Abstract
Ischemic stroke is the most common stroke, caused by occlusion of cerebral vessels and leading causes of disability. Erythropoietin (EPO) has non-hematopoietic effects as a neuroprotectant after ischemic event. This study aimed to learn the serum level of EPO in acute ischemic stroke. This cross-sectional study of ischemic stroke patients with onset < 24 h and consecutive sampling was used to collect the data from medical records review, physical examinations, head CT, 24-h EPO, 24-h and seventh-day NIHSS. A total of 47 patients consisting of 59.6% women, with a median age of 53 years old (21–70). The median 24 h EPO level was 808.6 pg/mL (134.2–2988.9). The relationship between 24 h-EPO and 24-h NIHSS were not significant (r = 0.101; p = 0.250), nor to 7th day NIHSS (r = − 0.0174; p = 0.121) and to delta NIHSS (r = 0.186; p = 0.106). The relationship of blood collection time (hour) and EPO was significant (r = − 0.260; p = 0.039). There was a statistically significant difference between serum EPO levels in ischemic stroke patients with lacunar stroke compared to non-lacunar stroke (288.5 vs. 855.4 ng/mL; p = 0.021). There was a relationship between the time of collection of blood and the level of EPO and also there was difference EPO level in lacunar stroke subtype compared with non-lacunar. The relationship between EPO and NIHSS lost significance after analysis. There is a need for a future study comparing each stroke risk factor and the same blood collection time.
Similar content being viewed by others
Introduction
Stroke is one of the most common causes of death1. The incidence of stroke will increase as much as 12% in developed countries and 20% in developing countries2. Data from The Indonesian Ministry of Health in 2018 showed the prevalence of stroke was 10@.9/1000, increasing from 7/1000 in 20133. Stroke is an acute condition related decrease to abrupt in causes disturbance or loss of neurological function for more than 24 h4. Ischemic stroke accounts for 87% of all stroke cases related to cerebral vessel occlusion1.
Decreasing cerebral blood flow is accompanied by a decrease in cerebral perfusion and led to an ischemic condition with a necrotic core and penumbra area surrounding it. There will be bioenergy failure in the necrotic core resulting in cell death, while the penumbra area carries out self-rescue with ischemic preconditioning. A disproportion in the supply and oxygen demand in cerebral tissue promotes a sequence of biochemical and molecular events that leads to neuron cell death. Following a hypoxia condition, brain responses in many ways. One of the most intriguing ways is by producing preconditioning hypoxia protein which includes transcription factors Hypoxia-inducible factor-1α (HIF-1α) in the penumbra area, and induce EPO and Vascular Endothelial Growth Factors (VEGF) a as a self-protect mechanism within minutes of the ischemic process and reaches its peak within 24 h of the ischemic stroke5,6,7,8,9. Therefore, a deeper understanding regarding the role of EPO, specifically in ischemic stroke, is required to improve recovery and brain repair process after stroke.
Methods
Study design and population
This cross-sectional study of ischemic stroke patients with onset < 24 h and consecutive sampling was used to collect the data from medical records review, physical examinations, head CT, 24-h EPO, 24-h and seventh-day NIHSS.
Clinical and imaging evaluation
The research subjects were patients diagnosed with ischemic stroke confirmed by CT scan (expertised by radiologist and confirmed by stroke neurologist) who came to the hospital within 24 h after stroke onset and were grouped into non-lacunar stroke and lacunar stroke. Inclusion criteria were a clinical diagnosis on the admission of < 24 h, aged 18–70 years old and have not got intravenous or oral drugs. We excluded patients with acute myocardial infarction, acute kidney injury, acute limb ischemia, dementia, respiratory failure, head trauma, history of malignancies, hematology disorders, hypoglycemia, history of surgery in the last three months, congestive heart failure, atrial fibrillation, liver dysfunction, significant of ischemic cerebral, a stroke of unknown onset, and hospital-acquired pneumonia. The results of the NIHSS criteria assessment are scores, namely a score of < 4 indicating a mild neurological deficit, a score of 5–12 indicating a moderate neurological deficit, and a score of > 12 indicating a severe neurological deficit10.
EPO measurement
Examination of serum EPO levels was on admission using Enzyme Link Immunosorbent Assay (ELISA). The serum formed was separated and put into a microtube and then stored at − 80 °C until a predetermined number of samples was obtained. Serum EPO (pg/mL) was determined using a human EPO Elisa kit (E-EL-H3640, Elabscience, USA).
Statistics
Pearson and spearman were used to see the correlation between 24-h onset EPO and NIHSS (24 h and seventh-day NIHSS). The t-Test and Mann Whitney U tests were used to compare EPO level in category severity of stroke. A p-value of less than 0.05 was considered statistically significant.
Ethical approval
Dr. Hasan Sadikin General Hospital Bandung Human Research Ethics Committee (LB.02.01/X/6.5.251.2019) had approved this study. This study had complied with all relevant ethical regulations (including The Declaration of Helsinki).
Result
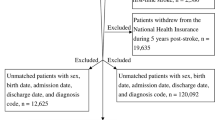
A total of 124 subject with ischemic stroke was enrolled from August to December 2019, subjects were excluded due to other diseases, 11 subjects with > 24 h onset, five deaths, and nine subjects with hospital-acquired pneumonia. In the end, we recruited 47 patients (see supplementary material). Of the total subjects, 59.6% were women and, the median age was 53 (21–70) years. The level of hemoglobin, leukocyte, platelet, erythrocyte was normal compared to the reference value. Risk factor included hypertension (74.5%), hyperlipidemia (74.5%), diabetes mellitus (25.5%), hyperuricemia (10.6%) (Table 1).
This study showed median EPO 24 h 808.6 pg/mL, with a median 24 h NIHSS of 6 and 76.6% accounted for moderate stroke. The median of the seventh day NIHSS was seventh and, 85.1% accounted for a mild stroke. The median of delta NIHSS was 4, with 4.3% subject without changing NIHSS (Table 1).
This study also showed us that there was no significant relationship between comorbidities and other basic characteristics in lacunar and non-lacunar groups (p > 0.05) except increasing of total cholesterol and hypertrigliseride (Table 2).
This study showed a positive trend between 24-h EPO and 24-h NIHSS (r = 0.101 , p = 0.250), negative trend between 24-h EPO and 7th day NIHSS (r = − 0.174, p = 0.121) and positive trend between 24-h EPO and delta NIHSS (r = 0.186, p = 0.106) (Table 3 and Fig. 1).
Data analysis showed us that in 24-h, median EPO in mild stroke was 705.5 (159.9–2441.8) pg/mL and in moderate stroke was 822.5 (134.2–2988.9) pg/mL, but statistically, there was no significant difference. There was a statistically significant difference between serum EPO levels in ischemic stroke patients with lacunar stroke compared to nonlacunar stroke ( 288.5 vs. 855.4 ng/mL; p = 0.021) (Table 4).
Data analysis showed us in seventh day, median EPO in mild stroke was 814.6 (134.2–2988.9) pg/mL and in moderate stroke was 428.3 (239.5–980) pg/mL, and statistically, there was no significant differentiation (Table 3 and Fig. 2).
The median blood collection time in this study was 12 (6–18) hours; data analysis showed a significant correlation between time to collect and EPO level, with a weak correlation and negative trend ( r = -0.260; p = 0.039) (Table 3 and Fig. 3).
Discussion
A total of 47 subjects was included in this study, acute ischemic stroke patients with 59.6% of women with the median age of 53 years. This data was similar with a characteristic patient of Sahlgrenska Academy Study on Ischemic Stroke (SAHLSIS) with a mean of 56 years. Hypertension and hyperlipidemia were the most common risk factor in this study, with hypertension could be a single risk factor and the most common risk factor for stroke11,12.
There was a positive trend of the relationship between 24 h EPO and delta NIHSS, but also statistically was not significant (r = 0.186, p = 0.106). This finding is consistent with a previous study from SAHLSIS that showed a negative relationship between 24 h EPO and Scandinavian Stroke Scale (SSS), but not significant statistically as well11.
Data analysis showed there was a negative correlation between blood collect time (hour) and EPO ( r = − 0.260; p = 0.039). There was no requirement time for exact blood collection time for this study, so the result for EPO might also be influenced. SAHLSIS study showed there is no significant differentiation between time (day) and EPO serum results12.
EPO serum could decrease in these situations such as chronic anemia, liver dysfunction, elderly, dementia but increase in hypoglycemia, head trauma or surgery history, hematologic disease, malignancy, atrial fibrillation, congestive heart failure. All of the mentioned were exclusion criteria in this study, but retinal disease, hyperglycemia, hyperlipidemia were not excluded13,14,15,16,17.
From this study results showed that there was no significant relationship between comorbidities and other basic characteristics in lacunar and non-lacunar groups (p > 0.05) except increasing of total cholesterol and hypertrigliseride. Some studies showed that EPO induces lipid catabolism activation via JAK2-STAT5 in adipose tissue, and later it produces triglycerides lipase (ATGL) so that decreased triglycerides serum level is expected, but there was no explanation on the relationship between cholesterol to EPO, according to this study there was a significant relationship between total cholesterol or triglyceride and EPO18. According to previously published results in chronic kidney disease patients, such inflammatory mediators as lipopolysaccharide and cytokines inhibit cholesterol efflux from cells by decreasing expression of the adenosine triphosphate–binding cassette A1 (ABCA1) gene. This effect leads to the decrease in reverse cholesterol transport and consequently the decrease in serum HDL-C levels. Furthermore, it was suggested that EPO expresses anti-inflammatory activities19. Further research is needed for investigating correlation between EPO level and lipid metabolism in acute ischemic stroke patients.
Sex in this study was dominated by women (59.6%). In previous study showed that disability and quality of life in stroke women would be worse than men. That could be a bias factor for increasing NIHSS. Men will also have a 10% higher differentiation erythrocyte mass index than women related to hormonal factors, iron needs due to menstruation, pregnancy, and lactation20.
A total of 74.5% of subjects in this study had hypertension and, 25.5% with diabetes mellitus. This condition is related to chronic inflammation, which would induce immune activation such as T cell and monocyte which secreted pro-inflammatory cytokine interferon-gamma (IFN). This pro-inflammatory cytokine will influence the metabolism of iron in the bone marrow and decrease the sensitivity of EPO. On the other hand, this cytokine will increase the severity level of stroke and contribute to some bias for this study18,21,22,23,24.
This study showed that the median level of 24-h EPO 808.6 pg/mL was higher than the SAHLSIS study (in Sweden population) with a mean of 9.3mIU/mL. There was a statistically significant difference between serum EPO levels in ischemic stroke patients with lacunar stroke compared to non-lacunar stroke (288.5 vs. 855.4 ng/mL; p = 0.021). Small lesions in lacunar stroke will certainly produce small ischemic lesions, this will result in a better clinical outcome in lacunar stroke than non-lacunar25. A wide brain damage also correlates with worsening of neurological deficit in acute ischemic stroke patients and wide ischemic changes were found in the non-lacunar group. This finding showed the extent of neuronal brain damage after ischemic stroke5. It should be borne in mind that lacunar infarcts are the stroke subtype with the best functional prognosis even in pure motor stroke, which is the lacunar syndrome with the greatest functional impairment, and we can asess using NIHSS on admission and discharge24.
Tissue hypoxia and cerebral ischemia activate HIF-1α, which in turn activates transcription of the EPO and Vascular Endothelial Growth Factor (VEGF) genes. HIF-1α, EPO, and VEGF increased as early as 1 h after acute ischemic stroke and may be used as marker of severity of neuronal damage and recovery in brain ischemic. There were strong correlations between HIF-1α and on admission and discharge NIHSS5. The main targets of EPO are neurons, while VEGF prevents apoptosis and induces endothelial cell proliferation, resulting in angiogenesis and improved tissue oxygenation5,26. EPO also contributes to endothelial cell proliferation, and VEGF also has a direct neuroprotective effect on neuronal cells. EPO and VEGF receptors are also generated on microglial cells and astrocytes, the targets of which are glial cells, but the effect on neuronal survival is unclear. EPO is thought to be able to stop the signal for cell death thereby reducing infarct volume14. There is a need to study further the possible influence of environmental and gene interactions with EPO levels in acute ischemic stroke24,27,28. Due to stroke, HIF-1α and EPO starts to accumulate as a response to inadequate oxygen level. HIF-1α mRNA expression and EPO after hypoxia could be detected within first thirty to sixty minutes. The regulation of HIF-1α and EPO, in penumbra, is rising until 7.5 h after onset of ischemia, so blood collection time (less then 7.5 h onset) could influence the EPO level in acute ischemic stroke5. Therefore, a deeper understanding regarding the role of EPO, specifically in ischemic stroke, is required to improve recovery and brain repair process after stroke29,30.
Limitation of study
This study did not have the same blood collection time, and this study also did not compare each stroke risk factor in every subject.
Conclusion
There was a relationship between the time of collection of blood and the level of EPO and also there was difference EPO level in lacunar stroke subtype compared with non-lacunar. Future research using prospective method is needed regarding EPO level and clinical outcome in acute ischemic stroke comparing each stroke risk factor and the same blood collection time.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Akbar, M. et al. Clinical features of transient ischemic stroke patients at high recurrence risk in Indonesia. Neurol. Asia 23(2), 107–113 (2018).
Mestre, H., Minian, Y. C., Fainsod, D. Z. & Ibara, A. Pharmacological treatment of acute ischemic stroke. Intech Open Sci. https://doi.org/10.5572/53774 (2013).
Wappler, E. A., Felszeghy, K., Varshney, M., Mehra, R. D. & Nyakas Csaba, D. K. K. Brain plasticity following ischemia. Dalam: Balestrino, M. (ed). Advances in the preclinical study of ischemic stroke 89–114 (2012).
Fann, D. Y. W. et al. Pathogenesis of acute stroke and the role of inflammasomes. Ageing Res. Rev. 12(4), 941–966. https://doi.org/10.1016/j.arr.2013.09.004 (2013).
Amalia, L., Sadeli, H. A., Parwati, I., Rizal, A. & Panigoro, R. Hypoxia-inducible factor-1α in acute ischemic stroke: neuroprotection for better clinical outcome. Heliyon 6(6), e04286 (2020).
Lo, E. H., Dalkara, T. & Moskowitz, M. A. Neurological diseases: Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 4(5), 399–414. https://doi.org/10.1038/nrn1106 (2003).
Simats, A., García-Berrocoso, T. & Montaner, J. Neuroinflammatory biomarkers: From stroke diagnosis and prognosis to therapy. Biochim. Biophys. Acta Mol. Basis Dis. 1862(3), 411–424. https://doi.org/10.1016/j.bbadis.2015.10.025 (2016).
Lai, T. W., Zhang, S. & Wang, Y. T. Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Prog. Neurobiol. 115(C), 157–188. https://doi.org/10.1016/j.pneurobio.2013.11.006 (2014).
Juul, S. & Pet, G. The potential of erythropoietin to treat asphyxia in newborns. Res. Rep. Neonatol. 4, 195. https://doi.org/10.2147/RRN.S52375 (2014).
Chalos, V. et al. National Institutes of Health Stroke Scale: An alternative primary outcome measure for trials of acute treatment for ischemic stroke. Stroke. 51(1), 282–290. https://doi.org/10.1161/STROKEAHA.119.026791 (2020).
Kenneth, M. Erytropoietin and diabetes mellitus. World J. Diabetes 6(4), 1259–1273. https://doi.org/10.4239/wjd.v6.i14.1259 (2015).
Li, J. et al. Kidney secreted erythropoietin lowers lipidemia via activating JAK2-STAT5 signaling in adipose tissue. EBioMedicine https://doi.org/10.1016/j.ebiom.2019.11.007 (2019).
Brines, M. & Cerami, A. Erythropoietin-mediated tissue protection: Reducing collateral damage from the primary injury response. J. Intern. Med. 264(5), 405–432. https://doi.org/10.1111/j.1365-2796.2008.02024 (2008).
Brines, M. L. et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc. Natl. Acad. Sci. 97(19), 10526–10531. https://doi.org/10.1073/pnas.97.19.10526 (2000).
Wang, B. et al. Beneficial effect of erythropoietin short peptide on acute traumatic brain injury. Neurotherapeutics. 13(2), 418–427. https://doi.org/10.1007/s13311-015-0418-y (2016).
Aberg, N. D. et al. Serum erythropoietin and outcome after ischemic stroke: A prospective study. Br. Med. J. https://doi.org/10.1136/bmjopen-2015-009827 (2015).
Iqbal, F., Hussain, S. & Hassan, M. Hypertension, diabetes mellitus, and hypercholesterolaemia as risk factors for stroke. Pak. J. Med. Res. 42(1), 17–22 (2003).
Macdougall, I. C. & Cooper, A. The inflammatory response and epoietin sensitivity. Nephrol. Dial. Transplant. https://doi.org/10.1093/ndt/17.suppl_1.48 (2002).
Siamopoulos, K. C. et al. Long-term treatment with EPO increases serum levels of high-density lipoprotein in patients with CKD. Am. J. Kidney Dis. 48(2), 242–249. https://doi.org/10.1053/j.ajkd.2006.04.071 (2006).
El-Kannishy, G. M. et al. Obesity may be eryhtropoietin dose saving in hemodialysis patients. Kidney Res. Clin. Pract. https://doi.org/10.23876/j.krcp.2018.37.2.148 (2018).
Di lorio, B. R., Stellato, D., De Santo, N. G. & Cirillo, M. Association of gender and age with eryhtropoietin resistance in hemodialysis patients: role of menstrual status. Blood Purif. 22, 423–427. https://doi.org/10.1159/000080234 (2004).
Faria, M. S. et al. Body mass index and resistance to recombinant human erythropoietin in maintenance hemodyalisis patients. J. Renal Fail. https://doi.org/10.3109/0886022X.2013.828267 (2013).
Zhang, X. B., Zeng, Y. M., Zeng, H. Q., Zhang, H. P. & Wang, H. L. Erythropoietin levels in patiens with sleep apnea: a meta-analysis. Eur. Arch. Otorhinolaryngol. https://doi.org/10.1007/s00405-017-4483-1 (2017).
Renz, H. et al. Gene-environment interactions in chronic inflammatory disease. Nat. Immunol. https://doi.org/10.1038/ni0411-273 (2011).
Arboix, A. et al. Attack to early neurological recovery after nonlacunar ischemic stroke. Cerebrovasc. Dis. 18, 304–311. https://doi.org/10.1159/000080356 (2004).
Lina, R. N., Rahmadi, M. & Khotib, J. Erythropoietin restores motor functions through angiogenesis in the thalamus area of ischemic stroke in rats. Folia Medica Indonesiana. https://doi.org/10.20473/fmi.v54i3.10011 (2018).
Benders, M. J. et al. Feasibility and safety of erythropoietin for neuroprotection after perinatal arterial ischemic stroke. J. Pediatr. 164(3), 481–486. https://doi.org/10.1016/j.jpeds.2013.10.084 (2014).
Åberg, N. D. et al. Serum erythropoietin and outcome after ischaemic stroke: a prospective study. BMJ Open https://doi.org/10.1136/bmjopen-2015-009827 (2017).
Yao, X. et al. Erythropoietin treatment in patients with acute ischemic stroke: A systematic review and meta-analysis of randomized controlled trials. Curr. Drug Deliv. 14(6), 853–860. https://doi.org/10.2174/1567201813666160822104813 (2017).
Ma, Y., Zhou, Z., Yang, G. Y., Ding, J. & Wang, X. The effect of erythropoietin and its derivatives on ischemic stroke therapy: A comprehensive review. Front. Pharmacol. 17(13), 743926. https://doi.org/10.3389/fphar.2022.743926 (2022).
Funding
Open access funding provided by University of Padjadjaran.
Author information
Authors and Affiliations
Contributions
L.A.: research preparation, collecting data, analyze research data, manuscript preparation, editing and submitting for published article. G.N.S.: collecting data, analyze research data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Amalia, L., Saputra, G.N. Serum erythropoietin in acute ischemic stroke: preliminary findings. Sci Rep 14, 2661 (2024). https://doi.org/10.1038/s41598-024-53180-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53180-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.