Abstract
This study aimed to evaluate the safety and efficacy of intra-arterial (IA) administration of low- dose tirofiban during endovascular therapy in patients with large ischemic core volumes on initial brain CT. Patients were divided into two groups based on the use of IA tirofiban. We identified 87 patients (16 and 71 patients in the tirofiban and no-tirofiban groups, respectively) with acute ischemic stroke due to intracranial artery occlusion who underwent endovascular therapy with a low Alberta Stroke Program Early CT scores (2–5). Multivariate logistic regression analysis revealed no association between IA tirofiban administration and serious postprocedural hemorrhagic complications (adjusted odds ratio (aOR), 0.720; 95% confidence interval (CI) 0.099–5.219; p = 0.960), any radiologic hemorrhage (aOR 0.076; 95% CI 0.003–2.323; p = 0.139), or 3-month mortality (aOR, 0.087; 95% CI 0.005–1.501; p = 0.093). However, IA tirofiban was associated with a lower 90-day mRS score (aOR, 0.197; 95% CI 0.015–1.306; p = 0.017) and change of NIHSS compared with baseline (aOR, 0.698; 95% CI 0.531–0.917; p = 0.010). IA tirofiban administration during endovascular therapy in patients with large ischemic core volumes may be effective and safe.
Similar content being viewed by others
Introduction
Endovascular therapy (EVT) such as intracranial mechanical thrombectomy (MT) is the first-line treatment strategy for selected patients with acute ischemic stroke (AIS) due to large vessel occlusion (LVO)1,2,3. Moreover, recent studies revealed that even patients with a large ischemic core with an Alberta Stroke Program Early CT Score (ASPECTS) < 6 may benefit from revascularization4,5, despite the currently validated eligibility criteria for MT6.
Tirofiban is a highly selective glycoprotein IIb/IIIa receptor antagonist, which can effectively block the final stages of the platelet aggregation pathway and prevent subsequent thrombus formation, which can potentially benefit successfully recanalized patients with AIS. Recent studies have reported the safety and efficacy of intra-arterial (IA) tirofiban in LVO stroke patients undergoing MT7,8,9,10. However, most of the available data consist of patients with a small ischemic core volume, whose ASPECTS ≥ 6 points on the initial CT scan7,8,9,10,11. Although IA tirofiban infusion is beneficial in cases involving stent deployment or ongoing thrombus formation, this treatment may increase the risk of bleeding complications12, particularly in patients with large ischemic cores.
Thus, we aimed to evaluate the safety and efficacy of the IA administration of low-dose tirofiban during endovascular therapy in patients with a large ischemic core volume on initial CT.
Methods
Patients
We retrospectively collected data from patients with AIS who underwent EVT at a single tertiary hospital between 2017 and 2022. The inclusion criteria for patients in this study were as follows: (1) patients who presented to the emergency department with AIS due to internal carotid artery (ICA), middle cerebral artery (MCA; M1 and M2), or tandem large vessel occlusion and underwent EVT; and (2) patients with an ASPECTS between 2 and 5 on the initial brain CT performed in the emergency department and analysed using the automated software tool (RAPID ASPECTS software (Vesion 4.9; iSchemaView, Menlo Park, Calif). RAPID ASPECTS software performs a series of operations to generate an automated ASPECTS evaluation13.
The exclusion criteria were as follows: (1) poor quality of the initial brain CT resulting in low reliability of the ASPECTS analysed using the RAPID program; (2) unknown mRS score at the three-month follow-up after EVT; and (3) stroke caused by arterial dissection. The study was approved by the Ajou University Hospital Institutional Review Board (AJOUIRB-DB-2023-097), and the requirement for written informed consent was waived because of the retrospective study design. All methods were performed in accordance with the relevant guidelines and regulations3,6.
Endovascular therapy and IA tirofiban use
Three neurointerventionalists performed EVT. All had over 10 years of experience in neurointervention and could perform EVT proficiently. All procedures were performed under local anaesthesia. Intravenous (IV) heparin was mandatory to maintain the activated clotting time between 200 and 300 s during the procedure, except in subjects treated with IV tissue plasminogen activator (t-PA). The EVT procedure was chosen at the discretion of the neurointerventionalists. Stent retrieval and contact aspiration were routinely used. In cases of failed EVT for LVO, rescue treatments, including emergency stent placement, balloon angioplasty, or tirofiban infusion, were administered. Tirofiban was administered only to patients with failed EVT based on the following criteria: (1) residual stenosis ≥ 70% in the occlusion site after thrombectomy with forward blood flow not maintained by modified Treatment in Cerebral Infarction (mTICI) ≥ 2b, (2) rescue treatment with stenting or balloon angioplasty, and (3) reocclusion after the first reperfusion. Treatment involved 0.5 mg (2 mL) tirofiban diluted in 8 mL of normal saline, which was injected at an infusion rate of 1 mL/min. On follow-up angiography immediately and 10 min after IA infusion, the same protocol was used if additional tirofiban was required. The total IA tirofiban infusion ranged from 0.5 to 2.0 mg.
Data collection and outcomes
We analysed the clinical characteristics, including age, sex, NIHSS score on admission, and baseline mRS scores. The baseline ASPECTS was determined using the RAPID program based on the initial non-contrast brain CT performed in the emergency department14. The scores of all patients were then reviewed, and if a score was unreliable, it was determined by consensus of the two neurointerventionalists. Follow-up brain CT was performed to evaluate hemorrhagic complications immediately and 12–24 h after EVT. Aetiology of LVO was determined by angiographic diagnosis, as previously reported15.
Clinical efficacy outcomes included the mRS score at 90 days and the changes in NIHSS compared with baseline at 7 days (or discharge if earlier). Technical efficacy outcomes included successful reperfusion and postprocedural early reocclusion within 48 h of follow-up CTA or MRA. Based on the final angiography, successful reperfusion was defined as mTICI grade 2b (partial filling ≥ 50% territory) or 3 (complete reperfusion). Safety outcomes included the incidence of symptomatic intracranial hemorrhage within 48 h, any radiologic intracranial hemorrhage, and mortality within 90 days of treatment. Symptomatic intracranial hemorrhage was defined as parenchymal haematoma type 1 and 2 and/or thick subarachnoid hemorrhage (SAH) with or without intraventricular hemorrhage (modified Fisher grade 3 or 4 of SAH) as per the Second European-Australasian Acute Stroke Study (ECASS II) criteria16.
Statistical analyses
Descriptive statistics were used for the between-group comparisons of patient characteristics and outcomes. Categoric variables were analysed using x2 tests or Fisher exact tests and were presented as percentages. Continuous variables were analysed using Student t test or Mann–Whitney U test and presented as median and interquartile range. Multivariate logistic regression analysis was performed to evaluate the efficacy and safety of tirofiban. Variables with p < 0.15 in the bivariate analysis were included in the multivariate logistic regression analysis. All statistical analyses were performed using the R statistical and computing software (R Foundation for Statistical Computing; Vienna, Austria), and statistical significance was set at p < 0.05.
Ethics approval
Ethical approval was obtained from the relevant Institutional Review Board (AJOUIRB-DB-2023-097). The requirement for written informed consent was waived because of the retrospective nature of this study.
Results
Baseline characteristics of patients
We analysed 87 patients who underwent EVT with low initial ASPECTS on brain CT. Sixteen patients were treated with tirofiban (tirofiban group), whereas 71 patients were not (no-tirofiban group) (Fig. 1). Table 1 presents the baseline patient characteristics. Compared with the no-tirofiban group, the tirofiban group had significantly more cases of intracranial atherosclerotic stenosis-related occlusion (ICAS-O) (9.9% vs. 81.2%, p < 0.001), higher rates of rescue treatment (1.4% vs. 31.2%, p < 0.001), and longer procedure times (45 min vs. 75 min, p = 0.035). There was no significant difference in the baseline NIHSS scores between the two groups.
Safety and efficacy outcomes
Table 2 presents the efficacy and safety outcomes of tirofiban treatment. There was no significant difference in symptomatic hemorrhagic complications (26.7% vs. 25.0%; unadjusted OR 0.621 (0.121–3.197, 95% CI); p = 0.956), any radiologic hemorrhage (49.3% vs. 31.2%; unadjusted OR 0.486 (0.051–4.763, 95% CI); p = 0.536), or 90 day mortality (23.9% vs. 12.5%; unadjusted OR 0.388 (0.054–3.307, 95% CI); p = 0.367) between the two groups. There was also no significant difference in the successful reperfusion rate (80.3% vs. 75.0%; unadjusted OR 0.995 (0.179–5.519, 95% CI); p = 0.995) or early reocclusion rate within 48 h on follow-up CTA or MRA (4.2% vs 18.7%; unadjusted OR 5.213 (0.949–28.827, 95% CI); p = 0.057). Multivariate logistic regression analysis revealed no association between IA tirofiban and serious hemorrhage (adjusted OR [aOR], 0.720; 95% confidence interval [CI] 0.099–5.219; p = 0.960), radiologic hemorrhage (aOR, 0076; 95% CI 0.003–2.323; p = 0.139), or mortality within three months (aOR: 0.087; 95% CI 0.005–1.501; p = 0.093).
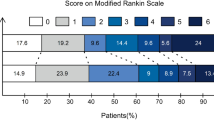
In the clinical efficacy outcome aspects, the median (IQR) 90-day mRS score was significantly lower in the tirofiban group than in the no tirofiban group (4(3–4.5) vs 5(4–5); p = 0.025), and the median (IQR) change in NIHSS compared with baseline also differed significantly between the tirofiban and no tirofiban group (− 3 (− 6.5 to − 0.5) vs 0 (− 4 to 2); p = 0.019, respectively). After adjustment, IA tirofiban was associated with a lower 90-day mRS score (aOR, 0.197; 95% CI 0.015–1.306; p = 0.017) and change in NIHSS compared with baseline (aOR, 0.698; 95% CI 0.531–0.917; p = 0.010).
Discussion
Recent studies have demonstrated that EVT could improve functional outcomes in patients with a large ischemic core volume without affecting the incidence of symptomatic hemorrhage4,5,17. Similarly, previous studies reported that the use of low-dose IA tirofiban during EVT did not increase the risk of ICH, symptomatic ICH, or 3-month mortality18,19,20. Our results indicate that low-dose IA tirofiban administration did not increase the risk of symptomatic hemorrhagic complications, radiologic intracranial hemorrhage, or 3-month mortality in AIS patients undergoing EVT, even in patients with a large ischemic core volume on the initial CT scan. In addition, IA tirofiban was associated with lowering the 90-day mRS score and improving the baseline NIHSS score. However, this did not significantly reduce the incidence of early reocclusion.
Tirofiban is a glycoprotein IIb/IIIa inhibitor that suppresses platelet aggregation in a dose-dependent manner. It has a short half-life, with platelet function normalising after 4 h21,22. Given these pharmacokinetics, tirofiban can be used safely during certain types of EVT, such as angioplasty or stenting for stenotic lesions, from the perspective of hemorrhagic complications. However, the use of tirofiban as rescue therapy during EVT produced varied results. One possible explanation could be the difference in the administration methods of the drug across studies. Kellert et al. suggested that IV tirofiban increases the risk of fatal intracranial hemorrhage and poor functional outcomes in patients treated with EVT23. In contrast to IV tirofiban, other studies have shown that low-dose IA tirofiban administered during EVT is safe7,8,9,10. Jang et al. reported that low-dose IA tirofiban did not increase the risk of bleeding in patients who underwent EVT with IV tPA7. On the contrary, Yang et al. demonstrated that IV tirofiban was not associated with any increase of sICH and IA tirofiban increased rate of sICH for patients with small core volume24. Another possible explanation for conflicting results could be associated with ischemic core volume rather than the administration methods.
In this study, we found that low-dose IA tirofiban did not increase the risk of bleeding in patients with an initial large ischemic core volume who underwent EVT. These results are consistent with those of previous studies on tirofiban, which did not consider the patients’ initial ischemic core volume7,8,9,10,11. The safety of tirofiban may be attributed to the several advantages of administering it via IA. IA tirofiban can be administered at a lower dose than intravenously. In the study by Kellert et al., tirofiban was administered IV, infused at 0.4 mg/kg/min for 30 min, followed by a continuous infusion of 0.1 mg/kg/min for 48 h. The total tirofiban dose was 18 mg for a 60-kg adult. Our dose (0.5–2.0 mg) was smaller than the IV tirofiban dose, and the drug could be administered in a target artery. Consequently, we speculated that the dose was an important factor, and that low-dose tirofiban was feasible during EVT in patients with a large ischemic core volume. However, it is also possible that the initial non-contrast brain CT scan did not accurately reflect the ischemic core volume and overestimated it.
In this study, the rate of successful reperfusion (mTICI = 2b/3) was lower, whereas the rate of early reocclusion within 48 h of follow-up imaging after EVT was higher in patients treated with low-dose IA tirofiban, though without statistical significance. This may be explained by the fact that the use of tirofiban was at the discretion of the treating interventionalists, who were prone to use tirofiban in patients with a high possibility of reocclusion after achieving partial recanalization of the occluded arteries during EVT, meaning that not all arteries were recanalized or ultimately achieved good reperfusion. Thus, the higher rate of early reocclusion and lower rate of successful reperfusion could have occurred due to the much higher rate of patients with underlying atherosclerotic stenosis of the occluded arteries in the tirofiban group. This bias likely explains the seemingly paradoxical study results, and further RCTs are needed to investigate whether tirofiban can improve reperfusion status.
Interestingly, the functional outcomes were better in patients treated with tirofiban, despite slightly lower rate of successful reperfusion. Previous studies have hypothesised that tirofiban can improve patient prognosis11,15,19,25,26. Even if the major arterial occlusion recanalizes after EVT, microvascular thrombosis may continue to occur at distal sites. Given their topographical localisation to microvessels distributed throughout the ischemic territory, the in-situ formation of microthrombi and microembolisms during thrombectomy procedures may contribute to the reduction of post-ischemic flow and infarct progression27. Studies have shown that glycoprotein IIb/IIIa antagonists are effective in preventing and treating microthrombosis and accelerating recovery from AIS27,28,29. Therefore, we speculate that tirofiban may improve functional outcomes by improving the reperfusion status of the microvasculature and maintain the reflow status.
Once the safety of treatment modalities is established, clinicians prioritize functional outcomes over radiological outcomes. Therefore, based on the results of this study, we anticipate that low-dose IA tirofiban could be a viable option for patients with low ASPECTS.
This study had several limitations. Firstly, the retrospective design of this study exposes it to gaps in prospective data collection, such as collecting mRS scores at three months for all patients. Secondly, the sample size was limited. In particular, only 16 patients received IA tirofiban. Thirdly, the use of IA tirofiban was at the discretion of the neurointerventionalist, and there might have been a selection bias. Specifically, neurointerventionalists may have decided to use IA tirofiban only if they consider it safe. Finally, the dose and use of tirofiban in this study were inconsistent, which necessitates further investigation to determine the optimal tirofiban treatment protocol for patients with AIS who are treated with EVT.
Conclusions
Our results indicate that low-dose IA tirofiban administration during EVT is safe and effective in patients with large ischemic core volume (low ASPECTS) and could be used to improve functional outcomes. To the best of our knowledge, this is the first study to assess the safety and efficacy of tirofiban in patients with AIS with a large ischemic core volume.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Campbell, B. C. et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N. Engl. J. Med. 372, 1009–1018 (2015).
Saver, J. L. et al. Stent-retriever thrombectomy after intravenous t-PA vs t-PA alone in stroke. N. Engl. J. Med. 372, 2285–2295 (2015).
Turc, G. et al. European Stroke Organisation (ESO)-European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke. J. Neurointerv. Surg. 11, 535–538 (2019).
Cagnazzo, F. et al. Mechanical thrombectomy in patients with acute ischemic stroke and ASPECTS </=6: A meta-analysis. J. Neurointerv. Surg. 12, 350–355 (2020).
Sarraj, A., Grotta, J. C., Pujara, D. K., Shaker, F. & Tsivgoulis, G. Triage imaging and outcome measures for large core stroke thrombectomy—A systematic review and meta-analysis. J. Neurointerv. Surg. 12, 1172–1179 (2020).
Powers, W. J. et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 50, e344–e418 (2019).
Jang, S. H. et al. The safety of intra-arterial tirofiban during endovascular therapy after intravenous thrombolysis. AJNR Am. J. Neuroradiol. 42, 1633–1637 (2021).
Kang, D. H. et al. Instant reocclusion following mechanical thrombectomy of in situ thromboocclusion and the role of low-dose intra-arterial tirofiban. Cerebrovasc. Dis. 37, 350–355 (2014).
Kwon, J. H., Shin, S. H., Weon, Y. C., Hwang, J. C. & Baik, S. K. Intra-arterial adjuvant tirofiban after unsuccessful intra-arterial thrombolysis of acute ischemic stroke: Preliminary experience in 16 patients. Neuroradiology 53, 779–785 (2011).
Goh, D. H., Jin, S. C., Jeong, H. W. & Ha, S. Y. Mechanical solitaire thrombectomy with low-dose booster tirofiban injection. Neurointervention 11, 114–119 (2016).
Zhao, W. et al. Low-dose tirofiban improves functional outcome in acute ischemic stroke patients treated with endovascular thrombectomy. Stroke 48, 3289–3294 (2017).
Wu, Y. et al. Endovascular thrombectomy. Stroke 49, 2783–2785 (2018).
Maegerlein, C. et al. Automated calculation of the alberta stroke program early CT score: Feasibility and reliability. Radiology 291, 141–148 (2019).
Ahmed, A. et al. Computed tomography perfusion stroke mimics on RAPID commercial software: A case-based review. Brain Circ. 9, 68–76 (2023).
Huo, X. et al. Safety and efficacy of tirofiban for acute ischemic stroke patients with large artery atherosclerosis stroke etiology undergoing endovascular therapy. Front. Neurol. 12, 630301 (2021).
Hacke, W. et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet 352, 1245–1251 (1998).
Kerleroux, B. et al. Perfusion imaging to select patients with large ischemic core for mechanical thrombectomy. J. Stroke 22, 225–233 (2020).
Yu, T. et al. Safety and efficiency of low dose intra-arterial tirofiban in mechanical thrombectomy during acute ischemic stroke. Curr. Neurovasc. Res. 15, 145–150 (2018).
Zhang, S. et al. Safety of intra-arterial tirofiban administration in ischemic stroke patients after unsuccessful mechanical thrombectomy. J. Vasc. Interv. Radiol. 30, 141-147.e141 (2019).
Zhao, W. et al. Low-dose tirofiban is associated with reduced in-hospital mortality in cardioembolic stroke patients treated with endovascular thrombectomy. J. Neurol. Sci. 427, 117539 (2021).
Harder, S., Klinkhardt, U. & Alvarez, J. M. Avoidance of bleeding during surgery in patients receiving anticoagulant and/or antiplatelet therapy: Pharmacokinetic and pharmacodynamic considerations. Clin. Pharmacokinet. 43, 963–981 (2004).
McClellan, K. J. & Goa, K. L. A review of its use in acute coronary syndromes. Drugs 56, 1067–1080 (1998).
Kellert, L. et al. Endovascular stroke therapy: Tirofiban is associated with risk of fatal intracerebral hemorrhage and poor outcome. Stroke 44, 1453–1455 (2013).
Yang, J. et al. Intraarterial versus intravenous tirofiban as an adjunct to endovascular thrombectomy for acute ischemic stroke. Stroke 51, 2925–2933 (2020).
Li, W. et al. Safety and preliminary efficacy of early tirofiban treatment after alteplase in acute ischemic stroke patients. Stroke 47, 2649–2651 (2016).
Choi, W., Hwang, Y. H. & Kim, Y. W. Long-term outcomes of local tirofiban infusion for intracranial atherosclerosis-related occlusion. Brain Sci. 12(8), 1089 (2022).
Fu, Z., Xu, C., Liu, X., Wang, Z. & Gao, L. Safety and efficacy of tirofiban in acute ischemic stroke patients receiving endovascular treatment: A meta-analysis. Cerebrovasc. Dis. 49, 442–450 (2020).
Choudhri, T. F. et al. Reduced microvascular thrombosis and improved outcome in acute murine stroke by inhibiting GP IIb/IIIa receptor-mediated platelet aggregation. J. Clin. Invest. 102, 1301–1310 (1998).
Philipps, J., Thomalla, G., Glahn, J., Schwarze, M. & Rother, J. Treatment of progressive stroke with tirofiban–experience in 35 patients. Cerebrovasc. Dis. 28, 435–438 (2009).
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea government (MSIT) (No. 2020R1G1A1102931).
Funding
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea government (MSIT) (No. 2020R1G1A1102931).
Author information
Authors and Affiliations
Contributions
K.-C.C. contributed to this work as first author. W.S.J contributed to this work as the corresponding author and responsible for the overall content as guarantor. K.-C.C., J.W.C., and W.S.J. gathered the data and collaboratively drafted the manuscript. N.-H.S. and S.H.G. contributed to the statistical analysis of the data. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cho, KC., Son, NH., Gwon, S.H. et al. The safety and efficacy of intra-arterial low-dose tirofiban administration during endovascular therapy in patients with large ischemic core volume. Sci Rep 14, 3353 (2024). https://doi.org/10.1038/s41598-024-53715-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53715-8
Keywords
This article is cited by
-
Efficacy of mechanical thrombectomy in the treatment of acute ischemic stroke patients with double aortic arches
Neurological Sciences (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.