the radiography findings. Similar findings were recorded before after peri-radicular surgery3. However, periapical radiography could not detect the difference. It can be inferred that minute differences such as reformation of PDL, cementum, and quality of bone cannot always be observed on periapical radiographs, even though the success criteria were based on the correlation between histologic findings and radiographic features as mentioned before3,28.
There are some limitations in this study such as the limited number of animals and assessment of healing only at 2 time points after surgery. Hence, the healing dynamic associated with the tested materials is unknown. The differences between MTA and BD might be more dramatic or non-significant at an earlier or extended follow-up period. Furthermore, bacteria profiles present in the canal may not be as complex as most clinical situations. Therefore, caution must be taken in directly applying these results in clinical conditions. Therefore, future studies are recommended, especially regarding an extended follow-up period and more complex bacterial profiles.
Conclusion
Biodentine exhibited favorable biocompatibility in the initial stage of healing than MTA and comparable biomineralization. Therefore, BD could be considered as an acceptable alternative to MTA in peri-radicular surgeries.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Kohli, M., Berenji, H., Setzer, F., Lee, S. & Karabucak, B. Outcome of endodontic surgery: A meta-analysis of the literature-part 3: Comparison of endodontic microsurgical techniques with 2 different root-end filling materials. J. Endod. 44(6), 923–931. https://doi.org/10.1016/j.joen.2018.02.021 (2018).
Kim, S. & Kratchman, S. Modern endodontic surgery concepts and practice: A review. J. Endod. 32(7), 601–623. https://doi.org/10.1016/j.joen.2005.12.010 (2006).
Basta, D. G., Abu-Seida, A. M., El-Batouty, K. M. & Tawfik, H. M. Effect of combining platelet-rich fibrin with synthetic bone graft on the healing of intrabony defects after apicectomy in dogs with periapical pathosis. Saudi Endod. J. 11, 300–307. https://doi.org/10.4103/sej.sej_191_20 (2021).
Gutmann, J. L. & Harrison, J. W. Surgical Endodontics (Blackwell Scientific Publications, 1991).
Nabeel, M., Tawfik, H. M., Abu-Seida, A. M. & Elgendy, A. A. Sealing ability of Biodentine versus ProRoot mineral trioxide aggregate as root-end filling materials. Saudi Dent. J. 31, 16–22. https://doi.org/10.1016/j.sdentj.2018.08.001 (2019).
Johnson, B. R. Considerations in the selection of a root-end filling material. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 87(4), 398–404. https://doi.org/10.1016/s1079-2104(99)70237-4 (1999).
Parirokh, M. & Torabinejad, M. Mineral trioxide aggregate: a comprehensive literature review—Part I: Chemical, physical, and antibacterial properties. J. Endod. 36(1), 16–27. https://doi.org/10.1016/j.joen.2009.09.006 (2010).
Tang, J., Shen, Z., Qin, W. & Lin, Z. A comparison of the sealing abilities between Biodentine and MTA as root-end filling materials and their effects on bone healing in dogs after periradicular surgery. J. Appl. Oral Sci. 27, e20180693. https://doi.org/10.1590/1678-7757-2018-0693 (2019).
Li, H. et al. Materials for retrograde filling in root canal therapy. Cochrane Database Syst. Rev. 2021(10), 517. https://doi.org/10.1002/14651858.CD005517.pub3 (2021).
Solanki, N. P., Venkappa, K. K. & Shah, N. C. Biocompatibility and sealing ability of mineral trioxide aggregate and biodentine as root-end filling material: A systematic review. J. Conserv. Dent. 21, 10–15. https://doi.org/10.4103/JCD.JCD_45_17 (2018).
Abboud, K. M., Abu-Seida, A. M., Hassanien, E. E. & Tawfik, H. M. Biocompatibility of NeoMTA Plus® versus MTA Angelus as delayed furcation perforation repair materials in a dog model. BMC Oral Health 21, 192. https://doi.org/10.1186/s12903-021-01552-w (2021).
Alazrag, M. A., Abu-Seida, A. M., El-Batouty, K. M. & AshryH, El. Marginal adaptation, solubility and biocompatibility of TheraCal LC compared with MTA-angelus and biodentine as a furcation perforation repair material. BMC Oral Health 20, 298. https://doi.org/10.1186/s12903-020-01289-y (2020).
Okasha, H., Abu-Seida, A. M., Hashem, A. A., El Ashry, S. H. & Nagy, M. M. Inflammatory response and immunohistochemical characterization of experimental calcium silicate-based perforation repair material. Int. J. Exp. Pathol. 103(4), 156–163. https://doi.org/10.1111/iep.12439 (2022).
Arandi, N. Z. & Thabet, M. Minimal intervention in dentistry: A literature review on Biodentine as a bioactive pulp capping material. Biomed. Res. Int. https://doi.org/10.1155/2021/5569313 (2021).
Nunez, C. M., Bosomworth, H. J., Field, C., Whitworth, J. M. & Valentine, R. A. Biodentine and mineral trioxide aggregate induce similar cellular responses in a fibroblast cell line. J. Endod. 40, 406–411. https://doi.org/10.1016/j.joen.2013.11.006 (2014).
Khedmat, S. et al. In vitro cytotoxicity of four calcium silicate-based endodontic cements on human monocytes, a colorimetric MTT assay. Restor. Dent. Endod. 39, 149–154. https://doi.org/10.5395/rde.2014.39.3.149 (2014).
Jung, S., Mielert, J., Kleinheinz, J. & Dammaschke, T. Human oral cells’ response to different endodontic restorative materials: An in vitro study. Head Face Med. 10, 55. https://doi.org/10.1186/s13005-014-0055-4 (2014).
Ceci, M., Beltrami, R., Chiesa, M., Colombo, M. & Poggio, C. Biological and chemical-physical properties of root-end filling materials: A comparative study. J. Conserv. Dent. 18, 94–99. https://doi.org/10.4103/0972-0707.153058 (2015).
Malhotra, S. & Hegde, M. Analysis of marginal seal of ProRoot MTA, MTA angleus, biodentine and glass ionomer cement as root end filling materials: An in vitro study. J. Oral Res. Rev. 7, 44–49. https://doi.org/10.4103/2249-4987.172493 (2015).
Hindlekar, A. & Raghavendra, S. S. Comparative evaluation of sealing ability of three root end filling materials-an in-vitro study. Int. J. Dent. Clin. 6, 4–7 (2014).
Radeva, E., Uzunov, T. & Kosturkov, D. Microleakage associated with retrograde filling after root end resection-in vitro study. J. IMAB 20, 578–583. https://doi.org/10.5272/jimab.2014203.578 (2014).
Han, L. & Okiji, T. Uptake of calcium and silicon released from calcium silicate-based endodontic materials into root canal dentine. Int. Endod. J. 44, 1081–1087. https://doi.org/10.1111/j.1365-2591.2011.01924.x (2011).
Kokate, S. & Pawar, A. An in-vitro comparative sterreomicroscopic evaluation of marginal seal between MTA, glass ionomer cement and biodentine as root end filling materials using 1% methylene blue as tracer. Endodontology 24, 36–42. https://doi.org/10.4103/0970-7212.352091 (2012).
Chalas, R., Mielko, E., Wrobel, J. Z. & Nowak, J. A chemical activity evaluation of two dental calcium silicate based materials. Curr. Issues Pharm. Med. Sci. 28, 89–91. https://doi.org/10.1515/cipms-2015-0051 (2015).
Bolhari, B., Ashofteh Yazdi, K., Sharifi, F. & Pirmoazen, S. Comparative scanning electron microscopic study of the marginal adaptation of four root-end filling materials in presence and absence of blood. J. Dent. (Tehran) 12, 226–234 (2015).
Soundappan, S., Sundaramurthy, J. L., Raghu, S. & Natanasabapathy, V. Biodentine versus mineral trioxide aggregate versus intermediate restorative material for retrograde root end filling: An in vitro study. J. Dent. (Tehran) 11, 143–149 (2014).
Shahi, S. et al. Portland cement: An overview as a root repair material. BioMed Res. Int. https://doi.org/10.1155/2022/3314912 (2022).
Molven, O., Halse, A. & Grung, B. Observer strategy and the radiographic classification of healing after endodontic surgery. Int. J. Oral Maxillofac. Surg. 16, 432–439. https://doi.org/10.1016/s0901-5027(87)80080-2 (1987).
Huang, G. T. J. Apexification: The beginning of its end. Int. Endod. J. 42, 855–866. https://doi.org/10.1111/j.1365-2591.2009.01577.x (2009).
El-Ashry, S., Abu-Seida, A. M., Al-Boghdady, H., El-Batouty, K. & Abdel-Fattah, M. The effect of different formulations of calcium hydroxide on healing of intentionally induced periapical lesions in dogs. Pak. Vet. J. 33, 48–52 (2013).
Eldessoky, A. E., Khalefa, M. M. & Abu-Seida, A. M. Regenerative endodontic therapy in mature teeth with necrotic pulp and apical periodontitis using two disinfection protocols. BMC Oral Health 23, 163. https://doi.org/10.1186/s12903-023-02863-w (2023).
El-Tayeb, M. M., Abu-Seida, A. M., El Ashry, S. H. & El-Hady, S. A. Evaluation of antibacterial activity of propolis on regenerative potential of necrotic immature permanent teeth in dogs. BMC Oral Health 19, 174. https://doi.org/10.1186/s12903-019-0835-0 (2019).
El Ashry, S. H., Abu-Seida, A. M., Bayoumi, A. A. & Hashem, A. A. Regenerative potential of immature permanent non-vital teeth following different dentin surface treatments. Exp. Toxicol. Pathol. 68, 181–190. https://doi.org/10.1016/j.etp.2015.12.001 (2016).
Gilheany, P. A., Figdor, D. & Tyas, M. J. Apical dentin permeability and microleakage associated with root end resection and retrograde filling. J. Endod. 20, 22–26. https://doi.org/10.1016/s0099-2399(06)80022-1 (1994).
Jang, Y., Hong, H. T., Roh, B. D. & Chun, H. Influence of apical root resection on the biomechanical response of a single-rooted tooth: A 3-dimensional finite element analysis. J. Endod. 40, 1489–1493. https://doi.org/10.1016/j.joen.2014.03.006 (2014).
Garip, H., Garip, Y., Oruçoglu, H. & Hatipoglu, S. Effect of the angle of apical resection on apical leakage, measured with a computerized fluid filtration device. Oral Surg. Oral Med. Oral Pathol. Oral. Radiol. Endod. 111, 50–55. https://doi.org/10.1016/j.tripleo.2010.10.034 (2001).
Gagliani, M., Taschieri, S. & Molinari, R. Ultrasonic root-end preparation: Influence of cutting angle on the apical seal. J. Endod. 24, 726–730. https://doi.org/10.1016/S0099-2399(98)80162-3 (1998).
Lee, B. et al. Effects of 3 endodontic bioactive cements on osteogenic differentiation in mesenchymal stem cells. J. Endod. 40, 1217–1222. https://doi.org/10.1016/j.joen.2014.01.036 (2014).
Margunato, S., Tasli, P. N., Aydin, S., Kazandag, M. K. & Sahin, F. In vitro evaluation of proRoot MTA, Biodentine and MM-MTA on human alveolar bone marrow stem cells in terms of biocompatibility and mineralization. J. Endod. 41, 1646–1652. https://doi.org/10.1016/j.joen.2015.05.012 (2015).
Zanini, M., Sautier, J. M., Berdal, A. & Simon, S. Biodentine induces immortalized murine pulp cell differentiation into odontoblast-like cells and stimulates biomineralization. J. Endod. 38, 1220–1226. https://doi.org/10.1016/j.joen.2012.04.018 (2012).
Mori, G. G., Teixeira, L. M., de Oliveira, D. L., Jacomini, L. M. & da Silva, S. R. Biocompatibility evaluation of biodentine in subcutaneous tissue of rats. J. Endod. 40, 1485–1488. https://doi.org/10.1016/j.joen.2014.02.027 (2014).
Jovanovic, L., Miljevic, I., Petrovic, B., Markovic, D. & Bajkin, B. Biocompatibility of three root end filling materials. J. Biomater. Tissue Eng. 4(3), 253–257. https://doi.org/10.1166/jbt.2014.1165 (2014).
Attik, G. N. et al. In vitro biocompatibility of a dentine substitute cement on human MG63 osteoblasts cells: Biodentine™ versus MTA®. Int. Endod. J. 47, 1133–1141. https://doi.org/10.1111/iej.12261 (2014).
Baek, S. H., Lee, W. C., Setzer, F. C. & Kim, S. Periapical bone regeneration after endodontic microsurgery with three different root-end filling materials: Amalgam, SuperEBA, and mineral trioxide aggregate. J. Endod. 36, 1323–1325. https://doi.org/10.1016/j.joen.2010.04.008 (2010).
Al-Sherbiny, I. M., Farid, M. H., Abu-Seida, A. M., Motawea, I. T. & Bastawy, H. Chemico-physical and mechanical evaluation of three calcium silicate based pulp capping materials. Saudi Dent. J. 33, 207–214. https://doi.org/10.1016/j.sdentj.2020.02.001 (2021).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This was self-funded research.
Author information
Authors and Affiliations
Contributions
M.N. carried out all surgeries. A.M.A. and A.A.E evaluated the radiographs. A.M.A. followed up the experimental animals. A.M.A. and H.M.T. wrote the main manuscript text and M.N. prepared figures. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nabeel, M., Abu-Seida, A.M., Elgendy, A.A. et al. Biocompatibility of mineral trioxide aggregate and biodentine as root-end filling materials: an in vivo study. Sci Rep 14, 3568 (2024). https://doi.org/10.1038/s41598-024-53872-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53872-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.