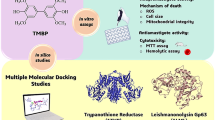
Abstract
Leishmania donovani is the causal organism of leishmaniasis with critical health implications affecting about 12 million people around the globe. Due to less efficacy, adverse side effects, and resistance, the available therapeutic molecules fail to control leishmaniasis. The mitochondrial primase of Leishmania donovani (LdmtPRI1) is a vital cog in the DNA replication mechanism, as the enzyme initiates the replication of the mitochondrial genome of Leishmania donovani. Hence, we target this protein as a probable drug target against leishmaniasis. The de-novo approach enabled computational prediction of the three-dimensional structure of LdmtPRI1, and its active sites were identified. Ligands from commercially available drug compounds were selected and docked against LdmtPRI1. The compounds were chosen for pharmacokinetic study and molecular dynamics simulation based on their binding energies and protein interactions. The LdmtPRI1 gene was cloned, overexpressed, and purified, and a primase activity assay was performed. The selected compounds were verified experimentally by the parasite and primase inhibition assay. Capecitabine was observed to be effective against the promastigote form of Leishmania donovani, as well as inhibiting primase activity. This study's findings suggest capecitabine might be a potential anti-leishmanial drug candidate after adequate further studies.
Similar content being viewed by others
Introduction
The intracellular protozoan parasite belonging to the genus Leishmania causes the consequential parasitic disease leishmaniasis, which substantially impacts human health1. Globally, the prevalence of Leishmania spp. infections have exceeded 12 million people, with 350 million individuals susceptible to infection2. Leishmaniasis exhibits a disproportionately higher incidence among underprivileged people in economically disadvantaged countries, affected by elimination programs with insufficient funding, minimal attention from pharma companies and inadequacies in healthcare facilities3,4,5.
Leishmaniasis is attributable to approximately 20 distinct Leishmania species, transmitted through various species of phlebotomine sandflies6. The infection spreads through female sandflies (Phlebotomus species) in the Old World (Asia, Africa, and Europe) and via Lutzomyia spp. in the New World (the Americas)7. The parasite subsists in two unique forms: the extracellular promastigote present in the female sandfly's lumen and the intracellular amastigote that proliferates inside the monocyte-macrophage cells of the mammalian host8,9. Parasite transmission unfolds through the hematophagous engagement of the sand fly, affecting the transfer of flagellated promastigotes into the human host10,11.
While many instances of Leishmania infections may proceed without apparent symptoms12, the clinical conditions of leishmaniasis include cutaneous leishmaniasis, mucocutaneous leishmaniasis and visceral leishmaniasis13,14,15. Visceral leishmaniasis considered the most severe form, involves the parasite infiltration of crucial organs such as the liver, spleen, and bone marrow16. This condition is widespread across 78 countries17. However, over 90% of global visceral leishmaniasis cases are collectively reported in Brazil, India, Kenya, Somalia, South Sudan, Sudan, and Ethiopia18,19. In India, the predominant cause of leishmaniasis was instigated by L. donovani transmitted via the sandfly vector Phlebotomus argentipes.
Duplex DNA replication is an exceedingly intricate and synchronized procedure comprising the activity of multiple enzymes together20. In the last few years, specific emphasis has been placed on DNA replication in subcellular organelles of eukaryotic cells21 and DNA replication within the cell. DNA primase enzyme is crucial in initiating DNA replication. The mitochondria of trypanosomatids contain a unique extra-chromosomal DNA, kinetoplast DNA or the kDNA, consisting of thousands of minicircles (0.5–10 kb each) and a few dozen maxicircles (20–40 kb each) topologically linked to form a catenated DNA network22.
The complex process of kinetoplast DNA (kDNA) replication relies on a specialized set of primase enzymes, PRI1 and PRI2, intricately involved in triggering the initiation of replication for both maxicircles and minicircles within kDNA of kinetoplastids. PRI1 plays a critical role in cell growth and kDNA replication, as RNA interference (RNAi) directed at PRI1 led to the depletion of maxicircle DNA23, aligning with PRI1's role in priming maxicircle DNA replication, encompassing the encoding of rRNA (12S and 9S) and protein-coding genes in the oxidative phosphorylation pathway. Conversely, RNA interference (RNAi) of PRI2 resulted in the loss of intermediate replication and free minicircles, indicating PRI2 is necessary for minicircle replication24. The replication of kDNA takes place during the S phase of the cell cycle, analogous to that of nuclear DNA replication25.
DNA replication stands as a fundamental event within the cell, and DNA primase's proficiency to initiate the replication of DNA is significant for the subsistence and sustenance of all organisms. The replication of the mitochondrial genome in Leishmania spp. follows an explicit mechanism started by a crucial enzyme known as mitochondrial primase. This enzyme is indispensable for replication initiation and facilitating the parasite's growth26. The significance of mitochondrial primase (PRI1) in kinetoplast replication plus essentiality for cell growth as demonstrated from pioneering work23, validating that loss of PRI1 results in inhibition of maxicircle DNA replication, thereby hindering cellular metabolism. Consequently, we were inclined to designate the mitochondrial primase enzyme (PRI1) as a promising candidate for drug targeting.
In drug discovery, chemical biology is employed with computational drug design techniques to facilitate the identification and optimization of lead compounds27. Leveraging computer-aided drug discovery (CADD) techniques in the early stages has played a crucial role in accelerating the drug discovery and development process, reducing costs, and mitigating the risk of failure in the penultimate stages28. Using coherent drug design by CADD gives valuable insights into interactions between molecules and binding affinity amongst proteins and ligands. High-performance computing facilities, parallel processing, and enhanced programs and algorithms have primarily contributed to discovering new drug candidates27.
Currently, a vaccine for leishmaniasis is absent, and the therapeutic approach is exclusively dependent on a restricted array of chemotherapeutic agents29. Pentavalent antimony (Sb5+) served as the conventional primary treatment, but its effectiveness was limited due to the emergence of resistance30. Substitute treatment options in Amphotericin B, miltefosine and paromomycin are available, but their utility is restricted as the drugs are costly, toxic and possess other side effects31. The absence of an economical and effective drug remains a concern, necessitating the development of novel therapeutic compounds32 with substantial antileishmanial effects and low toxicity to the host.
Given the formidable challenges associated with treating and controlling leishmaniasis, the imperative arises to discern and characterize new antileishmanial targets and novel pharmacological agents. This has spurred our research focus towards the mitochondrial DNA primase of Leishmania donovani (LdmtPRI1) as a prospective drug target for combating leishmaniasis; utilizing virtual screening and in-vitro methods in our research endeavours by strategically leveraging drug repurposing, we aim to introduce novel therapeutic alternatives that capitalize on the intrinsic potential of existing pharmaceutical compounds, presenting a more efficient and economically viable strategy for the management of leishmaniasis.
Methodology
LdmtPRI1 structure prediction and validation
LdmtPRI1 amino acid sequences (accession id: LdBPK_230850.1) were retrieved from the TriTrypDB database (https://tritrypdb.org/tritrypdb/app/)33 and were subjected to protein BLAST against human proteome. BLASTp results exhibited that primase does not possess a sequence related to any human proteins, and no crystal structure is available in the PDB database. De novo predictive models were built with a Robetta server (https://robetta.bakerlab.org/) and validated by PROCHECK34. Models were built employing the state-of-the-art three-track Neural Network RoseTTAfold35 available on the Robetta server (https://robetta.bakerlab.org/)36 and were validated by PROCHECK34. Further, the most optimal model underwent energy minimization to attain a low-energy and stable conformation, which was carried out using YASARA37.
Model evaluation
Post dynamism refinement, a conformational feature of the protein model was estimated via SAVESv6.0 (https://saves.mbi.ucla.edu/) and ProSa38. The structural conformation of the model was verified through PROCHECK34.
Active site prediction
Identifying ligand binding situates within proteins is a prerequisite for various applications in the procedure for drug design39. COFACTOR predicted potential ligand binding sites of LdmtPRI140 and was further validated by the FTSite server41.
Preparation of ligand coordinate files
Before the virtual screening process, ligands must be prepared to form three-dimensional shapes, determine the appropriate bond sequence, and generate available tautomeric and ionization conditions42. The initial requirement for a small molecule as a ligand is a stereo-chemically determined geometry with an appropriate protonation state, as the docking programme evaluates conformations concerning the binding residues within the LdmtPRI1 target43. A total of 4240 approved and clinical drug compounds (Drug Repurposing Library L9200) from Targetmol (https://www.targetmol.com/) were downloaded as structure data files (sdf format). Chemical information regarding the compounds was retrieved along with the SMILES files from the PubChem44 database, which was then converted to low-energy 3D dockable compounds as a pdbqt file with CORINA Classic45.
Molecular docking and interaction analysis
Molecular docking, a sophisticated computational technique at the forefront of structural biology and drug discovery, is utilized adeptly to predict and elucidate the intricate binding interactions between small molecules and target proteins. The study utilized the PyRx software, leveraging the Autodock Vina docking platform46 to conduct protein–ligand docking analyses. This approach facilitated the precise docking of proteins and ligands, enabling the discernment of compounds that could impede the target protein's function. Using AutoDock Vina, the macromolecule (LdmtPRI1) and ligands were docked into predefined binding sites within a grid box set along the X, Y, and Z axes, with dimensions of 25.43, 29.65, and 19.41 Angstrom, respectively. The docking procedure was routed to an exhaustiveness of 8 and fixed to yield a pose with the lowest energy. The best-conformity protein–ligand complexes were analyzed via Pymol molecular visualization software47, and Ligplot was employed to visualize the 2D interactions34.
Drug likeness and molecular properties of ligands
To evaluate the molecular characteristics and drug-like properties of the ligands, we used Lipinski's (http://www.scfbio-iitd.res.in/software/drugdesign/lipinski.jsp) and Molsoft's servers (http://www.scfbio-iitd.res.in/software/drugdesign/lipinski.jsp)44. Lipinski's rule states that for a drug to be orally effective, it should meet at least 4 out of the 5 known norms: molecular weight, octanol/water partition coefficient, H-bond donor, H-bond acceptor, and molar refractivity index that can differentiate between the drug and non-drug molecules48.
Admet profiling
Pharmacodynamics properties, namely Absorption, Distribution, Metabolism, Excretion and Toxicity (ADMET) of the compounds were calculated using the online server pkCSM (https://biosig.lab.uq.edu.au/pkcsm/). Amphotericin B & miltefosine served as controls to enhance the interpretation31.
Molecular dynamics (MD) simulation
Molecular dynamics simulations were conducted on the LdmtPRI1 docked complexes using the Desmond software package49 by Schrödinger, LLC50. The simulations extended for 300 ns (ns) to observe the formational changes in the protein resulting from the protein–ligand complex formation. The aim was to assess the impact of these conformational changes on the protein–ligand complex51 under simulated physiological conditions, employing Newton's classical equation of motion52. The proteins and ligands were individually pre-processed using Maestro's Protein Preparation Wizard. For simulation, the orthorhombic box was designated as a solvent model; the arrangement was built with the System Builder tool via solvation using TIP3P and OPLS_2005 force field53. Appropriate counter ions (Na+/Cl-) and a salt concentration (0.15 M NaCl) were utilized to neutralize the solvating system and to imitate physiological circumstances54. Throughout the simulation, the NPT (Nose–Hover Thermostat)55 maintained a temperature of 300 K and a pressure of 1 atm. Root mean square deviation (RMSD), Root mean square fluctuations (RMSF), Radius of gyration (Rg), Hydrogen-bonds (H-bonds) and Secondary structure elements (SSE) were assessed to ascertain the stability of the protein–ligand complex56.
Principal component analysis (PCA) and dynamic cross-correlation matrix (DCCM) analysis
This study delved into the flexibility of the LdmtPRI1 complex by examining the collective movements of the protein–ligand complex. The methodology involved removing translational and rotational motions linked with the proteins. Subsequently, the coordinates were aligned with a reference structure to compute the positional covariance matrix of atomic coordinates and their respective eigenvectors. This symmetric matrix underwent diagonalization using an orthogonal coordinate transformation matrix, resulting in a diagonal matrix showcasing eigenvalues. Each eigenvector in this matrix represented an eigenvalue denoting the total mean-square fluctuation of the system along the specific eigenvectors. The covariance matrix (C) was calculated using the following equation.
where N stands for the count of Cα-atoms, xi/j refers to the Cartesian coordinate of the ith/jth Cα-atom, and <xi/j> signifies the time-averaged value across all conformations. This PCA study was conducted to reconstruct the comprehensive arcs throughout the simulation of 300 ns, calculated by constructing a covariance matrix57. The DCCM was constructed through Cα-ATOMs throughout 300 ns MD simulation for LdmtPRI1 bound complex to explore domain relationships. PCA and DCCM were analyzed using the Bio3D package of R using a script written in R language58,59.
Molecular mechanics-generalized born surface area (MM-GBSA) calculations
The Python script thermal mmgbsa.pyn within the prime module60 was utilized to evaluate the binding free energies (Gbind) of the protein–ligand complex employing the MM-GBSA method. These free binding energies were computed using the OPLS 2005 force field, VSGB solvent model, and rotamer search method61. The following equation determines the calculation of the binding free energy upon receptor-ligand binding:
where ΔGbind = binding free energy, Gcomplex = free energy of the complex, Gprotein = free energy of the target protein, and Gligand = free energy of the ligand.
Parasite inhibition assay
Cytotoxicity of both the test compounds against L. donovani promastigote was performed via MTT tetrazolium reduction assay62. This colorimetric analysis is based on the reduction of MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] dye into an insoluble purple colour product, formazan by mitochondrial enzymes in viable cells. The compounds of interest, benfotiamine and capecitabine (Sigma-Aldrich, USA, catalogue number B9636 and SML0653, respectively), were dissolved in 0.1% DMSO and subjected to screening to assess their impact on cellular cytotoxicity63. Briefly, promastigote cell culture of L. donovani (2 × 106 cells/mL) was dispensed into flat-bottom 96-well clear polystyrene plates (Tarsons, India) at a volume of 100 µl/well for the assay and left to incubate overnight. Following incubation, the test compounds, solubilized in 0.1% dimethylsulfoxide (DMSO) at concentrations (1–40 μM), along with the positive control (Amphotericin B), were added to the promastigote cells. Concurrently, untreated cells were included as the negative control. After this, the cultures were transferred to an environment shielded from light and incubated overnight at 26° C. Post-incubation, cells were pelleted, and the culture medium was removed. These cells were treated with MTT reagent (5 mg/mL) obtained from the In Vitro Toxicology Assay Kit (TOX-1, Sigma-Aldrich, USA)64 and incubated in darkness at 26° C for 4 h. Subsequently, the MTT reagent was suctioned, and the MTT solubilizer (100 μl/well) was added to dissolve formazan crystals. The reduction of MTT was quantified by measuring absorbance at 570 nm using a Microplate Reader (Thermo Scientific, USA) to determine cell viability. IC50 values for each compound were calculated using GraphPad Prism version 9.0 (http://www.graphpad.com/). The assay was conducted in triplicate, and results from three independent experiments were analyzed.
Cloning and purification of LdmtPRI1
The LdmtPRI1gene was PCR amplified from Ld1s genomic DNA using end primers (5′CACCGAATTCCATATGCAGCGTCTTACGTCTGCC3′) and (5′TCGGATCCTCCAGCTCGACGGAACGCC3′) (Hysel, India) and cloned into SmaI site of pUC19 vector (Addgene, USA) and sequenced to ensure authenticity. The recombinant plasmid was cloned into the BamH1 and EcoR1 sites of the pET28a(+) vector (Addgene, USA). The protein was expressed in 1L LB broth (Himedia, India) with E. coli BL21 cells induced with 0.5 mM IPTG (Sigma-Aldrich, USA) and incubated for 20 h at 16°C. The expressed histidine-tagged protein was purified with Ni2+-NTA agarose (Takara, India) and eluted with buffer (50 mM Tri-Cl, pH 7.5, 300 mM NaCl, and 250 mM Imidazole) (Sigma-Aldrich, USA) and analyzed by Sodium dodecyl-sulphate polyacrylamide gel electrophoresis. The final eluted fraction was concentrated with Pierce™ Protein Concentrator PES, 10 K MWCO (Thermo Scientific) and aliquots were stored with 25% glycerol (V/V) at − 80° C for further studies.
Primase activity assay
The quantification of purified primase obtained through Ni2+-NTA chromatography was conducted using the Bradford assay65. Following the method devised by Biswas and colleagues, a coupled primase–pyrophosphatase assay was employed to evaluate and quantify primase activity66. Reactions were set up with 40 nM primase (LdmtPRI1), 1.25 µM M13mp18 Single-stranded DNA (7249 bp) (New England Biolabs, UK), 100 µM NTP (Takara, India), 50 mM NaCl (Sigma-Aldrich, USA), 150 mM Potassium glutamate (Sigma-Aldrich, USA), 20 mM CAPS (3-(Cyclohexylamino)-1-propanesulfonic acid) buffer pH 8.8 (Sigma-Aldrich, USA), 2 mM Mg2+ (Sigma-Aldrich, USA) and 1 U pyrophosphatase (Sigma-Aldrich, USA) in flat-bottom 96-well clear polystyrene plates (Tarsons, India) and maintained at 22° C for 1 h. PPiase selectively cleaves pyrophosphate (PPi) into two phosphates (Pi) and does not hydrolyze nucleotide triphosphates, thus allowing us to inspect PPi release through detection of Pi67. Three volumes of the malachite green reagent (Sigma-Aldrich, USA) were added to the reaction mix and maintained at RT for 5 min, followed by 10% sodium citrate (Sigma-Aldrich, USA) and kept at RT for a minute. Following a 30-min incubation to enable colour development, the absorbance was assessed at 650 nm66 using a Microplate Reader (Thermo Scientific). The colorimetric primase–pyrophosphatase assay offers a quantitative assessment of primase activity by detecting the release of PPi during the incorporation of NTP into developing RNA66. The experimental results were based on triplicates of the assay performed.
Primase inhibition assay
Assorted concentrations (1 nM–1 μM) of compounds (benfotiamine and capecitabine (Sigma-Aldrich, USA) prepared in 0.1% DMSO was used in triplicate for the primase inhibition studies, and the inhibition reaction was set up similarly to that of the primase assay. A negative control of 0.1% DMSO was introduced concerning the drug, whereas an optimized primase reaction (Methods 2.11) was taken as the positive control. The reaction mixture was maintained at 22° C for 1 h. Three volumes of the malachite green reagent (0.0812% malachite green 2.32% w/v polyvinyl alcohol, 5.72% in 6 M of HCl ammonium molybdate and water in the ratio 2:1:1:2 respectively) were added, trailed by 10% sodium citrate to the reaction to develop colour and absorbance was measured at 650 nm. GraphPad prism vs 9.0 (http://www.graphpad.com/) was utilized to calculate the IC50 values of enzyme inhibition66. The triplicate of the assay was used as an experimental parameter.
Statistical analysis
All the experiments were conducted autonomously, with each trial replicated at least three times in triplicate. Statistical analyses were performed utilizing the unpaired Student's t-test and executed on GraphPad Prism version 9.0 (http://www.graphpad.com/).
Results
De novo LdmtPRI1 structure prediction, evaluation and energy refinement-
Owing to the unavailability of LdmtPRI1 crystal structure, we built its predictive model (Figure S1, Supplementary data) using the Robetta server that predicts the 3-dimensional structure of a protein based upon the RoseTTA Fold algorithm35 utilizing the amino acid sequence as input (https://robetta.bakerlab.org/).
PROCHECK was deployed to validate the models using Ramachandran's plot. The 4th model was intended for further study as it covered 92.6% of residues in the favoured region, 7.4% in additional allowed regions, and no residues in the generously allowed or disallowed regions of the Ramachandran plot (Figure S2 & Table S1, Supplementary data).
The best model (Model No. 4) was refined via the Energy minimization server YASARA to enhance the model’s stereochemistry. The refined model (Model No. 4) was then analyzed via the ProSa server for validation. The Z-score of LdmtPRI1, determined using the ProSa, was found to be – 6.54 (Figure S3, Left, Supplementary data). In the energy plots, the average energy is shown by the thick line, which has a window size of 40 residues; additionally, the thin line within the plot’s background denotes the mean energy about each of the 10 residue fragments (Figure S3, Right, Supplementary data). The energy plot by ProSa signified a decent model for further study.
Molecular docking
Molecular docking plays an essential and vital function in conceptual drug development and is a powerful and efficient approach for computational screening68. Molecular docking studies how ligand molecules align and conform when they bind to target proteins, where actual positions produced by algorithms are ranked using scoring systems43. Determining the correct binding mode in which ligands bind within the protein cavity is an arduous task in computational chemistry69. Virtual screening based on ligands was used in this study. Virtual screening with a structure-based approach is significant and complements conventional screening methods70. The PyRx tool was used in the docking procedure. Based on the results from the docking processes, the top poses with the lowest dock scores were studied further and considered for visual representation. The ligands benfotiamine, capecitabine, febuxostat, rolipram, and varespladib exhibited the best binding energies, as shown in Table 1.
The top five compounds were analyzed post-molecular docking for their bond lengths, stable hydrogen bonds and interaction with residues within the protein. Active site residues used for docking of LdmtPRI1 against drug repurposing compounds are presented in Table 2. Pymol software was used to visualize the docked complexes in their three-dimensional configuration (Fig. 1A–E), whereas the two-dimensional interactions were observed with Ligplot (Fig. 1F–J). The ligand benfotiamine (dock score: − 7.6 kcal/mol) interacted and forged 4 stable H-bonds with the binding site residues, ARG 148, GLU 155, LYS 254 and THR 476 of the target protein (LdmtPRI1) (Fig. 1F), capecitabine (dock score: − 7.2 kcal/mol) form 2 stable H-bonds with ARG 148 and LYS 254 residues of the target protein (Fig. 1G), the ligand febuxostat (dock score: − 7.1 kcal/mol) interacted with the protein of interest in the residues ARG 148, GLU 155 and PHE 477 establishing four stable hydrogen bond (Fig. 1H), rolipram (dock score: − 7.5 kcal/mol) formed two hydrogen bonds at residue LYS 254 with target protein (Fig. 1I) and varespladib (dock score: − 8 kcal/mol) had only one stable hydrogen bond at residue ASN 451 of LdmtPRI1 (Fig. 1J).
As DNA primase requires nucleotides and single-stranded DNA as substrates for replication initiation, we also docked ATP and DCP (positive controls) against LdmtPRI1 to determine its substrate binding sites. Blind docking and interaction analysis exhibited docking scores of − 7.8 and − 6.4 kcal/mol for ATP and DCP, respectively, signifying substantial interaction and a pronounced affinity towards LdmtPRI1. Further 2D and 3D interaction analysis revealed DCP and ATP formed H-bonds with residues ARG 148 and THR 476 (Supplementary Figure S4A–D), thereby suggesting that these residues are also probable substrate binding sites in LdmtPRI1 other than active sites for ligand binding. Thus, selected drugs might also utilize the protein's substrate binding site to inhibit DNA replication.
LdmtPRI1’s in-silico dead variant was generated via a computational method. The amino acid residues ARG 148, GLU 155, ASN 246, LYS 254, LYS 259, VAL 266, ASN 451, CYS 470, THR 476, PHE 477, and LEU 481 in the substrate binding site were substituted with Alanine residues. The three-dimensional conformation of the in-silico mutant was predicted utilizing the Robetta web server, and the model's stereochemical integrity was assessed using PROCHECK71. A docking score of − 1.6 kcal/mol between the mutant protein and substrate ATP was found, suggesting a lowered affinity for the substrate binding site. The docked complex’s 3D and 2D analysis showed that mutations in the substrate binding site residues of the protein fail to establish any H-bonds with the substrate molecule. An in-silico study of the substrate binding residues revealed the probable amino acids responsible for substrate binding. Mutations in these residues can significantly decrease the protein's activity Supplementary Figure S5.
Drug likeness properties of ligands
The drug-likeness parameters and pharmacokinetic profiles were investigated where all ligands met Lipinski's rule of 5, indicating high permeability. All the ligands have molecular weights less than 500 Daltons and water/octanal partition coefficient (log P) values less than 5, indicating that the ligands are cell membrane permeable72. The ligand's drug-likeness scores were calculated via Molsoft L.L.C.: Drug-Likeness software and no ligands exhibited negative drug scores. The results of Lipinski's parameters and drug-likeness scores are listed in Table S2, Supplementary data.
ADMET profiling
Pharmacokinetic properties, i.e. (ADMET) profiles of the selected compounds, were analyzed via pkCSM software. Amphotericin B and miltefosine were taken as a control for better interpretations. The compounds are seen to be optimally water-soluble in terms of absorption, hence displaying an improved absorption rate in the human intestinal mucosa. The skin permeability of most of the compounds is positive, and they are non-inhibitors of human P-glycoprotein, suggesting that the compounds are uniformly adsorbed throughout the cell membrane (Table S3, Supplementary data). The tested compounds can be projected as ideal drug candidates (Table S3, Supplementary data) as they have been distributed efficiently over the cell membrane and are pervious to the brain barrier and central nervous system. The compounds also represent excellent metabolism (Table S3, Supplementary data) as they are non-substrate based and non-inhibitors of cytochrome p450. The total renal clearance (CLtot) values of the compounds are moderate, whereas the values of the human Organic cation transporter 2 (OCT2) substrate (Table S3, Supplementary data) were observed as negative. Apart from being non-inhibitors of human ether-a-go-go related gene (hERG) I & II, the compounds displayed negative potential towards Ames toxicity. These compounds did not induce skin sensitization and displayed a decent level of Oral rat chronic toxicity (LOAEL) (Table S3, Supplementary data).
MD simulation study
Molecular Dynamics Simulation tests were performed to evaluate the equipoise of ligands bound to LdmtPRI1 protein using Schrodinger Suite’s Desmond Simulation software to corroborate the conformational stability of the protein–ligand complexes. Backbone RMSDs were analyzed throughout a 300 ns simulation duration to determine the binding site stability of the compounds based on their projected affinity towards the binding site of LdmtPRI1. RMSD analysis was performed by assessing the Cα atoms of the LdmtPRI1 protein, which were then compared to the simulation time. RMSD plot for LdmtPRI1, as illustrated in Fig. 2A, reveals significant instability until 50 ns with deviations greater than 14 Å but attained stability post 100 ns and maintained stability throughout the simulation period. The obtained RMSD trajectories for the LdmtPRI1, with respect to its C-α backbone, rises with the RMSD > 14 Å at 40 ns but gradually decreases and was stable from (50–300) ns throughout (Fig. 2A). High RMSD values can affect the accuracy of predicting the binding site and interactions between the protein and ligand73. Moreover several study supports that functional regions of a protein often exhibit higher flexibility, leading to higher Root Mean Square Deviation (RMSD). Protein flexibility is frequently important for their ability to adapt to different biological activities. These dynamic character of the functional regions may be shown by the variation in backbone structure, as determined by RMSD73,74,75. Additionally, RMSD is just one measure of structural accuracy, and conjunction with other metrics for a comprehensive evaluation of a protein model73. The RMSD plot for benfotiamine confined to LdmtPRI1, as presented in Fig. 2B, conceals that in the initial 30 ns, the ligand-bound protein exhibited substantial stability, although it was marginally uneven within the period of 50–200 ns; conversely, the complexes stabilized after 200 ns simulation. On the contrary, the RMSD plot of capecitabine merged with LdmtPRI1 is presented in Fig. 2C, where between 30 and 120 ns simulation period, stabilization was achieved by ligand-binding complex. The complex displayed variability from 150 to 200 ns simulation time, although a slender deviation was witnessed in the complexes, which stabilized after 200 ns up to 300 ns. The validation of interaction stability between the ligand and protein is confirmed when the RMSD value of the backbone remains below 2.5 Å76. Protein–ligand exhibited a subtle deviation in RMSD, as evidenced by the backbone RMSD analysis. The observed deviance is likely attributed to conformational changes occurring in the rotatable bonds of the ligand, as evidenced by the presence of these bonds in the two-dimensional representation (Fig. 1F–J) of the protein–ligand interactions. These deviations arise from fluctuations in the torsion angles of the ligand77.
The root mean square deviation (RMSD) between the C-alpha atoms of LdmtPRI1 and ligands over time. (A) RMSD plot of LdmtPRI1 (protein-only), (B) RMSD plot of benfotiamine bounded with LdmtPRI1 and (C) RMSD plot of capecitabine bonded with LdmtPRI1. Differences in ligand root-mean-square deviation (RMSD) over time are presented on the right Y-axis.
The variation in individual amino acid residues and the degree of displacement or alteration of those residues during a simulation run is evaluated via RMSF. Minimal fluctuations in the atoms at the active site and the main chain suggest minimal conformational change, suggesting that the claimed top compound is well-maintained within the protein binding pocket cavity78. RMSF plot of the ligand–protein complexes indicated minimal fluctuations of all complexes that did not change significantly during the 300-ns simulation period and remained consistent across all complexes, as illustrated in Fig. 3A–C.
To investigate the conformational properties of the protein–ligand complex, we calculated the Rg plots associated with the protein's structural firmness (Fig. 4A). Results indicate that the Rg trajectories of LdmtPRI1 attain equilibrium during the early 20 ns of ∼ 31 Å and stay steady with negligible perturbation at 50–100 ns. In contrast, the arc of LdmtPRI1 complexed with benfotiamine achieved an equilibrium of 29–32 Å during the initial 0–40 ns, but the complex demonstrates sharp drifts of Rg approximately 29–32 Å during the ∼ 75–300 ns. The complex LdmtPRI1-capecitabine was perceived to remain stable around 0–20 ns but have marginal perturbation in radius of gyration with approximately 27–31 Å during the ∼ 50–300 ns period.
(A) Time progression graph of radius of gyration (Rg) during simulation LdmtPRI1 (black), LdmtPRI1-benfotiamine (purple) and LdmtPRI1-capecitabine (brown). (B) H-bonds within the protein–ligand complexes generated during simulation, LdmtPRI1-benfotiamine (purple) and LdmtPRI1-capecitabine (brown). (C) Solvent-accessible surface area (SASA) results of LdmtPRI1 (black) LdmtPRI1-benfotiamine (purple)& LdmtPRI1-capecitabine (brown).
The H-bonding interactions are vital for the molecular integrity of protein configuration and for ensuring the stable spatial localization of ligands within the protein’s active site. The occupancy of H-bonds by ligands, benfotiamine and capecitabine with LdmtPRI1 is illustrated in Fig. 4B. Benfotiamine is particularly effective against LdmtPRI1 and can establish more than ten H-bonds during the 300 ns simulation run with active site residues at ARG 148, GLU 155 and THR 479. At the same time, capecitabine maintained at least 1 H-bond in the active sites ARG 148 and LYS 254 throughout the 300 ns simulation period. Thus, benfotiamine was expected to be more efficient than capecitabine for probable inhibition of LdmtPRI1.
A solvent accessible surface area (SASA) study was also performed during a 300 ns MD simulation that quantifies interfaces of the protein–ligand complex with solvents79. Throughout the MD run, SASA for LdmtPRI1 alone was observed to range between 25,000–27,000 Å2, the complex LdmtPRI1-benfotiamine revealed an SASA value of 27,000–31,000 Å2, whereas LdmtPRI1-capecitabine had a SASA value of 28,000–32,000 Å2 (Fig. 4C). Protein–ligand complexes with high and relatively stable SASA values indicate that the ligand is available for solvents to interact with, devoid of any alterations in the protein structure80.
The secondary structural features, such as alpha-helices and beta-strands, were quantified during the simulation. The residual index was plotted contrary to the secondary structure elements graph to determine the distribution within the protein structure. LdmtPRI1 is composed of 9.67% helix and 5.33% strand, amounting to 15.00% total SSE (Fig. 5A) as compared to 17.79% alpha helix, 7.52% beta strand constituting 25.31% of total SSE in benfotiamine (Fig. 5B) whereas 11.6% alpha helices, 7.53% of beta strands leading to 19.21% of secondary structures elements (Fig. 5C) were observed with capecitabine. The proportion of alpha helix to beta-strand, too, impacts protein RMSD. Since protein structures comprise rigid regions, the RMSD of residues in these structures was significantly lower than that of the coils and loop residues81.
Elements of (A) LdmtPRI1(protein-only), (B) LdmtPRI1-benfotiamine and (C) LdmtPRI1-capecitabine, are distributed across protein–ligand complexes in relation to the residue index. The alpha helices are indicated by red columns, whereas the beta strands are represented by blue columns. The plot above (Protein-SSE Histogram) illustrates SSE distribution based upon residue index across the protein structure.
PCA and DCCM analysis
Random global mobility of amino acid residue’s atoms was interpreted using principal component analysis via MD simulation trajectories for ligands bound to LdmtPRI1. PCA evaluates the flexible dispersed trajectories caused by protein structural deformation. The movement of the internal coordinates into three dimensions during a spatial duration of 300 ns is documented in a covariance matrix. In contrast, orthogonal sets or eigenvectors infer coherent movement of individual trajectories. The conformational sampling was performed for LdmtPRI1 both independently and in the presence of benfotiamine and capecitabine, and the PC1, PC2 and PC3 projections were established using the Cα-atoms (Fig. 6A). Each blue, white and red dot represents maximum, intermediate, and reduced motility, respectively. The first three eigenvalues of LdmtPRI1, whether on its own or in the presence of benfotiamine and capecitabine, accounted for 74.6%, 67.7%, and 79.6% of the conformational variances, respectively. When LdmtPRI1 was without any ligand, its conformational space stretched from − 250 to + 50 along PC1 (48.84%), − 150 to + 100 along PC2 (21.21%), and − 100 to + 100 along PC3 (4.52%). Notably, the presence of benfotiamine and capecitabine induced changes in the flexibility of LdmtPRI1, which is evident in the PCA plot. LdmtPRI1 with benfotiamine occupied a subspace ranging from − 250 to + 50 along PC1 (44.87%), − 100 to + 100 along PC2 (15.31%), and − 100 to + 100 along PC3 (7.51%) (Fig. 6B); On the other hand, LdmtPRI1 with capecitabine occupied a subspace ranging from − 150 to + 100 along PC1 (53.03%), − 200 to + 100 along PC2 (18.56%), and − 50 to + 150 along PC3 (8.03%) (Fig. 6C).
Principal Component Analysis (PCA) & eigenvalue mapped versus the percentage of variance for 300 ns simulation trajectories: (A) LdmtPRI1 only (Variations in PC1, PC2, and PC3 add up to 48.84%, 21.21% and 4.52%), (B) LdmtPRI1-benfotiamine (Variations in PC1, PC2, and PC3 add up to 44.87%, 15.31% and 7.51%), (C) LdmtPRI1- capacitabine (Variations in PC1, PC2, and PC3 add up to 44.87%, 15.31% and 7.51%). Dynamic cross correlation matrix (DCCM) plots for (D) LdmtPRI1-protein only (E) LdmtPRI1-benfotiamine, (F) LdmtPRI1- capecitabine. The positive correlated motions are represented in dark blue, negative anti-correlated motions are represented in white and mixed correlation are represented in cyan.
DCCM plot was created to investigate the correlated movement of structural domains to achieve a steady conformational state of the complex following the binding to LdmtPRI1 (Fig. 6D–F). The varied colour patterns of the matrix plot correspond to different degrees of correlation. The positively correlated motions are represented in dark blue, negative anti-correlated motions are represented in white, and mixed correlations are represented in cyan.
MM-GBSA Calculations
The MM-GBSA method extensively evaluates the free binding energy between ligands and protein molecules82. It was employed here to assess the binding free energy within the LdmtPRI1 complex when bound to a ligand and examine additional non-bonded interaction energies. The binding energy of the ligands benfotiamine and capecitabine to LdmtPRI1 is − 69.79 kcal/mol and − 39.39 kcal/mol, respectively (Table 3). ΔGbind is governed by non-bonded interactions such as ΔGbind_Coulomb, ΔGbind_Packing, ΔGbind_Hbond, ΔGbind_Lipo, and ΔGbind_vdW (Table 3).
Parasite inhibition assay
The top two compounds (benfotiamine and capecitabine), which showed good ADMET and pharmacokinetic profiles, were selected for parasite inhibition by performing an MTT tetrazolium reduction assay62 to comprehend the effects of the benfotiamine and capecitabine on parasite growth. Drug concentrations (1 μM–40 μM) were prepared in 0.1% DMSO and added to the promastigote culture with an overnight incubation at 26° C. The cellular metabolic activity of the promastigote cells in the presence of drugs was assessed using the MTT assay. Amphotericin B was taken as a positive control drug to interpret the results better. Cell viability was quantified by checking absorbance at 570 nm and IC50 (Half maximum inhibitory concentration) values calculated using GraphPad Prism (http://www.graphpad.com/) from inhibition assay, where we observed IC50 values of benfotiamine as 19.79 ± 0.67 μM (R2 = 0.97; p = 0.0162) and capecitabine 12.40 ± 0.35 μM (R2 = 0. 97; p = 0.0003), in comparison to Amphotericin B with an IC50 value of 12.02 ± 0.09 μM (R2 = 0.96; p = 0.0132) (Fig. 7). These results validate the excellent repressive action of the chosen compounds against the parasite's promastigote form, where we observed that with a logarithmic increase in the concentration of the drugs, there was a substantial reduction in the number of viable cells, indicating inhibitory effects of the compounds in growth and proliferation of promastigote cell culture of L. donovani.
Purification of LdmtPRI1
Recombinant plasmid pET28a-LdmtPRI1 was transformed into E. coli BL21 cells and recombinant primase were expressed using 0.5 mM IPTG at 16 °C for 20 h. Cell harvesting was performed via centrifugation, followed by resuspension in lysis buffer (50 mM Tris-Cl, pH 7.5, 300 mM NaCl, 1 × protease inhibitor and 1 mg/mL lysozyme), and sonication was performed. The crude extract was centrifuged, and recombinant LdmtPRI1 were purified via Ni2+-NTA chromatography with buffer (50 mM Tris-Cl, pH 7.5, 300 mM NaCl, 250 mM Imidazole). SDS-PAGE was performed to detect the purified recombinant LdmtPRI1 protein (Supplementary Figure S6). The eluted fraction was concentrated, and aliquots were stored with 25% glycerol (V/V) at − 80 °C for further experiments.
Primase activity assay
With the ELISA plate reader set at 650 nm, the activity of the primase was identified through the detection of Pi (monophosphate) in the coupled primase-pyrophosphate assay where NTPs are utilized by primase to generate short stretches of oligonucleotide upon template releasing PPi (pyrophosphate). Released PPi are converted to Pi, leading to a light blue colour development upon adding MGR to the reaction. The oligo-synthesis activity of primase is directly signified by the presence of Pi during the primase-pyrophosphate assay. A significant primase activity was observed as the optimum extension of template DNA with 1.25uM M13mp18 single-stranded DNA was utilized during the assay (Fig. 8A). Maximal oligo-synthesis by primase is achieved upon adding 2 mM MgCl2 salts (Fig. 8B), whereas buffer at pH 8–10 is significant for primase activity (Fig. 8C), as observed during the experiments. The time of less than an hour (Fig. 8D) is viable for priming, as a reduction in the activity of the enzyme was witnessed with the more extended period; increased concentration of NTP (> 100 µM) does not significantly enhance the oligo-synthesis by primase as observed in due course of the primase assay (Fig. 8E). The assays were performed with 40 nM of LdmtPRI1 protein, and a Microplate Reader measured absorbance at 650 nm66.
Optimization of parameters for the primase-pyrophosphatase assay. (A) Activity of primase at varied DNA concentrations. (B) Primase activity assessed in presence of divalent metals salts at 2 mM concentration. (C) Primase activity with buffers at pH range 6–10. (D) Time course representation of the priming reaction over period of 0-70 min. (E) Rate PPi released by primase as a function of NTP concentrations. The experiments were conducted over a duration of 30 min.
Primase inhibition assay
Primase inhibition reaction was set up similarly to primase assay, evaluating the inhibition effects of the selected compounds (benfotiamine and capecitabine) with different concentrations ranging from 10 to 500 nM for the inhibition reactions. For their inhibitory effects on primase's oligo-synthesis capability, selected drugs benfotiamine and capecitabine were studied. The experiments were conducted using 1.25 µM of M13mp18 Single-stranded DNA, 100 µM of NTP and 40 nM of primase (LdmtPRI1), and absorbance was measured at 650 nm with a Microplate Reader. IC50 values of the selected compounds calculated via GraphPad Prism signifying a logarithmic rise in drug concentration resulted in a noteworthy decrease in oligo-synthesis activity by LdmtPRI1, indicating substantial inhibitory effects of the drugs (˂ 25 nM) employed to impede the activity of the LdmtPRI1 enzyme (Fig. 9A–C). The IC50 values calculated using GraphPad prism were predicted to be benfotiamine as 20.68 ± 0.03 nM (R2 = 0.98, i = 0.1179) and capecitabine as 15.27 ± 0.03 nM (R2 = 0.98, p = 0.2939), respectively.
Discussion
This study chose Leishmania kinetoplast (mitochondrial) DNA primase, essential for mitochondrial DNA replication, as a therapeutic target. Primase initiates DNA replication by synthesizing short oligo ribonucleotides on single-stranded DNA templates in the leading and lagging strand known as the Okazaki fragment20. The Leishmania genome has a unique kinetoplast DNA within the mitochondrion22. A kinetoplast mitochondrial DNA primase is necessary to create a mitochondrial DNA replication system in vitro because none of the known DNA polymerases can initiate a DNA strand23.
Here, we used drug repurposing approaches to identify the most effective therapeutics for combating Leishmaniasis caused by Leishmania donovani by targeting LdmtPRI1. Based on virtual drug screening, molecular docking, and MD simulation analysis, the study highlighted the possible inhibitory effect of benfotiamine and capecitabine. Further, the computational findings are validated by parasite inhibition assay and primase activity inhibition assay.
In this current investigation, we performed docking studies of 4240 approved and clinical drug compounds by PyRx utilizing the Autodock Vina docking platform46. The results revealed 5 compounds, namely benfotiamine, capecitabine, febuxostat, rolipram and varespladib, possessing the best binding affinity against LdmtPRI1. Amongst the top-scored docked compounds, benfotiamine (− 7.6 kcal/mol) formed 4 H-bond with the predicted active site residues ARG 148, GLU 155, LYS 254 and THR 476 of the target protein (Fig. 1F) followed by capecitabine (− 7.2 kcal/mol) with ARG 148 and LYS 254 residues (Fig. 1G) indicating possible top 2 inhibitors of LdmtPRI1. Desmond MD simulations were run for 300 ns to obtain information about the structural stability of the five best-docked compounds against LdmtPRI1 and the protein's apo form. The structure of LdmtPRI1-benfotiamine and LdmtPRI1-capecitabine hastily attained stable equilibrium, and stable conformation of RMSD trajectory was observed throughout 300 ns of MD simulation (Fig. 2), and LdmtPRI1-ligand complexes display spatial binding patterns. It can be inferred from Fig. 3 that the ligand molecules maintain favourable molecular interactions in the LdmtPRI1 binding pocket throughout the 300 ns simulation period.
Additionally, advanced MD simulations of MM-GBSA analysis (with 300 ns of run time) confirmed that the proposed LdmtPRI1-benfotiamine complex has a greater binding free energy (ΔGbind) score than LdmtPRI1-capecitabine (Table 3). The results of this study, which examined every detail from sequence levels to advanced structure dynamics, showed that benfotiamine may be able to block LdmtPRI1 and certainly has antileishmanial properties. Benfotiamine, a synthetic S-acyl derivative of thiamine, inhibits the formation of advanced glycation end products, alleviating severe diabetic complications such as neuropathy, nephropathy and retinopathy83. Whereas, capecitabine, an antimetabolite, exhibits activity against numerous types of neoplasms (oesophagus, larynx, gastrointestinal and genitourinary tracts) that is metabolized to form compounds that interfere with the synthesis of DNA, RNA and proteins, resulting in inhibition of the proliferating cancerous cells and other hastily burgeoning cells, ensuing to their death84. Leishmania spp., like cancer cells, can persist in the host organism for an extended period, and certain enzymes targeted by anticancer treatments can also be utilized to form antileishmanial compounds85,86. For instance, miltefosine is the first and only oral drug for the treatment of VL and was initially developed for breast cancer treatment87.
In this investigation, benfotiamine and capecitabine were specifically selected for post-molecular docking analysis due to their elevated binding affinity against the LdmtPRI1 binding site. The in-vitro study exclusively focused on benfotiamine and capecitabine, revealing a dose-dependent lethal effect against Leishmania donovani promastigotes, with IC50 values of 19.79 ± 0.67 μM and 12.40 ± 0.35 μM respectively as compared to Amphotericin B (12.02 ± 0.09 μM) (Fig. 7).
To validate the predicted computation results, the LdmtPRI1 homologue was overexpressed in E. coli, and the corresponding polypeptide was purified via Ni2+ NTA chromatography and validated via SDS-PAGE (Supplementary Figure S6) to perform primase assay (Fig. 8A–E) and primase inhibition study using benfotiamine and capecitabine. Inhibition of primase activity was detected at drug concentrations of > 10 nM, signifying the inhibitory effects of the drugs used to hinder the activity of the primase enzyme (Fig. 9) with predicted IC50 values of 20.68 ± 0.033 nM and 15.27 ± 0.031 nM for benfotiamine and capecitabine respectively.
Discovering and developing new therapeutics to treat human parasitic infections is challenging. Identifying new chemical entities is the focus of de novo drug discovery. Possible benefits of drug repurposing techniques include making drug development more efficient and cutting costs. An intriguing review deliberating on drug repurposing has recently been published, providing an overview of multi-functional drugs that are effective in the treatment of leishmaniasis88 with specific emphasis on the development of drug repurposing strategies for multi-target strategies in order to identify potential candidates for the treatment of leishmaniasis. Significantly, the majority of leishmaniasis drugs currently on the market or in the early stages of drug discovery were initially intended for other therapeutic uses.
Consequently, based upon the analysis of our work as discussed above, we can surmise that benfotiamine and capecitabine might be the preferable and more secure medication over the currently accessible treatment option for Leishmania and could be a potential option to counter leishmaniasis. Hence, these drugs might be potent inhibitors that can be further experimentally tested to treat leishmaniasis.
Conclusion
Repurposing drug molecules previously approved for treating specific diseases is a more effective, faster, and cost-effective disease treatment method. Overall, while it is certainly not the only answer, drug repurposing is an effective, relatively quick, and relatively inexpensive way to create critical new drugs, especially for neglected tropical infections like leishmaniasis. Our findings suggest that benfotiamine and capecitabine are probable drugs to counter leishmaniasis. Conversely, additional research using animal models would be required to conclude the safety and efficiency of these compounds. The present study's findings may provide crucial information for developing novel drugs to combat leishmaniasis.
Data availability
The datasets used and analyzed in this manuscript are available from the corresponding author on reasonable request.
References
Kaufer, A., Ellis, J., Stark, D. & Barratt, J. The evolution of trypanosomatid taxonomy. Parasit. Vectors 10, 287. https://doi.org/10.1186/s13071-017-2204-7 (2017).
Lockard, R. D., Wilson, M. E. & Rodríguez, N. E. Sex-related differences in immune response and symptomatic manifestations to infection with Leishmania species. J. Immunol. Res. 2019, 4103819. https://doi.org/10.1155/2019/4103819 (2019).
Choi, H. L., Jain, S., Ruiz Postigo, J. A., Borisch, B. & Dagne, D. A. The global procurement landscape of leishmaniasis medicines. PLOS Negl. Trop. Dis. 15, e0009181. https://doi.org/10.1371/journal.pntd.0009181 (2021).
Olías-Molero, A. I., de la Fuente, C., Cuquerella, M., Torrado, J. J. & Alunda, J. M. Antileishmanial drug discovery and development: Time to reset the model?. Microorganisms 9, 2500. https://doi.org/10.3390/microorganisms9122500 (2021).
Scarpini, S. et al. Visceral leishmaniasis: Epidemiology, diagnosis, and treatment regimens in different geographical areas with a focus on pediatrics. Microorganisms 10, 1887. https://doi.org/10.3390/microorganisms10101887 (2022).
Cecílio, P., Cordeiro-da-Silva, A. & Oliveira, F. Sand flies: Basic information on the vectors of leishmaniasis and their interactions with Leishmania parasites. Commun. Biol. 5, 305. https://doi.org/10.1038/s42003-022-03240-z (2022).
Abdellahi, L., Iraji, F., Mahmoudabadi, A. & Hejazi, S. H. Vaccination in leishmaniasis: A review article. Iran. Biomed. J. 26, 1–35. https://doi.org/10.52547/ibj.26.1.35 (2022).
Pace, D. Leishmaniasis. J. Infect. 69(Suppl 1), S10–S18. https://doi.org/10.1016/j.jinf.2014.07.016 (2014).
Sunter, J. & Gull, K. Shape, form, function and Leishmania pathogenicity: From textbook descriptions to biological understanding. Open Biol. 7, 170165. https://doi.org/10.1098/rsob.170165 (2017).
Georgiadou, S. P., Makaritsis, K. P. & Dalekos, G. N. Leishmaniasis revisited: Current aspects on epidemiology, diagnosis and treatment. J. Transl. Int. Med. 3, 43–50. https://doi.org/10.1515/jtim-2015-0002 (2015).
Steverding, D. The history of leishmaniasis. Parasit. Vectors 10, 82. https://doi.org/10.1186/s13071-017-2028-5 (2017).
Singh, O. P., Hasker, E., Sacks, D., Boelaert, M. & Sundar, S. Asymptomatic Leishmania infection: A new challenge for Leishmania control. Clin. Infect. Dis. 58, 1424–1429. https://doi.org/10.1093/cid/ciu102 (2014).
Alvar, J. et al. Kala-azar outbreak in Libo Kemkem, Ethiopia: Epidemiologic and parasitologic assessment. Am. J. Trop. Med. Hyg. 77, 275–282. https://doi.org/10.4269/ajtmh.2007.77.275 (2007).
Salam, N., Al-Shaqha, W. M. & Azzi, A. Leishmaniasis in the middle East: Incidence and epidemiology. PLOS Negl. Trop. Dis. 8, e3208. https://doi.org/10.1371/journal.pntd.0003208 (2014).
Aronson, N. et al. Diagnosis and treatment of leishmaniasis: Clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin. Infect. Dis. 63, 1539–1557. https://doi.org/10.1093/cid/ciw742 (2016).
Assis, T. M., Mancini, D. T., Ramalho, T. C. & da Cunha, E. F. F. In silico study of Leishmania donovani α-β tubulin and Inhibitors. J. Chem. 2014, 492579. https://doi.org/10.1155/2014/492579 (2014).
Wamai, R. G., Kahn, J., McGloin, J. & Ziaggi, G. Visceral leishmaniasis: A global overview. J. Glob. Health Sci. 2, e3. https://doi.org/10.35500/jghs.2020.2.e3 (2020).
Hailu, T., Yimer, M., Mulu, W. & Abera, B. Challenges in visceral leishmaniasis control and elimination in the developing countries: A review. J. Vector Borne Dis. 53, 193–198 (2016).
Burza, S., Croft, S. L. & Boelaert, M. Leishmaniasis. Lancet 392, 951–970. https://doi.org/10.1016/S0140-6736(18)31204-2 (2018).
Kornberg, A. & Baker, ΤA. DNA Replication (WΗ Freeman Press, 1992).
Murthy, V. & Pasupathy, K. Characterization of mitochondrial DNA primase from Saccharomyces cerevisiae. J. Biosci. 19, 1–8. https://doi.org/10.1007/BF02703462 (1994).
Shlomai, J. The structure and replication of kinetoplast DNA. Curr. Mol. Med. 4, 623–647. https://doi.org/10.2174/1566524043360096 (2004).
Hines, J. C. & Ray, D. S. A mitochondrial DNA primase is essential for cell growth and kinetoplast DNA replication in Trypanosoma brucei. Mol. Cell. Biol. 30, 1319–1328. https://doi.org/10.1128/MCB.01231-09 (2010).
Hines, J. C. & Ray, D. S. A second mitochondrial DNA primase is essential for cell growth and kinetoplast minicircle DNA replication in Trypanosoma brucei. Eukaryot. Cell 10, 445–454. https://doi.org/10.1128/EC.00308-10 (2011).
Woodward, R. & Gull, K. Timing of nuclear and kinetoplast DNA replication and early morphological events in the cell cycle of Trypanosoma brucei. J. Cell Sci. 95, 49–57. https://doi.org/10.1242/jcs.95.1.49 (1990).
Rai, P., Arya, H., Saha, S., Kumar, D. & Bhatt, T. K. Drug repurposing based novel anti-leishmanial drug screening using in-silico and in-vitro approaches. J. Biomol. Struct. Dyn. 40, 10812–10820. https://doi.org/10.1080/07391102.2021.1950574 (2022).
Macalino, S. J. Y., Gosu, V., Hong, S. & Choi, S. Role of computer-aided drug design in modern drug discovery. Arch. Pharm. Res. 38, 1686–1701. https://doi.org/10.1007/s12272-015-0640-5 (2015).
Yu, W. & MacKerell, A. D. Computer-Aided Drug Design Methods in Antibiotics 85–106 (Humana Press, 2017). https://doi.org/10.1007/978-1-4939-6634-9_5.
Norcliffe, J. L. et al. Identifying inhibitors of the Leishmania inositol phosphorylceramide synthase with antiprotozoal activity using a yeast-based assay and ultra-high throughput screening platform. Sci. Rep. 8, 3938. https://doi.org/10.1038/s41598-018-22063-9 (2018).
Sundar, S. Drug resistance in Indian visceral leishmaniasis. Trop. Med. Int. Health 6, 849–854. https://doi.org/10.1046/j.1365-3156.2001.00778.x (2001).
Ghorbani, M. & Farhoudi, R. Leishmaniasis in humans: Drug or vaccine therapy?. Drug Des. Dev. Ther. 12, 25–40. https://doi.org/10.2147/DDDT.S146521 (2018).
Gurung, A. B., Ali, M. A., Lee, J., Farah, M. A. & Al-Anazi, K. M. An updated review of computer-aided drug design and its application to COVID-19. BioMed Res. Int. 2021, 8853056. https://doi.org/10.1155/2021/8853056 (2021).
Amos, B. VEuPathDB: The eukaryotic pathogen, vector and host bioinformatics resource center. Nucleic Acids Res. 50, 898–911. https://doi.org/10.1093/nar/gkab929 (2021).
Laskowski, R. A. & Swindells, M. B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 51, 2778–2786. https://doi.org/10.1021/ci200227u (2011).
Baek, M. et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science 373, 871–876. https://doi.org/10.1126/science.abj8754 (2021).
Kim, D. E., Chivian, D. & Baker, D. Protein structure prediction and analysis using the Robetta server. Nucleic Acids Res. 32(Web Server Issue), W526–W531. https://doi.org/10.1093/nar/gkh468 (2004).
Krieger, E. et al. Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: Four approaches that performed well in CASP8. Proteins 77(9), 114–122. https://doi.org/10.1002/prot.22570 (2009).
Wiederstein, M. & Sippl, M. J. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 35, W407–W410. https://doi.org/10.1093/nar/gkm290 (2007).
Kozakov, D. et al. The FTMap family of web servers for determining and characterizing ligand-binding hot spots of proteins. Nat. Protoc. 10, 733–755. https://doi.org/10.1038/nprot.2015.043 (2015).
Zhang, C., Freddolino, P. L. & Zhang, Y. COFACTOR: Improved protein function prediction by combining structure, sequence, and protein-protein interaction information. Nucleic Acids Res. 45, W291–W299. https://doi.org/10.1093/nar/gkx366 (2017).
Ngan, C. H. et al. FTSite: High accuracy detection of ligand binding sites on unbound protein structures. Bioinformatics 28, 286–287. https://doi.org/10.1093/bioinformatics/btr651 (2012).
Sastry, G. M., Adzhigirey, M., Day, T., Annabhimoju, R. & Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aid. Mol. Des. 27, 221–234. https://doi.org/10.1007/s10822-013-9644-8 (2013).
Torres, P. H. M., Sodero, A. C. R., Jofily, P. & Silva-Jr-Jr, F. P. Key topics in molecular docking for drug design. Int. J. Mol. Sci. 20, 4574. https://doi.org/10.3390/ijms20184574 (2019).
Kim, S. et al. PubChem substance and compound databases. Nucleic Acids Res. 44, 1202–1213. https://doi.org/10.1093/nar/gkv951 (2016).
Sadowski, J., Gasteiger, J. & Klebe, G. Comparison of automatic three-dimensional model builders using 639 X-ray structures. J. Chem. Inf. Comput. Sci. 34, 1000–1008. https://doi.org/10.1021/ci00020a039 (1994).
Trott, O. & Olson, A. J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 31, 455–461. https://doi.org/10.1002/jcc.21334 (2010).
Schrödinger, L. & DeLano, W. PyMOL. http://www.pymol.org/pymol (2020).
Lipinski, C. A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 1, 337–341. https://doi.org/10.1016/j.ddtec.2004.11.007 (2004).
Bowers, K. J. et al. Scalable algorithms for molecular dynamics simulations on commodity clusters. In Proceedings of the ACM/IEEE Conference on Supercomputing. SC06 (Tampa, FL, 2006), 43–43. https://doi.org/10.1109/SC.2006.54.
Schrödinger Release. Desmond Molecular Dynamics System, D. E. Shaw Research, New York, NY, 2023. Maestro-Desmond Interoperability Tools 2023 (Schrödinger, 2023–2024).
Srivastava, N., Garg, P., Srivastava, P. & Seth, P. K. A molecular dynamics simulation study of the ACE2 receptor with screened natural inhibitors to identify novel drug candidate against COVID-19. PeerJ 9, e11171. https://doi.org/10.7717/peerj.11171 (2021).
Hospital, A., Goñi, J. R., Orozco, M. & Gelpí, J. L. Molecular dynamics simulations: advances and applications. Adv. Appl. Bioinform. Chem. AABC 8, 37–47. https://doi.org/10.2147/AABC.S70333 (2015).
Shivakumar, D. et al. Prediction of absolute solvation free energies using molecular dynamics free energy perturbation and the OPLS force field. J. Chem. Theor. Comput. 6, 1509–1519. https://doi.org/10.1021/ct900587b (2010).
Mark, P. & Nilsson, L. Structure and dynamics of the TIP3P, SPC, and SPC/E water models at 298 K. J. Phys. Chem. 105(43), 9954–9960. https://doi.org/10.1021/JP003020w (2001).
Tuckerman, M. B. B. J. M., Berne, B. J. & Martyna, G. J. Reversible multiple time scale molecular dynamics. J. Chem. Phys. 97, 1990–2001. https://doi.org/10.1063/1.463137 (1992).
Bharadwaj, K. K. et al. Potent bioactive compounds from seaweed waste to combat cancer through bioinformatics investigation. Front. Nutr. 9, 889276. https://doi.org/10.3389/fnut.2022.889276 (2022).
Grant, B. J., Skjaerven, L. & Yao, X. Q. The Bio3D packages for structural bioinformatics. Protein Sci. 30, 20–30. https://doi.org/10.1002/pro.3923 (2021).
Ichiye, T. & Karplus, M. Collective motions in proteins: A covariance analysis of atomic fluctuations in molecular dynamics and normal mode simulations. Proteins 11, 205–217. https://doi.org/10.1002/prot.340110305 (1991).
Palma, J. & Pierdominici-Sottile, G. On the uses of PCA to characterise molecular dynamics simulations of biological macromolecules: Basics and tips for an effective use. ChemPhysChem 24, e202200491. https://doi.org/10.1002/cphc.202200491 (2023).
Bharadwaj, K. K. et al. Nature Potential for COVID-19: Targeting SARS-CoV-2 Mpro Inhibitor with Bioactive Compound. ChemRxiv (Cambridge Open Engage, 2021).
Piao, L. et al. Molecular Dynamics simulations of wild type and mutants of SAPAP in complexed with Shank3. Int. J. Mol. Sci. 20, 224. https://doi.org/10.3390/ijms20010224 (2019).
Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 65, 55–63. https://doi.org/10.1016/0022-1759(83)90303-4 (1983).
Riss, T. L. et al. Cell viability assays in (ed. Markossian, S.). Assay Guidance Manual (Eli Lilly & Company and The National Center for Advancing Translational Sciences, 2013).
Denizot, F. & Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 89, 271–277. https://doi.org/10.1016/0022-1759(86)90368-6 (1986).
Kruger, N. J. The Bradford method for protein quantitation. The protein protocols handbook. Springer Protoc. Handb. https://doi.org/10.1007/978-1-59745-198-7_4 (2009).
Biswas, T., Resto-Roldán, E., Sawyer, S. K., Artsimovitch, I. & Tsodikov, O. V. A novel non-radioactive primase-pyrophosphatase activity assay and its application to the discovery of inhibitors of Mycobacterium tuberculosis primase DnaG. Nucleic Acids Res. 41, e56. https://doi.org/10.1093/nar/gks1292 (2013).
Josse, J. Constitutive inorganic pyrophosphatase of Escherichia coli. 1. Purification and catalytic properties. J. Biol. Chem. 241, 1938–1947. https://doi.org/10.1016/S0021-9258(18)96650-0 (1966).
Kuntz, I. D. Structure-based strategies for drug design and discovery. Science 257, 1078–1082. https://doi.org/10.1126/science.257.5073.1078 (1992).
Bhowmik, D. et al. Evaluation of potential drugs against leishmaniasis targeting catalytic subunit of Leishmania donovani nuclear DNA primase using ligand based virtual screening, docking and molecular dynamics approaches. J. Biomol. Struct. Dyn. 39, 1838–1852. https://doi.org/10.1080/07391102.2020.1739557 (2021).
Li, H. et al. Identification of novel falcipain-2 inhibitors as potential antimalarial agents through structure-based virtual screening. J. Med. Chem. 52, 4936–4940. https://doi.org/10.1021/jm801622x (2009).
Sonawane, P., Patel, K., Vishwakarma, R. K., Singh, S. & Khan, B. M. In Silico mutagenesis and docking studies of active site residues suggest altered substrate specificity and possible physiological role of Cinnamoyl CoA Reductase 1 (Ll-CCRH1). Bioinformation 9, 224–232. https://doi.org/10.6026/97320630009224 (2013).
Waring, M. J. Lipophilicity in drug discovery. Expert Opin. Drug Discov. 5, 235–248. https://doi.org/10.1517/17460441003605098 (2010).
Kufareva, I. & Abagyan, R. Methods of protein structure comparison. Methods Mol. Biol. 857, 231–257. https://doi.org/10.1007/978-1-61779-588-6_10 (2012).
Benson, N. C. & Daggett, V. Dynameomics: Large-scale assessment of native protein flexibility. Protein Sci. 12, 2038–2050. https://doi.org/10.1110/ps.037473.108 (2008).
Prabantu, V. M., Gadiyaram, V., Vishveshwara, S. & Srinivasan, N. Understanding structural variability in proteins using protein structural networks. Curr. Res. Struct. Biol. 4, 134–145. https://doi.org/10.1016/j.crstbi.2022.04.002 (2022).
Patel, S. Jr. CHARMM fluctuating charge force field for proteins: II protein/solvent properties from molecular dynamics simulations using a nonadditive electrostatic model. J. Comput. Chem. 25, 1504–1514. https://doi.org/10.1002/jcc.20077 (2004).
Hao, M. H., Haq, O. & Muegge, I. Torsion angle preference and energetics of small-molecule ligands bound to proteins. J. Chem. Inf. Model. 47, 2242–2252. https://doi.org/10.1021/ci700189s (2007).
Pawara, R., Ahmad, I., Surana, S. & Patel, H. Computational identification of 2, 4-disubstituted amino-pyrimidines as L858R/T790M-EGFR double mutant inhibitors using pharmacophore mapping, molecular docking, binding free energy calculation, DFT study and molecular dynamic simulation. In Silico Pharmacol. 9, 54. https://doi.org/10.1007/s40203-021-00113-x (2021).
Joshi, T., Joshi, T., Sharma, P., Chandra, S. & Pande, V. Molecular docking and molecular dynamics simulation approach to screen natural compounds for inhibition of Xanthomonas oryzae pv. Oryzae by targeting peptide deformylase. J. Biomol. Struct. Dyn. https://doi.org/10.1080/07391102.2020.1719200 (2020).
Liao, K. H. et al. Ligand-based and structure-based investigation for Alzheimer’s disease from traditional Chinese medicine. Evid. Based Complement. Alternat. Med. 2014, 364819. https://doi.org/10.1155/2014/364819 (2014).
Zhang, G. & Su, Z. Inferences from structural comparison: Flexibility, secondary structure wobble and sequence alignment optimization. BMC Bioinform 13(15), S12. https://doi.org/10.1186/1471-2105-13-S15-S12 (2012).
Godschalk, F., Genheden, S., Söderhjelm, P. & Ryde, U. Comparison of MM/GBSA calculations based on explicit and implicit solvent simulations. Phys. Chem. Chem. Phys. 15, 7731–7739. https://doi.org/10.1039/c3cp00116d (2013).
Balakumar, P., Rohilla, A., Krishan, P., Solairaj, P. & Thangathirupathi, A. The multifaceted therapeutic potential of Benfotiamine. Pharmacol. Res. 61, 482–488. https://doi.org/10.1016/j.phrs.2010.02.008 (2010).
Walko, C. M. & Lindley, C. Capecitabine: A review. Clin. Ther. 27, 23–44. https://doi.org/10.1016/j.clinthera.2005.01.005 (2005).
Klinkert, M. Q. & Heussler, V. The use of anticancer drugs in antiparasitic chemotherapy. Mini Rev. Med. Chem. 6, 131–143. https://doi.org/10.2174/138955706775475939 (2006).
Uliana, S. R. B. & Barcinski, M. A. Repurposing for neglected diseases. Science 326, 935. https://doi.org/10.1126/science.326.5955.935-a (2009).
Charlton, R. L., Rossi-Bergmann, B., Denny, P. W. & Steel, P. G. Repurposing as a strategy for the discovery of new anti-leishmanials: The-state-of-the-art. Parasitology 145, 219–236. https://doi.org/10.1017/S0031182017000993 (2018).
Braga, S. S. Multi-target drugs active against leishmaniasis: A paradigm of drug repurposing. Eur. J. Med. Chem. 183, 111660. https://doi.org/10.1016/j.ejmech.2019.111660 (2019).
Acknowledgements
The lab is supported by the Department of Biotechnology (India) (Order No: BT/PR16224/NER/95/176/2015) to Dr. Diwakar Kumar. Mitul Nath acknowledges the financial support of DBT-Twinning, GOI Grant (Order No: BT/PR16224/NER/95/176/2015 and Non-NET Fellowship from Assam University, Silchar. Deep Bhowmik and Rajat Nandi acknowledges the financial support of ICMR (Fellowship/95/2022-ECD-II & Letter No. 45/06/2022-DDI/BMS, dated 17/05/2022, respectively). Satabdi Saha recognize the financial support from the Inspire Fellowship (IF180806).
Author information
Authors and Affiliations
Contributions
M.N., D.B., S.S., R.N. and D.K. conducted the experiment. M.N., D.B., R.N. and D.K. wrote the manuscript. M.N., D.B., S.S., R.N. and D.K. contributed to the analysis of the results. D.K. supervised the project and conceived the original idea.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nath, M., Bhowmik, D., Saha, S. et al. Identification of potential inhibitor against Leishmania donovani mitochondrial DNA primase through in-silico and in vitro drug repurposing approaches. Sci Rep 14, 3246 (2024). https://doi.org/10.1038/s41598-024-53316-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53316-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.