Abstract
Death with a functioning graft is important cause of graft loss after kidney transplantation. However, little is known about factors predicting death with a functioning graft among kidney transplant recipients. In this study, we evaluated the association between post-transplant creatinine-cystatin C ratio and death with a functioning graft in 1592 kidney transplant recipients. We divided the patients into tertiles based on sex-specific creatinine-cystatin C ratio. Among the 1592 recipients, 39.5% were female, and 86.1% underwent living-donor kidney transplantation. The cut-off value for the lowest creatinine-cystatin C ratio tertile was 0.86 in males and 0.73 in females. The lowest tertile had a significantly lower 5-year patient survival rate and was independently associated with death with a functioning graft (adjusted hazard ratio 2.574, 95% confidence interval 1.339–4.950, P < 0.001). Infection was the most common cause of death in the lowest tertile group, accounting for 62% of deaths. A low creatinine-cystatin C ratio was significantly associated with an increased risk of death with a functioning graft after kidney transplantation.
Similar content being viewed by others
Introduction
Kidney transplantation (KT) is the preferred treatment for patients with end-stage kidney disease. Although short-term outcomes of KT have continued to improve over the past couple of decades, long-term outcomes remain suboptimal1,2. Previous studies on long-term outcomes post-KT have focused on death-censored graft failure3,4. Graft outcomes have improved in recent years through evolutions in clinical practice, including accurate measurement of graft renal function, immunologic monitoring, and histologic evaluation5,6,7. However, death with a functioning graft (DWFG)—an important cause of overall graft loss—has received little attention8,9,10. Given that the number of elderly KT recipients with multiple comorbidities continues to rise, DWFG is becoming an increasingly relevant issue9,10. Therefore, clinical parameters identifying patients at higher risk for DWFG are urgently required.
Serum concentration of cystatin C, a cysteine protease inhibitor produced by all nucleated cells, has been proposed as a measure that may be superior to creatinine as a proxy for glomerular filtration of the kidneys11. Interestingly, evidence suggests that cystatin C is linked to mortality risk independently of renal function12,13. Furthermore, several studies have investigated the serum creatinine-cystatin C ratio as a surrogate marker for mortality14,15. This ratio is easy to obtain and minimizes the influence on renal function. Nevertheless, the clinical relevance of serum creatinine-cystatin C ratio in KT recipients has not been heretofore evaluated.
In the present study, we investigated the association between post-transplantation creatinine-cystatin C ratio and DWFG in a large KT cohort.
Results
Baseline characteristics
Among the 1592 recipients included in this study, 39.5% were female, and 86.1% underwent living-donor KT. Donor, recipient, and transplantation characteristics are summarized in Table 1. The mean age of recipients at the time of KT was 42.8 years, and their mean body mass index (BMI) was 22.4 kg/m2. Recipients in the lowest tertile received dialysis for a longer time before KT, were more likely to be underwent re-transplantation, and were more likely to be older at the time of KT. There were also significant differences in donor age and donor sex between creatinine-cystatin C ratio tertiles.
Baseline patient characteristics at the time of creatinine-cystatin C ratio measurement are shown in Table 2. Overall, the median time from transplantation to ratio measurement was 121 months (IQR, 24–196 months). Recipients in the lowest creatinine-cystatin C ratio tertile were significantly older and had a higher prevalence of diabetes mellitus and hypertension. The majority of recipients received calcineurin inhibitors (98.7%) in combination with a glucocorticoid (83.7%) and antimetabolite (75.8%) after KT. Cyclosporin was used more frequently in the lowest tertile, whereas tacrolimus and an antimetabolite were used more frequently in the highest tertile. The median serum creatinine increased (first to third tertile: 1.07, 1.18, and 1.36 mg/dL, respectively) and the median cystatin C level decreased (first to third tertile: 1.47, 1.32, and 1.29 mg/L, respectively) as the creatinine-cystatin C ratio increased. The lowest creatinine-cystatin C ratio tertile had significantly lower hemoglobin, albumin, total cholesterol, and uric acid, compared with the other tertiles.
Creatinine-cystatin C ratio
There was a strong positive correlation between serum creatinine and cystatin C levels (males: r = 0.850, P < 0.001; females: r = 0.871, P < 0.001) (Fig. S1). The cut-off value for the lowest creatinine-cystatin C ratio tertile was 0.86 in males and 0.73 in females. Distributions of the creatinine-cystatin C ratio and the age and creatinine-cystatin C based eGFR are shown in Fig. 1.
To investigate risk factors associated with a low creatinine-cystatin C ratio, we performed multivariable logistic regression analysis using the lowest tertile as a binary, dependent variable. In multivariable analysis, the following were independently associated with the lowest creatinine-cystatin C ratio tertile: older recipient age at the time of creatinine-cystatin C ratio measurement (adjusted odds ratio [aOR] 1.082, 95% confidence interval [CI] 1.069–1.095; P < 0.001), re-transplant (aOR 2.215, 95% CI 1.526–3.214; P < 0.001), and longer duration of dialysis (aOR 1.005, 95% CI 1.002–1.007; P = 0.001) (Table 3).
Death with a functioning graft
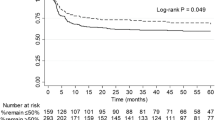
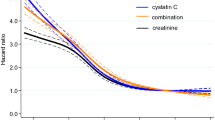
The median duration of follow-up after creatinine-cystatin C ratio measurement was 28 months (IQR, 11.0–87.0 months). During follow-up, DWFG occurred in 78 patients. There was a significant difference in patient survival between creatinine-cystatin C ratio groups. The 5-year patient survival rate after creatinine-cystatin C measurement was 87.3% in the lowest tertile, 96.0% in the middle tertile, and 96.7% in the highest tertile (P < 0.001) (Fig. 2). In multivariable Cox proportional hazard analysis, the lowest creatinine-cystatin C ratio tertile was significantly associated with DWFG (adjusted hazard ratio [aHR] 2.574, 95% CI 1.339–4.950; P = 0.005) (Table 4, model 3). This association was further evaluated using cubic spline analysis (Fig. 3). In the competing risk analysis, the lowest tertile group showed a significant increase in the hazard for DWFG, considering the competing risk of death-censored graft failure (HR 1.478, p < 0.001) (Fig. S2).
Causes of death
Causes of patient death are shown in Table 5. Overall, infection was the most common cause of death (50%), followed by cardiovascular disease (30.8%) and malignancy (12.8%). In the lowest tertile, infection was the most common cause of death, accounting for 62% of all deaths. By contrast, cardiovascular disease was the most common cause of death in the middle plus highest tertiles (39.3%). In multivariable Cox analysis, the lowest creatinine-cystatin C ratio tertile was associated with an increased risk of infection-related death (aHR 3.763, 95% CI 1.481–9.561; P = 0.005) (Table 4, model 3).
Subgroup analysis
We performed subgroup analyses, stratified by recipient sex (male or female), age at creatinine-cystatin C measurement (< 55 or ≥ 55 y), BMI (< 23 or ≥ 23 kg/m2), creatinine-cystatin C–based eGFR (< 60 or ≥ 60 mL/min/1.73 m2), serum albumin level (< 4.0 or ≥ 4.0 mg/dL), and donor status (living or deceased donor). There were no significant interactions between subgroups and lowest creatinine-cystatin C tertile for DWFG, indicating that the association between a low creatinine-cystatin C ratio and an increased risk of DWFG persisted, regardless of recipient sex, age, BMI, creatinine-cystatin C–based eGFR, serum albumin, and donor status.
Discussion
In this large single-center study, we investigated the clinical associations between sex-specific creatinine-cystatin C ratio and long-term outcomes after KT. We found that a low creatinine-cystatin C ratio was significantly associated with an increased risk of DWFG in KT recipients. Compared with the highest creatinine-cystatin C ratio group, the lowest creatinine-cystatin C ratio group had a 2.6-fold higher risk of DWFG, even after adjusting for potential confounding factors, including age, sex, diabetes mellitus, eGFR, and serum albumin. Infection was the most common cause of death in the lowest creatinine-cystatin C ratio group.
DWFG presents as distinctly different clinical phenotypes when compared with death-censored graft failure (the focus of most previous research and a condition caused by many different pathogenic processes)8,16. However, relatively few studies have examined risk factors for DWFG, despite DWFG accounting for approximately half of overall graft losses9,17. Infection, cardiovascular disease, and malignancy are common causes of DWFG. Recipient age, diabetes mellitus, and pre-transplantation duration of dialysis are known risk factors for DWFG, but these factors are not modifiable18. Little is known about post-transplantation factors predictive of DWFG. Only a limited number of studies have investigated the relationship between post-KT eGFR and DWFG, and the relationship remains unclear8,18. Therefore, clinical parameters that reliably identify patients at higher risk for DWFG are urgently required.
Significant graded associations between reduced eGFR and adverse outcomes, including cardiovascular events, hospitalization, and mortality, have been well established19. Although creatinine is the most frequently used biomarker for eGFR, creatinine-based eGFR has an “inverted J-shape” association with all-cause mortality20,21. By contrast, cystatin C has a strong and linear association with all-cause mortality12. Accumulating recent evidence suggests that the creatinine-cystatin C ratio may have utility as a surrogate marker for mortality beyond renal function14,15. Previous studies reported that a low creatinine-cystatin C ratio is associated with an increased risk of mortality in various conditions, such as diabetes mellitus22, chronic kidney disease23,24, malignancy15, and chronic obstructive pulmonary disease25. Our data demonstrated that a low sex-specific creatinine-cystatin C ratio was an independent risk factor for all-cause mortality in KT recipients. To our knowledge, this is the first study to evaluate clinical outcomes according to creatinine-cystatin C ratio in a large population of KT recipients.
One plausible explanation for the relationship between creatinine-cystatin C ratio and DWFG observed in the present study is that the ratio might reflect muscle mass. Since creatinine is an end-product of muscle catabolism, serum creatinine is affected by muscle mass26,27. In contrast, cystatin C is produced by all nucleated cells28. Therefore, a low creatinine-cystatin C ratio might be a marker of low muscle mass. In addition, the risk factors for low creatinine-cystatin C ratio found in our study were older age, longer dialysis duration, and re-transplant, which are all known risk factors for low muscle mass. Low muscle mass has emerged as a strong predictor of mortality in various populations. Several investigators, including our group, have reported an association between low muscle mass and poor transplant outcomes after KT29,30.
Another possible explanation for our findings is that cystatin C might reflect inflammatory status. Several studies have suggested that cystatin C is directly linked to adverse outcomes beyond renal function11,13, and previous studies reported that cystatin C is associated with inflammatory markers31,32,33. A low creatinine-cystatin C ratio, resulting from an elevated cystatin C level, may indicate an inflammatory state rather than a decline in renal function.
In addition, previous various studies have reported that patients with low muscle mass have increased vulnerability to infection and an increased risk of infection-related mortality34,35,36. Taking into account that the lowest tertile group might be associated with low muscle mass, this is consistent with our finding that infection is the most common cause of death in the lowest creatinine-cystatin C ratio group.
Our findings have potentially important clinical implications. Creatinine-based eGFR may overestimate GFR in patients with a low muscle mass, whereas cystatin C is less affected by muscle mass. Accordingly, the National Kidney Foundation (NKF) and the American Society of Nephrology have recommended the use of cystatin C to estimate GFR37. In situations where cystatin C and creatinine are measured concurrently, the creatinine-cystatin C ratio could be used as a potential clinical parameter for predicting DWFG, an outcome that is otherwise difficult to predict.
This study has several limitations. First, it was a retrospectively designed single-institution study, and the results of the study were not validated in an independent cohort. Therefore, further external validation of these study results is necessary. However, since it was conducted at a single center, the study has the advantages of consistent use of immunosuppressive drugs, standardized patient management protocols, and more complete and reliable data regarding mortality (including causes). Second, this study investigated cystatin C data measured at least 6 months after KT. It is therefore unknown whether the creatinine-cystatin C ratio has similar significance in predicting early mortality after KT. Third, because of the late introduction of cystatin C measurements, creatinine-cystatin C ratios were not obtained on the same day after KT across patients. In 2015, the NKF published guidelines recommending the use of cystatin C to evaluate kidney function post-KT, and cystatin C measurements were begun relatively recently in the Republic of Korea. Nevertheless, our data on the creatinine-cystatin C ratio have the advantage of representing real-world data. Fourth, since muscle mass was not measured, we were unable to directly assess the relationship between low muscle mass and creatinine-cystatin C ratio.
In conclusion, a low creatinine-cystatin C ratio was significantly associated with an increased risk of DWFG in KT recipients.
Methods
Patient selection
We screened consecutive adults who received a kidney transplant between January 1991 and December 2016 at Severance Hospital, Seoul, Republic of Korea. In this retrospective observational cohort study, we included patients with concurrent measurements of serum creatinine and cystatin C obtained at least 6 months after KT and excluded patients with graft failure or loss of follow-up before 2009 (at the time of initiation of cystatin C measurement). We also excluded patients who underwent multi-organ transplantation or experienced graft failure within 1 month after the creatinine-cystatin C ratio measurement. After excluding ineligible patients, 1592 KT recipients were included in this study. The patients were grouped into three groups based on sex-specific creatinine-cystatin C ratio tertiles (Fig. 4). The first creatinine (in mg/dL) to cystatin C (in mg/L) ratio at least 6 months after KT was used.
All study procedures were conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Severance Hospital (4-2022-1560). The need for informed consent was waived owing to the retrospective study design.
Data collection
Demographic and clinical data were retrieved from the Severance Kidney Transplant database. Laboratory and medication data were retrieved from the patients’ electronic medical records. Laboratory data included complete blood cell count (including hemoglobin), blood urea nitrogen, and serum levels of creatinine, cystatin C, albumin, calcium, total cholesterol, phosphorus, and uric acid. Serum creatinine was measured using the Jaffe kinetic method, and cystatin C was measured using the immunoturbidimetric assay. Estimated glomerular filtration rate (eGFR) was calculated using the recently published Chronic Kidney Disease Epidemiology Collaboration 2021 creatinine-cystatin C equation5.
Immunosuppression and follow-up protocol
Maintenance immunosuppression consisted of a calcineurin inhibitor (tacrolimus or cyclosporine), prednisolone, and mycophenolate mofetil or a mammalian target of rapamycin inhibitor. All patients were followed at our transplant center. Patients visited the outpatient clinic every month for the first post-transplantation year and every 3 months thereafter. At each visit, history and physical examination were performed, and routine laboratory tests were obtained. At our institution, serum cystatin C levels have been measured annually in KT recipients at the physicians’ discretion since 2009.
Clinical outcomes
The primary outcome of this study was DWFG. Secondary outcomes were cause of death and infection-related death. Patient survival was calculated from the date of creatinine-cystatin C ratio measurement to the date of death, loss to follow-up, or December 31, 2022 (end of the follow-up period). We also censored patients with graft failure (return to long-term dialysis or re-transplantation). Mortality data were obtained from both the electronic medical records of our transplant center and the Korean National Statistical Office, using a unique identifier.
Statistical analyses
Data were expressed as frequency, mean ± standard deviation, or median and interquartile range (IQR), depending on the type of data. Chi-square test or Fisher’s exact test was used as appropriate to compare categorical variables. Continuous variables were compared using a one‐way analysis of variance for parametric data and the Kruskal–Wallis test for nonparametric data. Correlations between parameters were evaluated with the Pearson test. Multivariable logistic regression analysis was performed using the lowest creatinine-cystatin C ratio tertile as a binary outcome variable. Patient survival rates were analyzed using Kaplan–Meier curves and the log-rank test. Cox proportional hazard regression models were used to evaluate associations between creatinine-cystatin C ratio and study outcomes. Three models were created: model 1, unadjusted; model 2, adjusted for age, sex, and diabetes mellitus at the time of creatinine-cystatin C ratio measurement; and model 3, adjusted for the model 2 factors plus creatinine-cystatin C–based eGFR and serum albumin. A competing risk analysis for death with a functioning graft (DWFG) was conducted using the Fine and Gray subdistribution hazard model, with death-censored graft failure set as the competing risk. All tests were performed as two-tailed tests, and P values < 0.05 were considered statistically significant. Statistical analyses were performed using SPSS software (version 26.0; IBM Corp., Armonk, NY, USA) and R (version 3.6.3; R Foundation for Statistical Computing, Vienna, Austria).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Coemans, M., Callemeyn, J. & Naesens, M. Long-term survival after kidney transplantation. N. Engl. J. Med. 386, 497–498. https://doi.org/10.1056/NEJMc2115207 (2022).
Hariharan, S., Israni, A. K. & Danovitch, G. Long-term survival after kidney transplantation. N. Engl. J. Med. 385, 729–743. https://doi.org/10.1056/NEJMra2014530 (2021).
Shabir, S. et al. Predicting 5-year risk of kidney transplant failure: A prediction instrument using data available at 1 year posttransplantation. Am. J. Kidney Dis. 63, 643–651. https://doi.org/10.1053/j.ajkd.2013.10.059 (2014).
Loupy, A. et al. Prediction system for risk of allograft loss in patients receiving kidney transplants: International derivation and validation study. BMJ 366, l4923. https://doi.org/10.1136/bmj.l4923 (2019).
Inker, L. A. et al. New creatinine- and cystatin c-based equations to estimate GFR without race. N. Engl. J. Med. 385, 1737–1749. https://doi.org/10.1056/NEJMoa2102953 (2021).
Loupy, A. et al. The Banff 2019 Kidney Meeting Report (I): Updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am. J. Transplant. 20, 2318–2331. https://doi.org/10.1111/ajt.15898 (2020).
Tambur, A. R. et al. Sensitization in transplantation: Assessment of risk (STAR) 2019 Working Group Meeting Report. Am. J. Transplant. 20, 2652–2668. https://doi.org/10.1111/ajt.15937 (2020).
Gaston, R. S. et al. Late graft loss after kidney transplantation: Is “death with function” really death with a functioning allograft?. Transplantation 104, 1483–1490. https://doi.org/10.1097/tp.0000000000002961 (2020).
Ying, T. et al. Death after kidney transplantation: An analysis by era and time post-transplant. J. Am. Soc. Nephrol. 31, 2887–2899. https://doi.org/10.1681/asn.2020050566 (2020).
Awan, A. A. et al. Trends in the causes of death among kidney transplant recipients in the United States (1996–2014). Am. J. Nephrol. 48, 472–481. https://doi.org/10.1159/000495081 (2018).
Shlipak, M. G., Mattes, M. D. & Peralta, C. A. Update on cystatin C: Incorporation into clinical practice. Am. J. Kidney Dis. 62, 595–603. https://doi.org/10.1053/j.ajkd.2013.03.027 (2013).
Shlipak, M. G. et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N. Engl. J. Med. 352, 2049–2060. https://doi.org/10.1056/NEJMoa043161 (2005).
Peralta, C. A. et al. Cystatin C identifies chronic kidney disease patients at higher risk for complications. J. Am. Soc. Nephrol. 22, 147–155. https://doi.org/10.1681/asn.2010050483 (2011).
Jung, C. Y. et al. Creatinine-cystatin C ratio and mortality in patients receiving intensive care and continuous kidney replacement therapy: A retrospective cohort study. Am. J. Kidney Dis. 77, 509-516.e501. https://doi.org/10.1053/j.ajkd.2020.08.014 (2021).
Jung, C. Y. et al. Creatinine-cystatin C ratio and mortality in cancer patients: A retrospective cohort study. J. Cachexia Sarcopenia Muscle 13, 2064–2072. https://doi.org/10.1002/jcsm.13006 (2022).
Mayrdorfer, M. et al. Exploring the complexity of death-censored kidney allograft failure. J. Am. Soc. Nephrol. 32, 1513–1526. https://doi.org/10.1681/asn.2020081215 (2021).
Pascual, M., Theruvath, T., Kawai, T., Tolkoff-Rubin, N. & Cosimi, A. B. Strategies to improve long-term outcomes after renal transplantation. N. Engl. J. Med. 346, 580–590. https://doi.org/10.1056/NEJMra011295 (2002).
Lorenz, E. C. et al. Kidney allograft function and histology in recipients dying with a functioning graft. Am. J. Transplant. 14, 1612–1618. https://doi.org/10.1111/ajt.12732 (2014).
Go, A. S., Chertow, G. M., Fan, D., McCulloch, C. E. & Hsu, C. Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 351, 1296–1305. https://doi.org/10.1056/NEJMoa041031 (2004).
Levey, A. S. et al. The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int. 80, 17–28. https://doi.org/10.1038/ki.2010.483 (2011).
Matsushita, K. et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375, 2073–2081. https://doi.org/10.1016/s0140-6736(10)60674-5 (2010).
Osaka, T. et al. Decreased the creatinine to cystatin C ratio is a surrogate marker of sarcopenia in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 139, 52–58. https://doi.org/10.1016/j.diabres.2018.02.025 (2018).
Hyun, Y. Y. et al. Serum creatinine to cystatin C ratio and clinical outcomes in adults with non-dialysis chronic kidney disease. Front. Nutr. 9, 996674. https://doi.org/10.3389/fnut.2022.996674 (2022).
Lin, Y. L. et al. Serum indices based on creatinine and cystatin C predict mortality in patients with non-dialysis chronic kidney disease. Sci. Rep. 11, 16863. https://doi.org/10.1038/s41598-021-96447-9 (2021).
Chen, Z. et al. Serum creatinine/cystatin C ratio as a predictor of in-hospital mortality in patients hospitalized with acute exacerbation of chronic obstructive pulmonary disease. Lung 200, 609–617. https://doi.org/10.1007/s00408-022-00568-5 (2022).
Onopiuk, A., Tokarzewicz, A. & Gorodkiewicz, E. Cystatin C: A kidney function biomarker. Adv. Clin. Chem. 68, 57–69. https://doi.org/10.1016/bs.acc.2014.11.007 (2015).
Rule, A. D. et al. For estimating creatinine clearance measuring muscle mass gives better results than those based on demographics. Kidney Int. 75, 1071–1078. https://doi.org/10.1038/ki.2008.698 (2009).
Stevens, L. A. et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int 75, 652–660. https://doi.org/10.1038/ki.2008.638 (2009).
Kim, H. J. et al. Low skeletal muscle mass is associated with mortality in kidney transplant recipients. Am. J. Transplant. 23, 239–247. https://doi.org/10.1016/j.ajt.2022.11.016 (2023).
Deliège, P. G. et al. Skeletal Muscle Index as a prognostic marker for kidney transplantation in older patients. J. Ren. Nutr. 31, 286–295. https://doi.org/10.1053/j.jrn.2020.08.014 (2021).
Okura, T. et al. Association between cystatin C and inflammation in patients with essential hypertension. Clin. Exp. Nephrol. 14, 584–588. https://doi.org/10.1007/s10157-010-0334-8 (2010).
Shlipak, M. G. et al. Cystatin-C and inflammatory markers in the ambulatory elderly. Am. J. Med. 118, 1416. https://doi.org/10.1016/j.amjmed.2005.07.060 (2005).
Singh, D., Whooley, M. A., Ix, J. H., Ali, S. & Shlipak, M. G. Association of cystatin C and estimated GFR with inflammatory biomarkers: The Heart and Soul Study. Nephrol. Dial. Transplant. 22, 1087–1092. https://doi.org/10.1093/ndt/gfl744 (2007).
Arase, H. et al. Modified creatinine index and risk for long-term infection-related mortality in hemodialysis patients: Ten-year outcomes of the Q-Cohort Study. Sci. Rep. 10, 1241. https://doi.org/10.1038/s41598-020-58181-6 (2020).
Krell, R. W. et al. Association between sarcopenia and the risk of serious infection among adults undergoing liver transplantation. Liver Transpl. 19, 1396–1402. https://doi.org/10.1002/lt.23752 (2013).
Pereira, R. A. et al. Sarcopenia in chronic kidney disease on conservative therapy: Prevalence and association with mortality. Nephrol. Dial. Transplant. 30, 1718–1725. https://doi.org/10.1093/ndt/gfv133 (2013).
Delgado, C. et al. A unifying approach for GFR estimation: Recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. Am. J. Kidney Dis. 79, 268-288.e261. https://doi.org/10.1053/j.ajkd.2021.08.003 (2022).
Funding
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI20C1234).
Author information
Authors and Affiliations
Contributions
Research idea and study design: M.C.C., J.L.; data acquisition: M.C.C., S.H.Y., H.J.K., J.L.; data analysis/interpretation: M.C.C., D.G.K., S.H.Y., H.J.K., H.W.K., J.L.; statistical analysis: M.C.C., D.G.K.; supervision and mentorship: J.Y., B.S.K., K.H.H., M.S.K., J.L. Each author contributed important intellectual content during manuscript drafting or revision and agrees to be personally accountable for the individual’s own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved, including with documentation in the literature if appropriate.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Choi, M.C., Kim, D.G., Yim, S.H. et al. Creatinine-cystatin C ratio and death with a functioning graft in kidney transplant recipients. Sci Rep 14, 1966 (2024). https://doi.org/10.1038/s41598-024-52649-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-52649-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.