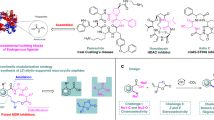
Abstract
Hybrid molecules maintain their stronghold in the drug market, with over 60% of drug candidates in pharmaceutical industries. The substantial expenses for developing and producing biologically privileged drugs are expected to create opportunities for producing hybrid molecule-based drugs. Therefore, we have developed a simple and efficient copper-catalyzed approach for synthesizing a wide range of triazole-linked glycohybrids derived from pyrazolo[1,5-a]pyrimidines. Employing a microwave-assisted copper-catalyzed approach, we developed a concise route using various 7-O-propargylated pyrazolo[1,5-a]pyrimidines and 1-azidoglycosides. This strategy afforded a series of twenty-seven glycohybrids up to 98% yield with diverse stereochemistry. All were achieved within a remarkably shortened time frame. Our investigation extends to evaluating the anticancer potential of these synthesized triazole-linked pyrazolo[1,5-a] pyrimidine-based glycohybrids. In-vitro assays against MCF-7, MDA-MB231, and MDA-MB453 cell lines reveal intriguing findings. (2R,3S,4S,5R,6R)-2-(acetoxymethyl)-6-(4-(((5-(4-chlorophenyl)pyrazolo[1,5-a]pyrimidin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate emerges as a standout with better anticancer activity against MDA-MB231 cells (IC50 = 29.1 µM), while (2R,3R,4S,5R,6R)-2-(acetoxymethyl)-6-(4-(((5-(4-chlorophenyl)pyrazolo[1,5-a]pyrimidin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate demonstrates the best inhibitory effects against MCF-7 cells (IC50 = 15.3 µM) in all derived compounds. These results align with our docking analysis and structure–activity relationship (SAR) investigations, further validating the in-vitro outcomes. This work not only underscores the synthetic utility of our devised protocol but also highlights the promising potential of these glycohybrids as candidates for further anticancer therapeutic exploration.
Similar content being viewed by others
Introduction
Nitrogen-containing heterocycles are abundant in nature, necessary for life, and play a crucial role in the metabolism of all living cells1,2,3,4. The pyrazolo[1,5-a]pyrimidine moiety is one of the several nitrogen-containing heterocycles and is a crucial pharmacophore found in many biologically active molecules5. Pyrazolo-pyrimidines and related heterocyclic compounds have a wide range of applications in medicine and agriculture6,7. These compounds have been found to exhibit diverse pharmacological activities, and their ability to mimic the structural features of biogenic purines makes them promising candidates for drug development8. Additionally, pyrazolo[1,5-a]pyrimidines are bioisosteres for compounds such as triazolothienopyrimidines, imidazoquinazolines, pyrimidoquinazolines, and imidazo-quinolinones, all of these have demonstrated good anticancer activity9. The literature has recently emphasized the cancer chemopreventive properties of pyrazolo[1,5-a]pyrimidine derivatives, which have been found to exhibit apoptosis and differentiation-induced anticancer activities in various in-vitro cell line models. Cancer chemoprevention is mainly aimed to preventing, delaying, or suppressing tumor incidence using synthetic or natural bioactive agents. Encouraging outcomes have been observed, and the derivatives have demonstrated potential in preventing cancer10,11. Figure 1 represents the pyrazolopyrimidine core containing privileged scaffolds (A–E), which shows various biological potentials such as (A) Zaleplon is hypnotic generally used as a treatment of sleeping disorder (Insomnia), (B) Indiplon, hypnotic and sedative, (C) Formycin as antibacterial agent, (D) Sildenafil, used as treatment of erectile disfunction (E) Oxoformycin, antibacterial drug12,13,14,15,16,17.
On the other hand, carbohydrates are a type of molecule with diverse stereochemistry and play essential roles in molecular recognition and various intracellular functions18,19,20,21,22. When combined with bioactive molecules, carbohydrates can be used to create chemical libraries for drug discovery and development23,24,25,26,27. To improve the bioavailability of carbohydrate-derived drugs, the hydroxyl group in the molecule is often masked with a hydrophobic acyl group, which is later cleaved in the blood to create a pro-drug that can be more easily absorbed. In this particular scenario, the notion of hybrid drug design is in its early stages and shows promise, as it enables the utilization of current anticancer agents to create combinations that feature two or more pharmacophores28. These combinations can target multiple distinct sites within the infected tissues.
Thus, the process of adding glycone molecules to bioactive aglycone molecules to produce new glycohybrids is ongoing research29,30,31. The process is widely used in drug development to enhance the pharmacological properties and ADMET parameters of drugs32. Examples of drug development include glycosylated paclitaxel33,34 and demethylepipodophyllotoxin35,36, which have increased water solubility and reduced toxicity. Additionally, glycosylated diphyllin37 is a more potent topoisomerase II inhibitor compared to the parent compound, and acyl-protected sugar units in etopophos (tafluposide) have been shown to enhance biological activity compared to etopophos alone38. Thus, adding the pharmacophoric moiety (N-heterocycles) with the glycone unit has become an effective tool for the medication of rapidly growing cancerous cells39,40. We conceived that a fresh combination of such compounds could exhibit greater potency than the original one, making it considerably more effective in inhibiting cancerous growth in the body.
These scaffolds stand out as a privileged heterocycle in drug discovery41, exhibiting a plethora of biological activities, with a particular emphasis on its significance in anticancer properties42. Nevertheless, further extensive studies are required to unveil potential compounds with target-based therapeutic efficacy. In light of the aforementioned literature, It was planned to synthesize the triazole-linked glycohybrids by using the structural motifs of pyrazolo[1,5-a]pyrimidine with acetylated glucose, galactose and mannose through a triazole ring as a linker and evaluate their anticancer activity. The copper(I)-catalyzed 1,2,3-triazole formation was chosen as the linking tool due to its stability, specificity, and biocompatibility43,44. The 1,2,3-triazole moiety was considered an ideal bioisosteric replacement for the amide due to its similarity in size, dipolar character, and H-bond acceptor properties, and its high chemical stability further supported its use in this context45. Azido glycosides derived from d-glucose, d-galactose and d-mannose were used as diversity expedient to couple with various substituted alkyne-modified pyrazolo[1,5-a]pyrimidine. These hybrid molecules offered structural diversity, improved solubility and valuable anticancer potentials.
Results and discussion
Chemistry
For the synthesis of designed molecules as triazole-linked glycohybrids of pyrazolo[1,5-a]pyrimidines, it was planned to prepare aglycone and glycone intermediates. Initially, we recognized commercially available diverse acetophenones 1a–1i as a starting material, which can be transformed into diverse β-keto esters 2a–2i (SI, Scheme S1). The β-keto esters 2a–2i were obtained by the esterification of acetophenones 1a–1i, using diethyl carbonate in the presence of a strong base25,46. We synthesized β-keto esters and noticed that the product yield varied depending on the various substitutions at the aryl ring. We noticed that upon substitution with electron-withdrawing groups such as fluoro- and trifluoro-methyl at the aryl ring afforded the lower yield of β-keto esters, upon the substitution with electron donating group such as methyl- and methoxy- at the aryl ring favors the process, with a higher yield of β-keto esters. To obtain diverse pyrazolopyrimidin-7-ol, β-keto esters 2a–2i were treated with 3-amino pyrazole 3 under reflux conditions in acetic acid for 12–14 h, which afforded diverse pyrazolopyrimidin-7-ol 4a–4i in good to very good yields (Fig. 2)47. Thus, the following variants of pyrazolo[1,5-a]pyrimidin-7-ol (4a–4i) were synthesized, and it was noticed that the product yield varied depending on the various substitutions at the aryl ring. We found that aryl rings with trifluoro methyl and isopropyl substitution had a lower yield. On the other hand, when we used the aprotic solvent toluene instead of the protic solvent acetic acid, we noticed that pyrazolo[1,5-a]pyrimidinone 5 was obtained as a primary product (Fig. 3). The proton NMR confirmed that protic solvent is essential for producing pyrazolo[1,5-a]pyrimidin-7-ol. 1H NMR value of OH in 5-(4-isopropylphenyl)pyrazolo[1,5-a]pyrimidin-7-ol (4g) appeared at 12.44 ppm has disappeared in 5-(4-isopropylphenyl)pyrazolo[1,5-a]pyrimidin-7(4H)-one (5). 1H NMR (500 MHz, DMSO-d6) for δ 8.43 (d, J = 8.0 Hz, 2H), 8.38 (s, 1H), 7.96 (d, J = 8.0 Hz, 2H), 6.72 (d, J = 2.7 Hz, 1H), 6.60–6.57 (m, 1H), 3.54 (dt, J = 14.4, 6.8 Hz, 1H), 1.83 (d, J = 6.9 Hz, 6H).
After successfully synthesizing diverse pyrazolopyrimidin-7-ol 4a–4i, it was planned to synthesize alkyne-modified pyrazolopyrimidine-7-ol. Diversely substituted pyrazolopyrimidin-7-ol 4a–4i were treated with propargyl bromide in DMF using K2CO3 as a base; at elevated temperatures, it furnishes 7-O-propargyl pyrazolo[1,5-a]pyrimidines 6a–6i (Fig. 4) as a significant product. It is worth mentioning here that minor products were formed under these reaction conditions, which were N-propargylated derivatives.
After preparation of aglycone intermediate by performing propargylation of pyrazolo[1,5-a]pyrimidines, our focus was drawn on the synthesis of glycone intermediate, which were different azido glycosides of glucose, galactose, and mannose, respectively. The commercially available glucose, galactose, and mannose were acetylated to synthesize these azido glycosides to afford pentaacetylated products 7a, 7b, and 7c, respectively. Further, these pentaacetylated products 7a, 7b, and 7c were treated with trimethylsilyl azide in the presence of SnCl4 in dichloromethane at room temperature furnished selective β-1-azido derivatives 8a, 8b, and α-1-azido derivatives 8c in good to excellent yield (SI, Scheme S2)24.
After the successful synthesis and complete characterization of glycone and aglycone parts, it was decided to synthesize designed triazole-linked pyrazolo[1,5-a]pyrimidine-based glycohybrids. Herein, we have used the Cu(I) catalyzed 1,3 dipolar cycloaddition reaction (click condition) with our synthesized glycone and aglycone intermediates to transform our designed triazole-linked pyrazolo[1,5-a]pyrimidine-based glycohybrids. Initially, the click reaction was carried out between 7-O-propargylated pyrazolo[1,5-a]pyrimidine 6a and azido glycoside 8a adopting standard method using CuSO4·5H2O and sodium ascorbate in t-BuOH–H2O (1:1, v/v) at 50 °C afforded glycohybrid 9a in 80% isolated yield. This yield was not outstanding from the perspective of click chemistry, so to get a good to excellent yield in a shorter time, we chose a new synthetic strategy, in which we carried out the subsequent reactions utilizing the microwave irradiation technique. Here, the reaction was carried out between 7-O-propargylated pyrazolo[1,5-a]pyrimidine 6a and azido glucoside 8a using CuSO4·5H2O and sodium ascorbate in t-BuOH–H2O (1:1, v/v), at 50 °C and 100 W for 20 min, we obtained desired glycohybrid product 9a in excellent yield (98%). By using microwave irradiation and adopting similar reaction conditions, the diverse examples of glucohybrids of pyrazolo[1,5-a]pyrimidines 9b–9i have been prepared in good to excellent yields (Fig. 5). To explore the advantages of microwave heating, the reaction was also performed in an oil bath at heating conditions. Still, it was a more extensive, time-consuming reaction (6 h) than the microwave conditions (20 min). Moreover, the yield obtained with conventional heating was lower, reaching 80% compared to the microwave irradiation of 98%. So, it is evident that the microwave approach offers several advantages, such as high yields, mild reaction conditions, short reaction time, and good tolerance of functional groups.
Applying a similar synthetic protocol using microwave irradiation conditions, we were also interested in synthesizing galactohybrids, combining propargylated pyrazolo[1,5-a]pyrimidines 6a–6i and 1-azido galactoside 8b. Good to excellent isolated yields of galactohybrids 10a–10i were obtained when propargylated pyrazolo[1,5-a]pyrimidines 6a–6i reacted with 1-azido galactoside 8b in the presence of CuSO4·5H2O and sodium ascorbate in t-BuOH-H2O (1:1, v/v) using microwave irradiation under optimized reaction conditions (Fig. 6).
We were also interested in investigating the biological potential of mannohybrids. Thus, we have synthesized mannohybrids, integrating propargylated pyrazolo[1,5-a]pyrimidines 6a–6i and 1-azido mannoside 8c, using a similar synthetic approach under microwave reaction conditions. When propargylated pyrazolo[1,5-a]pyrimidines 6a–6i interacted with 1-azido mannoside 8c in the presence of CuSO4·5H2O and sodium ascorbate in t-BuOH–H2O (1:1, v/v) under microwave irradiation conditions, good to excellent isolated yields of mannohybrids 11a–11i were achieved (Fig. 7).
Anticancer activity
Several previous reports showed that the nucleus of pyrazolo[1,5-a]pyrimidine may serve as a potent anticancer activity. Thus, we screened the synthesized library of pyrazolo[1,5-a]pyrimidin-7-ols 4a–4i. Anticancer activity (in terms of growth inhibition/decreased cell viability with respect to control) of all synthesized compounds (n = 9) was investigated by MTT assay in MDA-MB-231 (human breast cancer) cell line at different concentrations for 72 h. Anticancer drugs YM155 and menadione were used as positive controls, and activity is summarized in Table 1.
The results summarized in Table 1, showed that among the library of pyrazolo[1,5-a]pyrimidin-7-ols, none of these compounds did have any growth inhibitory activity against MDA-MB 231 (human breast cancer). Thus, it was aimed to investigate the anticancer activity of a synthesized library of triazole-linked glycohybrids of pyrazolo[1,5-a]pyrimidines. Hence, after synthesizing the library of triazole-linked glycohybrids of pyrazolo[1,5-a]pyrimidines, they were screened for their anticancer activity using different cell lines. The MTT assay was used to investigate the anticancer activity of twenty-seven synthesized compounds on the growth inhibition and decrement in cell viability of MDA-MB-231 (human breast cancer). Different concentrations were tested for 72 h, and the results were compared to a control. Positive controls, namely YM155 and menadione, were also utilized, and the findings are presented in Table 2.
The results summarized in Table 2 showed moderate growth inhibition in all tested compounds with better activity with compounds 9a, 9d, 9h, 10d, 10f and 11g in MDA-MB-231 breast cancer cells. These compounds show almost 50% inhibition at 25 μM concentration. These six compounds were further screened, and results were taken to identify IC50 (Fig. 8).
MDA-MB-231 represents a specific subtype known as triple-negative breast cancer (TNBC). The investigation study was extended with active six hit compounds (9a, 9d, 9h, 10d, 10f, and 11g) in two other different cancer cell lines (MCF-7 and MDA-MB-453), each representing a separate class of breast cancers (MCF-7: hormone receptor/HR-positive; MDA-MB-453: human epidermal growth factor 2 receptor/HER2 positive). The IC50 values for the most active compounds 9a, 9d, 9h, 10d, 10f, and 11g were calculated and presented in Fig. 9 against MDA-MB-231 breast cancer cell line were found 29.6 µM, 43.1 µM, 35.9 µM, 29.1 µM, 31.2 µM and 38.8 µM, respectively.
IC50 of the most active compounds. To calculate half maximal inhibitory concentration (IC50) of compounds 9a, 9d, 9h, 10d, 10f, and 11g MDA-MB-231 cells were treated for 72 h with different concentrations (10, 25 and 50 µM) of the respective drugs and tested for cell viability by the MTT assay. IC50 values were determined by plotting values of percent cell viability against the concentration of each of these compounds. IC50 values for compounds 9a, 9d, 9h, 10d, 10f and 11g against MDA-MB-231 breast cancer cell line were found 29.6 µM, 43.1 µM, 35.9 µM, 29.1 µM, 31.2 µM and 38.8 µM respectively. The experiments were performed in triplicates, n = 3 and ± SD value was calculated for each data point.
We also included normal mammary epithelial MCF-10A cells in our experiments to confirm if the growth-inhibitory activities of the selected compounds are truly cancer cell-specific. The activity data from MCF-7 and MDA-MB-453 cell lines (tested at 1 µM, 10 µM and 25 µM) and activity data from MCF-10A cell line (tested at 25 µM, 50 µM and 100 µM) are summarized in Table 3.
The results summarized in Table 3 showed better inhibition of cell viability was observed with compounds 9a, 9d, 9h, 10d, 10f and 11g at 25 µM in MCF-7 (hormone receptor/HR-positive breast cancer cells). These compounds show almost 50% inhibition at 25 μM concentration. The IC50 values of the most active compounds are calculated and presented in Fig. 10.
IC50 of the most active compounds. To calculate half maximal inhibitory concentration (IC50) of compounds 9a, 9d, 9h, 10d, 10f and 11g, MCF-7 cells were treated for 72 h with different concentrations (10 µM, 25 µM and 50 μM) of the respective drugs and tested for cell viability by the MTT assay. The IC50 values for compounds 9a, 9d, 9h, 10d, 10f and 11g against MCF-7 breast cancer cells line were found 35.4 µM, 15.3 µM, 21.1 µM, 40.1 µM, 42.1 µM, and 18.3 μM, respectively. While these compounds did not reach up to IC50 value against MDA-MB-453 cells. Furthermore, none of these compounds did have any growth inhibitory activity against normal breast epithelial cell (MCF-10A).
Thus, through screening against various cell lines such as MDA-MB231, MCF-7, MDA-MB453 and MCF-10A, it has been found that among the derived library of compounds, most of the compounds show moderate to good anticancer activity. While compound 10d shows the best inhibitory activity against MDA-MB231 cell line with an IC50 value at 29.1 µM, and compound 9d shows the best inhibitory activity against the MCF-7 cell line with an IC50 value of 15.3 µM.
Molecular docking and SAR studies
Docking plays a crucial role in drug design by analyzing the binding interactions between a protein and a ligand. As a result, it necessitates the use of 3-D structures for both the ligand and protein. This is important in identifying potential targets for developing substrate and cofactor-based inhibitors, which may be effective as anticancer and antiproliferative drugs. To this end, we utilized the Schrödinger maestro tool to perform SAR studies and develop a ligand-based pharmacophore model hypothesis for the determination of ligand potencies and pharmacological properties. Our analysis revealed that the designed molecule exhibits diverse constitutional molecular descriptors, including aromatic rings (R1–R3), hydrogen bond acceptor sites (A1–A14), and hydrophobic site (H1). By substituting various electron-donating or withdrawing groups (H1) on the R3 aromatic ring, we achieved a range of ligand potency and intrinsic activity. The aromatic rings (R1–R3) aided in successful binding to the hydrophobic pocket of the target protein, as shown in Fig. 11.
3D-Pharmacophore model showing different constitutional molecular descriptors triazole linked hybrids of pyrazolo[1,5-a]pyrimidines. The grey circle with the arrow represents the hydrogen acceptor sites (A1–A14), the brown circle represents various aromatic rings (R1–R3), green circle represents the hydrophobic (H1) site.
In several previous reports, it has been reported that heterocycle containing pyrazolo[1,5-a]pyrimidine nucleus displays crucial drug-like properties such as anticancer, anti-neoplastic, antiproliferative, and many more. These molecules were found to have potential anticancer activity against MCF-7, which are HER2 positive cell lines. Thus, to identify the anticancer properties of pyrazolo[1,5-a]pyrimidine nucleus, we performed molecular docking between the designed compounds with the Human HER2 protein of tyrosine kinase domain (Protein Data Bank ID: 3PP0). The HER2 (Human epidermal growth factor receptor-2) protein of the tyrosine kinase domain is a membrane oncogene and serves as a major driver for the tumor development and proliferation of breast cancer. Thus, HER2 protein has always served as a desirable target site for researchers to evaluate the potency of most anticancer and anti-proliferative drugs. Most of the anticancer drugs inhibit the kinase activity of HER2 protein, suppressing the proliferation of cancerous cells.
Active site residues of the protein receptors have been identified as LEU785, LYS753, LEU852, GLY804, LEU800, MET801, ALA751, THR862, ASP863, THR798, VAL734, SER783, and PHE864. The parameters of the molecule in the active region were determined with the following values: grid box sizes of 34, 25, and 32 Å3, and x, y, z centers of 17.882, 15.918, and 28.054, respectively. The docking study reveals that the interaction between synthesized triazole-linked pyrazolo[1,5-a]pyrimidine-based glycohybrids and catalytic site HER2 protein occurs primarily through hydrogen bonds, hydrophobic bonds and π-stacking (as shown in Fig. 12).
Docking analysis of triazole-linked pyrazolo[1,5-a] pyrimidine-based glycohybrids (Compounds 9a, 9d, 9h, 10d, 10f and 11g) and catalytic site of HER2 protein with a 3D representation of hydrogen bond donor/acceptor surface (shown in pink and green color) and hydrogen bond (green color). The docking analysis was conducted using the collected set of compounds (gray) into the proposed binding pocket of the X-ray crystallographic structure of HER2 protein (Protein Data Bank ID: 3PP0, resolution: 2.4 Å).
Based on the docking results, the best docking poses, docking score with high binding affinity/energies and a number of favorable interactions suggest that most of the designed triazole-linked pyrazolo[1,5-a]pyrimidine-based glycohybrids may serve as effective anticancer candidates. Among the derived library of compounds, compound 9d displays the best docking pose with binding energy – 42.73 kcal/mol in mode 1 with minimum root mean square deviation value and perfectly fits into the active binding pocket of the protein. In Fig. 13 shows four conventional hydrogen bonds between the LYS736, and LYS724 active residues, respectively. Along with this, π-alkyl interactions have been found between the CYS805 and LEU726 active residues and the triazole ring and heterocyclic ring of the designed molecule. Also, other interactions, such as alkyl C–H interactions, have also been found. These hydrophobic and hydrophilic interactions displayed the binding versatility of the designed molecules, and the results obtained from in-silico docking suggest that most of the molecules perfectly fit into the binding pocket of the docked protein and may possess anticancer activity.
These results obtained from in-silico studies further validate the biological potential of our designed molecules as effective anticancer agents and support the results obtained from in-vitro studies.
Conclusions
In this investigation, we have designed and developed a series of pyrazolo[1,5-a] pyrimidine-based triazole-linked glycohybrids. The process involved microwave-assisted synthesis adopting copper-catalyzed click reaction, in which newly synthesized different 7-O-propargylated pyrazolo[1,5-a]pyrimidines were developed in excellent yields. This 7-O-propargylated pyrazolo[1,5-a]pyrimidines reacted with 1-azido-2,3,4,6-tetra-O-acetyl- d-glucose, d-galactose and d-mannose respectively under click reaction conditions they afforded diverse library of glycohybrids. Herein, we have synthesized a series of twenty-seven diverse substituted glycohybrids containing electron-donating and electron-withdrawing groups with inherited stereochemical diversity in a shorter reaction time. In this investigation, we have also evaluated the anticancer potential of our newly synthesized pyrazolo[1,5-a]pyrimidine-based triazole-linked glycohybrids. Anticancer activity was performed in-vitro against MCF-7, MDA-MB231, and MDA-MB453 cell-lines in cell-based assays. It was found that among the derived library of compounds, 10d shows better anticancer activity with IC50 value of 29.1 µM against MDA-MB231 cell line and 9d shows the best inhibitory activity against MCF-7 cell line with IC50 value of 15.3 µM. The in-silico docking analysis and SAR studies well supported the outcomes obtained from in-vitro study.
Experimental
General experimental methods
All experiments were conducted using anhydrous solvents and oven-dried glassware and were carried out using a CEM microwave synthesizer. High-resolution mass spectra were recorded using an ESI source and a quadrupole/TOF mass spectrometer. Solvents were distilled using standard methods and stored in 4Å and 3Å molecular sieves. JEOL JNM-ECZ500R/S1 instrument was used to record 1H (500 MHz) and 13C (126 MHz) spectra. The chemical shifts for 1H and 13C were referenced to the residual signals of CDCl3 1H NMR δ 7.26 and δ 77.16 for 13C NMR, and DMSO-d6 1H NMR δ 2.5 and δ 39.52 for 13C NMR, reported in parts per million (ppm) at 25 °C. Coupling constants were expressed in hertz (Hz). Thin-layer chromatography was used to monitor reactions, carried out on 0.25 mm Merck silica gel plates (60F-254), with spots visualized using phosphomolybdic acid and 10% H2SO4 in ethanol. Reagents were purchased from TCI, Merck, Sigma Aldrich, and other sources.
Synthesis of triazole-linked glucohybrids of pyrazolo[1,5-a]pyrimidines 9a–9i
In a microwave vial, a mixture of 50 mg (0.189 mmol) of O-propargylated pyrazolo[1,5-a]pyrimidine 6a and 70.89 mg (0.189 mmol) of 1-azido glucoside 8a in 2 ml of solvent (1:1, v/v mixture of t-BuOH-H2O) was treated with CuSO4.5H2O (1.24 mg, 0.0056 mmol) and sodium ascorbate (2.23 mg, 0.011 mmol), and then subjected to microwave heating for 20 min at 50 °C (100 W). The reaction was monitored by TLC to confirm completion, and upon completion, the reaction mixture was extracted with 5 ml of water and 3 ml of EtOAc. The organic layer was then dried over Na2SO4 and evaporated via a rotary evaporator to obtain the crude residue, which was purified by flash column chromatography to yield pure compound 9a in 98% isolated yield. Using similar methods, with 50 mg of propargylated reactants compounds 9b–9i were synthesized in good to excellent yields utilizing 1-azido glucoside.
(2R,3R,4S,5R,6R)-2-(acetoxymethyl)-6-(4-(((5-(p-tolyl)pyrazolo[1,5-a]pyrimidin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (9a): yellow colored sticky solid; yield 118.39 mg (98%), Rf = 0.32 (EtOAc); 1H NMR (500 MHz, CDCl3) δ 8.01 (s, 1H), 7.86 (d, J = 3.3 Hz, 1H), 7.82 (d, J = 7.9 Hz, 2H), 7.21 (d, J = 7.3 Hz, 2H), 6.50 (s, 1H), 6.39 (d, J = 3.8 Hz, 1H), 6.10 (d, J = 15.7 Hz, 1H), 5.99 (d, J = 15.6 Hz, 1H), 5.79 (d, J = 8.0 Hz, 1H), 5.36 (t, J = 8.0 Hz, 1H), 5.35–5.32 (m, 1H), 5.21 (t, J = 9.3 Hz, 1H), 4.23 (dd, J = 12.7, 5.5 Hz, 1H), 4.09 (dd, J = 11.3 Hz, 3.5 Hz, 1H), 3.97–3.94 (m, 1H), 2.35 (s, 3H), 2.01 (s, 3H), 2.00 (s, 3H), 1.96 (s, 3H), 1.62 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 170.5, 169.9, 169.3, 168.4, 162.2, 158.3, 153.9, 141.5, 140.2, 139.9, 134.9, 129.4, 127.1, 123.4, 100.2, 98.6, 85.8, 75.2, 72.4, 70.3, 67.6, 61.4, 47.0, 21.3, 20.6, 20.5, 19.8. HRMS (ESI-TOF), m/z calcd. C30H33N6O10 [M + H]+ 637.2253; Found: 637.2228.
(2R,3R,4S,5R,6R)-2-(acetoxymethyl)-6-(4-(((5-(4-methoxyphenyl)pyrazolo[1,5-a]pyrimidin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (9b): red colored sticky solid; yield: 113.32 mg (97%), Rf = 0.33 (EtOAc); 1H NMR (500 MHz, CDCl3) δ 7.98 (s, 1H), 7.92 (d, J = 8.0 Hz, 2H), 7.83 (d, J = 4.0 Hz, 1H), 6.95 (d, J = 8.0 Hz, 2H), 6.48 (s, 1H), 6.39 (d, J = 4.0 Hz, 1H), 6.10 (d, J = 14.7 Hz, 1H), 6.00 (d, J = 14.7 Hz, 1H), 5.76 (d, J = 9.3 Hz, 1H), 5.36 (t, J = 10.0 Hz, 1H), 5.34–5.33 (m, 1H), 5.20 (t, J = 9.5 Hz, 1H), 4.25 (dd, J = 13.3, 5.5 Hz, 1H), 4.11 (dd, J = 12.0 Hz, 3.5 Hz, 1H), 3.96–3.93 (m, 1H), 3.84 (s, 3H), 2.03 (s, 6H), 1.98 (s, 3H), 1.65 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 170.6, 170.0, 169.3, 168.5, 161.9, 161.4, 158.4, 153.9, 141.6, 139.9, 130.3, 128.8, 123.3, 114.1, 100.3, 98.0, 85.9, 75.3, 72.5, 70.3, 67.7, 61.5, 55.4, 47.1, 20.7, 20.6, 19.9. HRMS (ESI-TOF), m/z calcd. C30H33N6O11[M + H]+ 653.2202; Found: 653.2179.
(2R,3R,4S,5R,6R)-2-(acetoxymethyl)-6-(4-(((5-(4-bromophenyl)pyrazolo[1,5-a]pyrimidin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (9c): light yellow colored sticky solid; yield 99.57 mg (96%), Rf = 0.31 (EtOAc); 1H NMR (500 MHz, CDCl3) δ 7.99 (s, 1H), 7.87 (d, J = 5.3 Hz, 1H), 7.83 (d, J = 8.0 Hz, 2H), 7.56 (d, J = 8.0 Hz, 2H), 6.50 (s, 1H), 6.42 (d, J = 5.3 Hz, 1H), 6.14 (d, J = 14.7 Hz, 1H), 6.00 (d, J = 14.7 Hz, 1H), 5.77 (d, J = 9.3 Hz, 1H), 5.36 (t, J = 10.0 Hz, 1H), 5.32 (t, J = 10.0 Hz, 1H), 5.20 (t, J = 10.0 Hz, 1H), 4.26 (dd, J = 13.3, 5.5 Hz, 1H), 4.11 (dd, J = 12.0 Hz, 3.5 Hz, 1H), 3.96–3.93 (m, 1H), 2.04 (s, 6H), 1.99 (s, 3H), 1.65 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 170.5, 169.9, 169.3, 168.4, 161.0, 158.2, 153.9, 141.5, 140.0, 136.7, 131.8, 128.8, 124.6, 123.3, 100.2, 98.8, 85.9, 75.2, 72.4, 70.3, 67.7, 61.5, 47.1, 20.7, 20.5, 19.8. HRMS (ESI-TOF), m/z calcd. C29H30BrN6O10 [M + H]+ 701.1201; Found: 701.1177.
(2R,3R,4S,5R,6R)-2-(acetoxymethyl)-6-(4-(((5-(4-chlorophenyl)pyrazolo[1,5-a]pyrimidin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (9d): off white colored sticky solid; yield 116.42 mg (97%), Rf = 0.31 (EtOAc); 1H NMR (500 MHz, CDCl3) δ 7.99 (s, 1H), 7.88 (m, 3H), 7.39 (d, J = 8.1 Hz, 2H), 6.49 (s, 1H), 6.40 (d, J = 4.0 Hz, 1H), 6.13 (d, J = 14.8 Hz, 1H), 5.99 (d, J = 15.4 Hz, 1H), 5.79 (d, J = 8.3 Hz, 1H), 5.37 (t, J = 9.5 Hz, 1H), 5.32 (t, J = 9.5 Hz, 1H), 5.21 (t, J = 9.5 Hz, 1H), 4.25 (dd, J = 13.3, 5.0 Hz, 1H), 4.11 (dd, J = 12.0 Hz, 3.5 Hz, 1H), 3.97–3.94 (m, 1H), 2.03 (s, 6H), 1.97 (s, 3H), 1.63 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 170.5, 169.9, 169.3, 168.5, 160.9, 158.2, 153.9, 141.5, 140.0, 136.3, 136.2, 128.9, 128.6, 123.3, 100.2, 98.9, 85.9, 75.3, 72.4, 70.3, 67.7, 61.4, 47.2, 20.7, 20.5, 19.8. HRMS (ESI-TOF), m/z calcd. C29H30ClN6O10 [M + H]+ 657.1706; Found: 657.1687.
(2R,3R,4S,5R,6R)-2-(acetoxymethyl)-6-(4-(((5-(4-fluorophenyl)pyrazolo[1,5-a]pyrimidin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (9e): white colored sticky solid; yield 132.74 mg (95%) Rf = 0.31 (EtOAc); 1H NMR (500 MHz, CDCl3) δ 8.00 (s, 1H), 7.92 (dd, J = 9.0, 5.1 Hz, 2H), 7.87 (d, J = 3.9 Hz, 1H), 7.09 (m, 2H), 6.46 (s, 1H), 6.39 (d, J = 3.9 Hz, 1H), 6.12 (d, J = 14.8 Hz, 1H), 5.99 (d, J = 14.8 Hz, 1H), 5.80 (d, J = 8.1 Hz, 1H), 5.37 (t, J = 9.5 Hz, 1H), 5.32 (t, J = 9.0 Hz, 1H), 5.21 (t, J = 9.5 Hz, 1H), 4.23 (dd, J = 13.3, 5.0 Hz, 1H), 4.11 (dd, J = 12.0 Hz, 3.5 Hz, 1H), 3.98–3.95 (m, 1H), 2.02 (s, 3H), 2.01 (s, 3H), 1.96 (s, 3H), 1.62 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 170.52, 169.9, 169.3, 168.4, 165.0, 163.1, 161.1, 158.2, 153.9, 141.5, 140.0, 134.0, 129.2, 129.2, 123.3, 115.7, 115.5, 100.1, 98.6, 85.8, 75.2, 72.4, 70.3, 67.7, 61.4, 47.1, 20.6, 20.5, 20.5, 19.8. 13C–19F couplings in 13C NMR (125 MHz, DMSO-d6) δ 164.1 (d, JC-F = 249.48 Hz, C1), 129.2 (d, JC-F = 8.82 Hz, C3), 115.6 (d, JC-F = 21.42 Hz, C2). HRMS (ESI-TOF), m/z calcd. C29H30FN6O10 [M + H]+ 641.2002; Found: 641.1975.
(2R,3R,4S,5R,6R)-2-(acetoxymethyl)-6-(4-(((5-(4-(trifluoromethyl)phenyl)pyrazolo[1,5-a]pyrimidin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (9f): off-white colored sticky solid; yield: 117.47 mg (95%), Rf = 0.29 (EtOAc); 1H NMR (500 MHz, CDCl3) 1H NMR (500 MHz, CDCl3) δ 8.03 (m, 3H), 7.91 (d, J = 4.0 Hz, 1H), 7.65 (d, J = 8.0 Hz, 2H), 6.52 (s, 1H), 6.41 (d, J = 4.0 Hz, 1H), 6.16 (d, J = 14.7 Hz, 1H), 5.99 (d, J = 14.7 Hz, 1H), 5.82 (d, J = 8.0 Hz, 1H), 5.37 (t, J = 9.5 Hz, 1H), 5.32 (t, J = 9.5 Hz, 1H), 5.21 (t, J = 9.0 Hz, 1H), 4.23 (dd, J = 12.7, 4.0 Hz, 1H), 4.11 (dd, J = 12.0 Hz, 3.5 Hz, 1H), 3.99–3.96 (m, 1H), 2.01 (s, 3H), 2.00 (s, 3H), 1.95 (s, 3H), 1.60 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 170.5, 169.9, 169.3, 168.4, 160.5, 158.1, 153.9, 141.4, 141.3, 140.1, 132.0, 131.7, 131.5, 131.2, 127.6, 125.6, 125.1, 123.3, 123.0, 100.2, 99.5, 85.8, 75.2, 72.3, 70.3, 67.7, 61.5, 47.1, 20.6, 20.5, 20.5, 19.8. 13C–19F couplings in 13C NMR (125 MHz, DMSO-d6) δ 131.6 (q, JC-F = 31.5 Hz, C2), 125.5 (d, JC-F = 3.78 Hz, C3), 124.0 (q, JC-F = 272.16 Hz, C1). HRMS (ESI-TOF), m/z calcd. C30H30F3N6O10 [M + H]+ 691.1970; Found: 691.1949.
(2R,3R,4S,5R,6R)-2-(acetoxymethyl)-6-(4-(((5-(4-isopropylphenyl)pyrazolo[1,5-a]pyrimidin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (9g): gray colored sticky solid; yield: 123.81 mg (97%), Rf = 0.32 (EtOAc); 1H NMR (500 MHz, CDCl3) δ 8.00 (s, 1H), 7.85 (d, J = 8.0 Hz, 3H), 7.27 (d, J = 8.0 Hz, 2H), 6.50 (s, 1H), 6.39 (d, J = 3.9 Hz, 1H), 6.11 (d, J = 15.3 Hz, 1H), 5.99 (d, J = 15.0 Hz, 1H), 5.79 (d, J = 9.0 Hz, 1H), 5.37 (t, J = 10.0 Hz, 1H), 5.33 (t, J = 9.5 Hz, 1H), 5.20 (t, J = 9.5 Hz, 1H), 4.24 (dd, J = 13.0, 5.0 Hz, 1H), 4.09 (dd, J = 12.0 Hz, 3.5 Hz, 1H), 3.97–3.94 (m, 1H), 2.92 (hept, J = 7.5, 1H), 2.01 (s, 3H), 2.01 (s, 3H), 1.97 (s, 3H), 1.63 (s, 3H), 1.23 (d, J = 7.5 Hz, 6H). 13C NMR (125 MHz, CDCl3) δ 170.5, 169.9, 169.3, 168.4, 162.3, 158.4, 153.9, 151.1, 141.5, 139.8, 135.4, 127.3, 126.8, 123.4, 100.2, 98.6, 85.8, 75.2, 72.4, 70.3, 67.7, 61.4, 47.1, 34.0, 23.9, 20.6, 20.5, 19.8. HRMS (ESI-TOF), m/z calcd. C32H37N6O10 [M + H]+ 665.2566; Found: 665.2540.
(2R,3R,4S,5R,6R)-2-(acetoxymethyl)-6-(4-(((5-(naphthalen-2-yl)pyrazolo[1,5-a]pyrimidin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (9h): white colored sticky solid; yield: 123.57 mg (96%), Rf = 0.32 (EtOAc); 1H NMR (500 MHz, CDCl3) δ 8.47 (s, 1H), 8.09 (s, 1H), 8.01 (d, J = 9.3 Hz, 1H), 7.91 (d, J = 5.3 Hz, 2H), 7.87 (d, J = 8.0 Hz, 1H), 7.83–7.80 (m, 1H), 7.50–7.45 (m, 2H), 6.68 (s, 1H), 6.46 (d, J = 4.0 Hz, 1H), 6.15 (d, J = 15.8 Hz, 1H), 6.00 (d, J = 15.8 Hz, 1H), 5.81 (d, J = 9.1 Hz, 1H), 5.38–5.36 (m, 2H), 5.23–5.20 (m, 1H), 4.24 (dd, J = 12.7, 4.0 Hz, 1H), 4.10 (dd, J = 12.0 Hz, 3.5 Hz, 1H), 3.97–3.94 (m, 1H), 2.01 (s, 3H), 2.00 (s, 3H), 1.95 (s, 3H), 1.61 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 170.5, 169.9, 169.3, 168.4, 162.0, 158.4, 153.9, 141.5, 140.0, 135.0, 134.1, 133.2, 128.9, 128.3, 127.6, 127.3, 127.0, 126.4, 124.4, 123.6, 100.1, 99.2, 85.8, 75.1, 72.4, 70.3, 67.6, 61.4, 47.1, 20.6, 20.5, 19.8. HRMS (ESI-TOF), m/z calcd. C33H33N6O10 [M + H]+ 673.2253; Found: 673.2235.
(2R,3R,4S,5R,6R)-2-(acetoxymethyl)-6-(4-(((5-(benzo[d][1,3]dioxol-5-yl)pyrazolo[1,5-a]pyrimidin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (9i): white colored sticky solid; yield: 126.67 mg (97%), Rf = 0.31 (EtOAc); 1H NMR (500 MHz, CDCl3) δ 7.98 (s, 1H), 7.84 (d, J = 5.3 Hz, 1H), 7.49 (d, J = 9.3 Hz, 1H), 7.44 (s, 1H), 6.84 (d, J = 8.0 Hz, 1H), 6.43 (s, 1H), 6.38 (d, J = 4.0 Hz, 1H), 6.10 (d, J = 14.7 Hz, 1H), 6.00 (d, J = 8.0 Hz, 1H), 5.99 (s, 2H) 5.78 (d, J = 9.3 Hz, 1H), 5.37 (t, J = 9.0 Hz, 1H), 5.33 (t, J = 9.5 Hz, 1H), 5.20 (t, J = 9.5 Hz, 1H), 4.24 (dd, J = 12.7, 5.5 Hz, 1H), 4.11 (dd, J = 12.0 Hz, 3.5 Hz, 1H), 3.97–3.93 (m, 1H), 2.04 (s, 3H), 2.03 (s, 3H), 1.98 (s, 3H), 1.65 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 170.5, 170.0, 169.3, 168.5, 161.7, 158.3, 153.8, 149.3, 148.2, 141.5, 139.9, 132.1, 123.3, 121.8, 108.4, 107.6, 101.5, 100.2, 98.3, 85.9, 75.3, 72.4, 70.3, 67.7, 61.4, 47.1, 20.7, 20.6, 19.9. HRMS (ESI-TOF), m/z calcd. C30H31N6O12 [M + H]+ 667.1994; Found: 667.1964.
Synthesis of triazole-linked galactohybrids of pyrazolo[1,5-a]pyrimidines 10a–10i
In a microwave vial, a mixture of 50 mg (0.189 mmol) of O-propargylated pyrazolo[1,5-a]pyrimidine 6a and 70.89 mg (0.189 mmol) of 1-azido galactoside 8b in 2 ml of solvent (1:1, v/v mixture of t-BuOH-H2O) was treated with CuSO4·5H2O (1.24 mg, 0.005 mmol) and sodium ascorbate (2.23 mg, 0.011 mmol), and then subjected to microwave heating for 20 min at 50 °C (100 W). The reaction was monitored by TLC to confirm completion, and upon completion, the reaction mixture was extracted with 5 ml of water and 3 ml of EtOAc. The organic layer was then dried over Na2SO4 and evaporated via a rotary evaporator to obtain the crude residue, which was purified by flash column chromatography to yield pure compound 10a in 98% isolated yield. Using similar methods, with 50 mg of propargylated reactants, compounds 10b–10i were synthesized in good to excellent yields utilizing 1-azido galactose tetraacetate.
(2R,3S,4S,5R,6R)-2-(acetoxymethyl)-6-(4-(((5-(p-tolyl)pyrazolo[1,5-a]pyrimidin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (10a): yellow colored sticky solid; yield: 117.19 mg (97%), Rf = 0.32 (EtOAc);1H NMR (500 MHz, CDCl3) δ 8.01 (s, 1H), 7.86 (d, J = 4.0 Hz, 1H), 7.84 (d, J = 8.0 Hz, 2H), 7.23 (d, J = 8.0 Hz, 2H), 6.52 (s, 1H), 6.41 (d, J = 4.0 Hz, 1H), 6.05 (br s, 2H), 5.72 (d, J = 9.0 Hz, 1H), 5.49 (dd, J = 9.0 Hz, 4.0 Hz, 1H), 5.42 (t, J = 10.0 Hz, 1H), 5.18 (dd, J = 10.7, 4.0 Hz, 1H), 4.18–4.13 (m, 2H), 4.10–4.06 (m, 1H), 2.37 (s, 3H), 2.21 (s, 3H), 1.96 (s, 3H), 1.95 (s, 3H), 1.66 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 170.3, 170.1, 169.8, 168.6, 162.2, 158.4, 153.9, 141.4, 140.2, 140.0, 135.0, 129.4, 127.2, 123.2, 100.3, 98.6, 86.4, 74.2, 70.6, 67.8, 66.8, 61.2, 47.0, 21.3, 20.7, 20.6, 20.5, 19.9. HRMS (ESI-TOF), m/z calcd. C30H33N6O10 [M + H]+ 637.2253; Found: 637.2281.
(2R,3S,4S,5R,6R)-2-(acetoxymethyl)-6-(4-(((5-(4-methoxyphenyl)pyrazolo[1,5-a]pyrimidin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (10b): yellow colored sticky solid; yield 113.32 mg (97%), Rf = 0.31 (EtOAc); 1H NMR (500 MHz, CDCl3) δ 8.01 (s, 1H), 7.90 (d, J = 8.0 Hz, 2H), 7.85 (d, J = 5.3 Hz, 1H), 6.93 (d, J = 8.0 Hz, 2H), 6.48 (s, 1H), 6.39 (d, J = 4.0 Hz, 1H), 6.04 (s, 2H), 5.72 (d, J = 9.3 Hz, 1H), 5.49 (dd, J = 9.0 Hz, 4.0 Hz, 1H), 5.42 (t, J = 10.0 Hz, 1H), 5.17 (dd, J = 10.0, 4.0 Hz, 1H), 4.17–4.12 (m, 2H), 4.10–4.04 (m, 1H), 3.82 (s, 3H), 2.21 (s, 3H), 1.96 (s, 3H), 1.95 (s, 3H), 1.66 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 170.3, 170.1, 169.8, 168.7, 161.8, 161.3, 158.4, 153.9, 141.4, 140.0, 130.2, 128.7, 123.2, 114.0, 100.2, 98.0, 86.4, 74.2, 70.6, 67.8, 66.8, 61.2, 55.4, 47.0, 20.7, 20.6, 20.5, 19.9. HRMS (ESI-TOF), m/z calcd. C30H33N6O11[M + H]+ 653.2202; Found: 653.2311.
(2R,3S,4S,5R,6R)-2-(acetoxymethyl)-6-(4-(((5-(4-bromophenyl)pyrazolo[1,5-a]pyrimidin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (10c): yellow colored sticky solid; yield: 101.64 mg (98%), Rf = 0.33 (EtOAc); 1H NMR (500 MHz, CDCl3) δ 8.01 (s, 1H), 7.89 (d, J = 3.7 Hz, 1H), 7.81 (d, J = 8.0 Hz, 2H), 7.55 (d, J = 9.2 Hz, 2H), 6.50 (s, 1H), 6.41 (d, J = 3.9 Hz, 1H), 6.06 (br s, 2H), 5.73 (d, J = 9.3 Hz, 1H), 5.50 (dd, J = 9.5 Hz, 4.0 Hz, 1H), 5.42 (t, J = 10.0 Hz, 1H), 5.18 (dd, J = 10.0, 4.0 Hz, 1H), 4.18–4.13 (m, 2H), 4.10–4.05 (m, 1H), 2.21 (s, 3H), 1.97 (s, 3H), 1.96 (s, 3H), 1.66 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 170.3, 170.1, 169.8, 168.7, 161.0, 158.3, 153.9, 141.4, 140.1, 136.8, 131.9, 128.8, 124.6, 123.2, 100.2, 98.9, 86.5, 74.3, 70.6, 67.9, 66.8, 61.2, 47.1, 20.7, 20.6, 20.5, 19.9. HRMS (ESI-TOF), m/z calcd. C29H30BrN6O10 [M + H]+ 701.1201; Found: 701.1226.
(2R,3S,4S,5R,6R)-2-(acetoxymethyl)-6-(4-(((5-(4-chlorophenyl)pyrazolo[1,5-a]pyrimidin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (10d): yellow colored sticky solid; yield: 116.42 mg (97%), Rf = 0.33 (EtOAc); 1H NMR (500 MHz, CDCl3) δ 8.00 (s, 1H), 7.88 (d, J = 5.3 Hz, 2H), 7.86 (s, 1H), 7.37 (d, J = 8.0 Hz, 2H), 6.48 (s, 1H), 6.39 (d, J = 4.0 Hz, 1H), 6.05 (br s, 2H), 5.74 (d, J = 9.3 Hz, 1H), 5.49 (dd, J = 9.0 Hz, 4.0 Hz, 1H), 5.40 (t, J = 10.5 Hz, 1H), 5.18 (dd, J = 10.4, 4.0 Hz, 1H), 4.19–4.12 (m, 2H), 4.08–4.04 (m, 1H), 2.20 (s, 3H), 1.95 (s, 3H), 1.94 (s, 3H), 1.64 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 170.2, 170.0, 169.8, 168.6, 160.8, 158.2, 153.9, 141.4, 140.1, 136.3, 136.1, 128.8, 128.5, 123.2, 100.2, 98.8, 86.4, 74.2, 70.5, 67.9, 66.8, 61.1, 47.1, 20.7, 20.6, 20.4, 19.9. HRMS (ESI-TOF), m/z calcd. C29H30ClN6O10 [M + H]+ 657.1706; Found: 657.1728.
(2R,3S,4S,5R,6R)-2-(acetoxymethyl)-6-(4-(((5-(4-fluorophenyl)pyrazolo[1,5-a]pyrimidin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (10e): light yellow colored sticky solid; yield: 131.34 mg (96%), Rf = 0.30 (EtOAc); 1H NMR (500 MHz, CDCl3) δ 7.99 (s, 1H), 7.90 (t, J = 6.8 Hz, 2H), 7.87 (d, J = 4.0 Hz, 1H), 6.03 (br s, 2H), 5.74 (d, J = 9.0 Hz, 1H), 5.49 (dd, J = 9.0 Hz, 5.0 Hz, 1H), 5.39 (t, J = 10.0 Hz, 1H), 5.18 (dd, J = 10.1, 4.0 Hz, 1H), 4.19–4.10 (m, 2H), 4.07–4.03 (m, 1H), 2.17 (s, 3H), 1.92 (s, 6H), 1.62 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 170.2, 170.0, 169.7, 168.6, 165.0, 163.0, 161.0, 158.2, 153.8, 141.3, 140.0, 133.9, 129.2, 129.1, 123.1, 115.6, 115.4, 100.1, 98.5, 86.3, 74.1, 70.5, 67.8, 66.8, 61.1, 47.0, 20.6, 20.5, 20.4, 19.8. 13C–19F couplings in 13C NMR (125 MHz, DMSO-d6) δ 164.0 (d, JC-F = 250.74 Hz, C1), 129.1 (d, JC-F = 8.82 Hz, C3), 115.5 (d, JC-F = 21.42 Hz, C2). HRMS (ESI-TOF), m/z calcd. C29H30FN6O10 [M + H]+ 641.2002; Found: 641.2027.
(2R,3S,4S,5R,6R)-2-(acetoxymethyl)-6-(4-(((5-(4-(trifluoromethyl)phenyl)pyrazolo[1,5-a]pyrimidin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (10f): white colored sticky solid; yield: 115 mg (93%), Rf = 0.29 (EtOAc); 1H NMR (500 MHz, CDCl3) δ 8.06 (d, J = 8.0 Hz, 2H), 8.03 (s, 1H), 7.91 (d, J = 4.0 Hz, 1H), 6.08 (br s, 2H), 5.74 (d, J = 9.0 Hz, 1H), 5.51 (dd, J = 9.0 Hz, 4.0 Hz, 1H), 5.42 (t, J = 10.0 Hz, 1H), 5.19 (dd, J = 10.0, 5.5 Hz, 1H), 4.19–4.14 (m, 2H), 4.11–4.06 (m, 1H), 2.22 (s, 3H), 1.98 (s, 3H), 1.96 (s, 3H), 1.67 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 170.3, 170.1, 169.8, 168.7, 160.6, 158.3, 154.0, 141.4, 141.4, 140.1, 131.9, 131.6, 131.4, 127.6, 125.7, 125.2, 123.2, 100.3, 99.6, 86.5, 74.3, 70.6, 67.9, 66.8, 61.2, 47.2, 20.8, 20.6, 20.5, 20.0. 13C–19F couplings in 13C NMR (125 MHz, DMSO-d6) δ 131.53 (q, JC-F = 31.5 Hz, C2), 125.70 (d, JC-F = 3.78 Hz, C3), 124.13 (q, JC-F = 272.16 Hz, C1). HRMS (ESI-TOF), m/z calcd. C30H30F3N6O10 [M + H]+ 691.1970; Found: 691.1990.
(2R,3S,4S,5R,6R)-2-(acetoxymethyl)-6-(4-(((5-(4-isopropylphenyl)pyrazolo[1,5-a]pyrimidin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (10g): yellow colored sticky solid; yield: 122.53 mg (96%), Rf = 0.33 (EtOAc); 1H NMR (500 MHz, CDCl3) δ 8.01 (s, 1H), 7.86 (s, 1H), 7.85 (s, 2H), 7.27 (d, J = 8.0 Hz, 2H), 6.52 (s, 1H), 6.39 (d, J = 4.0 Hz, 1H), 6.04 (br s, 2H), 5.72 (d, J = 9.0 Hz, 1H), 5.49 (dd, J = 10.0 Hz, 4.0 Hz, 1H), 5.41 (t, J = 10.0 Hz, 1H), 5.18 (dd, J = 10.5, 5.5 Hz, 1H), 4.18–4.12 (m, 2H), 4.10–4.05 (m, 1H), 2.91 (hept, J = 6.7 Hz, 1H), 2.20 (s, 3H), 1.95 (s, 6H), 1.65 (s, 3H), 1.23 (d, J = 6.8 Hz, 6H). 13C NMR (125 MHz, CDCl3) δ 170.3, 170.1, 169.8, 168.6, 162.3, 158.4, 153.9, 151.1, 141.4, 139.9, 135.4, 127.2, 126.8, 123.2, 100.3, 98.7, 86.4, 74.2, 70.6, 67.8, 66.8, 61.2, 47.0, 34.0, 23.8, 20.7, 20.6, 20.5, 19.9. HRMS (ESI-TOF), m/z calcd. C32H37N6O10 [M + H]+ 665.2566; Found: 665.2656.
(2R,3S,4S,5R,6R)-2-(acetoxymethyl)-6-(4-(((5-(naphthalen-2-yl)pyrazolo[1,5-a]pyrimidin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (10h): yellow colored sticky solid; yield: 124.86 mg (97%), Rf = 0.32 (EtOAc); 1H NMR (500 MHz, CDCl3) δ 8.48 (s, 1H), 8.02 (d, J = 12.0 Hz, 2H), 7.89 (dt, J = 17.3, 6.7 Hz, 3H), 7.84–7.80 (m, 1H), 7.50–7.44 (m, 2H), 6.68 (s, 1H), 6.45 (d, J = 5.3 Hz, 1H), 6.06 (br s, 2H), 5.74 (d, J = 9.5 Hz, 1H), 5.49 (dd, J = 10.0 Hz, 4.0 Hz, 1H), 5.43 (t, J = 9.5 Hz, 1H), 5.19 (dd, J = 10.7, 5.5 Hz, 1H), 4.18–4.12 (m, 2H), 4.10–4.05 (m, 1H), 2.20 (s, 3H), 1.93 (s, 6H), 1.65 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 170.2, 170.0, 169.8, 168.6, 161.9, 158.3, 153.9, 141.4, 140.0, 135.0, 134.1, 133.2, 128.9, 128.3, 127.6, 127.2, 127.0, 126.4, 124.4, 123.2, 100.2, 99.3, 86.3, 74.1, 70.5, 67.8, 66.8, 61.2, 47.0, 20.7, 20.5, 20.4, 19.9. HRMS (ESI-TOF), m/z calcd. C33H33N6O10 [M + H]+ 673.2253; Found: 673.2346.
(2R,3S,4S,5R,6R)-2-(acetoxymethyl)-6-(4-(((5-(benzo[d][1,3]dioxol-5-yl)pyrazolo[1,5-a]pyrimidin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (10i): yellow colored sticky solid; yield: 127.97 mg (98%), Rf = 0.33 (EtOAc); 1H NMR (500 MHz, CDCl3) δ 8.00 (s, 1H), 7.84 (d, J = 3.1 Hz, 1H), 7.46 (d, J = 8.3 Hz, 1H), 7.42 (s, 1H), 6.82 (d, J = 8.0 Hz, 1H), 6.41 (s, 1H), 6.35 (d, J = 3.8 Hz, 1H), 6.02 (br s, 2H), 5.73 (d, J = 9.0 Hz, 1H), 5.48 (dd, J = 10.0 Hz, 3.5 Hz, 1H), 5.41 (t, J = 9.5 Hz, 1H), 5.17 (dd, J = 10.5, 3.0 Hz, 1H), 4.18–4.11 (m, 2H), 4.08–4.04 (m, 1H), 2.19 (s, 3H), 1.95 (s, 3H), 1.93 (s, 3H), 1.64 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 170.2, 170.0, 169.8, 168.6, 161.5, 158.3, 153.7, 149.3, 148.1, 141.4, 140.0, 132.0, 123.2, 121.7, 108.3, 107.5, 101.4, 100.1, 98.2, 86.3, 74.1, 70.6, 67.8, 66.8, 61.1, 47.0, 20.7, 20.5, 20.4, 19.9. HRMS (ESI-TOF), m/z calcd. C30H31N6O12 [M + H]+ 667.1994; Found: 667.2114.
Synthesis of triazole-linked mannohybrids of pyrazolo[1,5-a]pyrimidines 11a–11i
In a microwave vial, a mixture of 50 mg (0.189 mmol) of O-propargylated pyrazolo[1,5-a]pyrimidine 6a and 70.89 mg (0.189 mmol) of 1-azido mannoside 8c in 2 ml of solvent (1:1, v/v mixture of t-BuOH–H2O) was treated with CuSO4·5H2O (1.24 mg, 0.005 mmol) and sodium ascorbate (2.238 mg, 0.011 mmol), and then subjected to microwave heating for 20 min at 50 °C (100 W). The reaction was monitored by TLC to confirm completion, and upon completion, the reaction mixture was extracted with 5 ml of water and 3 ml of EtOAc. The organic layer was then dried over Na2SO4 and evaporated via a rotary evaporator to obtain the crude residue, which was purified by flash column chromatography to yield pure compound 11a in 98% isolated yield. Using similar methods, with 50 mg of propargylated reactants, compounds 11b–11i were synthesized in good to excellent yields utilizing 1-azido mannose tetraacetate.
(2R,3R,4S,5S,6S)-2-(acetoxymethyl)-6-(4-(((5-(p-tolyl)pyrazolo[1,5-a]pyrimidin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (11a): brown colored sticky solid; yield: 115.98 mg (96%), Rf = 0.31 (EtOAc); 1H NMR (500 MHz, CDCl3) δ 7.95 (s, 1H), 7.89 (s, 1H), 7.82 (d, J = 8.0 Hz, 2H), 7.21 (d, J = 8.0 Hz, 2H), 6.46 (s, 1H), 6.42 (s, 1H), 6.16 (d, J = 14.7 Hz, 1H), 5.91 (d, J = 2.5 Hz, 1H), 5.36 (t, J = 4.0 Hz, 1H), 5.76 (dd, J = 8.0 Hz, 4.0 Hz, 1H), 5.27 (t, J = 9.5 Hz, 1H), 4.30 (dd, J = 12.6, 6.0 Hz, 1H), 3.97 (dd, J = 12.0 Hz, 3.0 Hz, 1H), 3.77–3.74 (m, 1H), 2.35 (s, 3H), 2.07 (s, 3H), 2.01 (s, 3H), 2.00 (s, 3H), 1.99 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 170.4, 169.6, 169.5, 169.3, 162.3, 158.2, 153.8, 141.8, 140.3, 139.6, 134.9, 129.4, 127.1, 124.9, 100.0, 98.1, 83.6, 72.3, 68.6, 68.0, 66.0, 61.4, 47.0, 21.3, 20.6, 20.5. HRMS (ESI-TOF), m/z calcd. C30H33N6O10 [M + H]+ 637.2253; Found: 637.2230.
(2R,3R,4S,5S,6S)-2-(acetoxymethyl)-6-(4-(((5-(4-methoxyphenyl)pyrazolo[1,5-a]pyrimidin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (11b): yellow colored sticky solid; yield: 108.65 mg (93%) Rf = 0.30 (EtOAc); 1H NMR (500 MHz, CDCl3) δ 7.95 (s, 1H), 7.91 (d, J = 8.5 Hz, 2H), 7.88 (d, J = 3.9 Hz, 1H), 6.94 (d, J = 8.2 Hz, 2H), 6.44 (s, 1H), 6.43 (d, J = 2.9 Hz, 1H), 6.18 (d, J = 14.8 Hz, 1H), 5.99 (d, J = 15.3 Hz, 1H), 5.92 (d, J = 3.5 Hz, 1H), 5.87 (t, J = 4.5 Hz, 1H), 5.78 (dd, J = 8.8 Hz, 4.0 Hz, 1H), 5.29 (t, J = 9.5 Hz, 1H), 4.32 (dd, J = 12.2, 5.5 Hz, 1H), 4.00 (dd, J = 12.0 Hz, 2.5 Hz, 1H), 3.84 (s, 3H), 3.79–3.76 (m, 1H), 2.10 (s, 3H), 2.03 (s, 3H), 2.02 (s, 3H), 2.01 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 170.5, 169.6, 169.6, 169.4, 162.1, 161.4, 158.4, 153.8, 141.8, 139.7, 130.1, 128.8, 125.0, 114.1, 100.1, 97.5, 83.7, 72.5, 68.7, 68.1, 66.1, 61.5, 55.4, 47.1, 20.7, 20.6. HRMS (ESI-TOF), m/z calcd. C30H33N6O11[M + H]+ 653.2202; Found: 653.2157.
(2R,3R,4S,5S,6S)-2-(acetoxymethyl)-6-(4-(((5-(4-bromophenyl)pyrazolo[1,5-a]pyrimidin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (11c): yellow colored sticky solid; yield: 97.49 mg (94%), Rf = 0.32 (EtOAc); 1H NMR (500 MHz, CDCl3) δ 7.95 (s, 1H), 7.92 (d, J = 4.0 Hz, 1H), 7.80 (d, J = 8.0 Hz, 2H), 7.54 (d, J = 9.3 Hz, 2H), 6.43 (s, 2H), 6.20 (d, J = 15.7 Hz, 1H), 5.98 (d, J = 14.8 Hz, 1H), 5.91 (d, J = 2.5 Hz, 1H), 5.85 (t, J = 3.5 Hz, 1H), 5.77 (dd, J = 9.2 Hz, 4.0 Hz, 1H), 5.29 (t, J = 9.5 Hz, 1H), 4.30 (dd, J = 12.6, 5.5 Hz, 1H), 3.99 (dd, J = 12.5 Hz, 2.5 Hz, 1H), 3.79–3.76 (m, 1H), 2.09 (s, 3H), 2.02 (s, 3H), 2.01 (s, 3H), 2.00 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 170.5, 169.6, 169.3, 161.1, 158.2, 153.7, 141.8, 139.7, 136.6, 131.8, 128.8, 124.9, 124.6, 100.0, 98.3, 83.7, 72.4, 68.6, 68.1, 66.0, 61.4, 47.2, 20.6, 20.6. HRMS (ESI-TOF), m/z calcd. C29H30BrN6O10 [M + H]+ 701.1201; Found: 701.1158.
(2R,3R,4S,5S,6S)-2-(acetoxymethyl)-6-(4-(((5-(4-chlorophenyl)pyrazolo[1,5-a]pyrimidin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (11d): brown colored sticky solid; yield: 114.02 mg (95%), Rf = 0.31 (EtOAc); 1H NMR (500 MHz, CDCl3) δ 7.94 (s, 1H), 7.92 (d, J = 4.0 Hz, 1H), 7.87 (d, J = 9.3 Hz, 2H), 7.38 (d, J = 8.0 Hz, 2H), 6.43 (s, 2H), 6.19 (d, J = 14.7 Hz, 1H), 5.92 (d, J = 2.5 Hz, 1H), 5.85 (t, J = 4.0 Hz, 1H), 5.77 (dd, J = 9.0 Hz, 4.0 Hz, 1H), 5.29 (t, J = 9.0 Hz, 1H), 4.30 (dd, J = 12.6, 5.5 Hz, 1H), 3.99 (dd, J = 12.5 Hz, 2.5 Hz, 1H), 3.79–3.76 (m, 1H), 2.08 (s, 3H), 2.02 (s, 3H), 2.01 (s, 3H), 2.00 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 170.5, 169.6, 169.3, 161.1, 158.2, 153.7, 141.8, 139.7, 136.2, 136.2, 128.9, 128.6, 124.9, 100.0, 98.3, 83.7, 72.4, 68.6, 68.0, 66.0, 61.4, 47.1, 20.6, 20.6. HRMS (ESI-TOF), m/z calcd. C29H30ClN6O10 [M + H]+ 657.1706; Found: 657.1640.
(2R,3R,4S,5S,6S)-2-(acetoxymethyl)-6-(4-(((5-(4-fluorophenyl)pyrazolo[1,5-a]pyrimidin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (11e): off-white colored sticky solid; yield: 127.16 mg (91%), Rf = 0.31 (EtOAc); 1H NMR (500 MHz, CDCl3) δ 7.94 (s, 1H), 7.92 (m, 3H), 7.09 (d, J = 8.7 Hz, 2H), 6.42 (d, J = 2.7 Hz, 1H), 6.41 (s, 1H), 6.19 (d, J = 16.0 Hz, 1H), 5.98 (d, J = 14.7 Hz, 1H), 5.92 (d, J = 4.0 Hz, 1H), 5.85 (t, J = 4.0 Hz, 1H), 5.77 (dd, J = 9.0 Hz, 4.0 Hz, 1H), 5.29 (t, J = 9.0 Hz, 1H), 4.30 (dd, J = 12.0, 5.5 Hz, 1H), 3.98 (dd, J = 12.0 Hz, 2.5 Hz, 1H), 3.78–3.75 (m, 1H), 2.08 (s, 3H), 2.02 (s, 3H), 2.00 (s, 3H), 1.99 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 170.5, 169.6, 169.3, 165.1, 163.1, 161.3, 158.2, 153.7, 141.8, 139.7, 133.9, 129.3, 129.2, 124.9, 115.7, 115.5, 100.0, 98.1, 83.7, 72.4, 68.6, 68.0, 66.0, 61.4, 47.1, 20.6, 20.6. 13C–19F couplings in 13C NMR (125 MHz, DMSO-d6) δ 164.16 (d, JC-F = 249.48 Hz, C1), 129.49 (d, JC-F = 8.82 Hz, C3), 115.67 (d, JC-F = 21.42 Hz, C2). HRMS (ESI-TOF), m/z calcd. C29H30FN6O10 [M + H]+ 641.2002; Found: 641.1956.
(2R,3R,4S,5S,6S)-2-(acetoxymethyl)-6-(4-(((5-(4-(trifluoromethyl)phenyl)pyrazolo[1,5-a]pyrimidin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (11f): yellow colored sticky solid; 111.29 mg (90%), Rf = 0.30 (EtOAc); 1H NMR (500 MHz, CDCl3) δ 8.03 (s, 1H), 8.01 (s, 1H), 7.96 (d, J = 5.3 Hz, 2H), 7.65 (d, J = 8.0 Hz, 2H), 6.46 (s, 1H), 6.44 (d, J = 4.0 Hz, 1H), 6.20 (d, J = 16.0 Hz, 1H), 5.99 (d, J = 14.7 Hz, 1H), 5.92 (d, J = 4.0 Hz, 1H), 5.84 (t, J = 4.0 Hz, 1H), 5.76 (dd, J = 9.0 Hz, 4.0 Hz, 1H), 5.28 (t, J = 9.0 Hz, 1H), 4.29 (dd, J = 12.0, 5.5 Hz, 1H), 3.98 (dd, J = 12.0 Hz, 2.5 Hz, 1H), 3.78–3.76 (m, 1H), 2.07 (s, 3H), 2.01 (s, 3H), 1.99 (s, 3H), 1.98 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 170.4, 169.5, 169.3, 160.6, 158.1, 153.7, 141.7, 141.2, 139.8, 132.0, 131.8, 131.5, 131.3, 127.5, 127.3, 125.6, 125.1, 124.9, 122.9, 120.8, 100.0, 98.9, 83.6, 72.4, 68.6, 68.0, 65.9, 61.4, 47.1, 20.6, 20.5. 13C–19F couplings in 13C NMR (125 MHz, DMSO-d6) δ 131.69 (q, JC-F = 32.76 Hz, C2), 125.60 (d, JC-F = 3.78 Hz, C3), 124.05 (q, JC-F = 273.42 Hz, C1). HRMS (ESI-TOF), m/z calcd. C30H30F3N6O10 [M + H]+ 691.1970; Found: 691.1908.
(2R,3R,4S,5S,6S)-2-(acetoxymethyl)-6-(4-(((5-(4-isopropylphenyl)pyrazolo[1,5-a]pyrimidin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (11g): yellow colored sticky solid; yield: 120 mg (94%), Rf = 0.33 (EtOAc); 1H NMR (500 MHz, CDCl3) δ 7.93 (s, 1H), 7.88 (d, J = 4.0 Hz, 1H), 7.83 (d, J = 8.0 Hz, 2H), 7.25 (d, J = 8.0 Hz, 2H), 6.44 (s, 1H), 6.41 (d, J = 4.0 Hz, 1H), 6.14 (d, J = 16.0 Hz, 1H), 5.90 (d, J = 4.0 Hz, 1H), 5.84 (t, J = 4.0 Hz, 1H), 5.74 (dd, J = 9.0 Hz, 4.0 Hz, 1H), 5.26 (t, J = 9.0 Hz, 1H), 4.29 (dd, J = 12.5, 5.5 Hz, 1H), 3.96 (dd, J = 12.0 Hz, 3.0 Hz, 1H), 3.75–3.72 (m, 1H), 2.90 (hept, J = 6.7 Hz, 1H), 2.06 (s, 3H), 2.00 (s, 3H), 1.98 (s, 6H), 1.22 (d, J = 6.7 Hz, 6H). 13C NMR (125 MHz, CDCl3) δ 170.5, 169.6, 169.5, 169.3, 162.4, 158.3, 153.7, 151.2, 141.8, 139.6, 135.2, 127.3, 126.8, 124.9, 100.1, 98.1, 83.6, 72.4, 68.7, 68.0, 66.0, 61.4, 47.1, 34.0, 23.8, 20.6, 20.5. HRMS (ESI-TOF), m/z calcd. C32H37N6O10 [M + H]+ 665.2566; Found: 665.2513.
(2R,3R,4S,5S,6S)-2-(acetoxymethyl)-6-(4-(((5-(naphthalen-2-yl)pyrazolo[1,5-a]pyrimidin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (11h): yellow colored sticky solid; yield: 122.28 mg (95%), Rf = 0.32 (EtOAc); 1H NMR (500 MHz, CDCl3) δ 8.47 (s, 1H), 8.01 (d, J = 8.0 Hz, 1H), 7.98 (s, 1H), 7.94–7.90 (m, 2H), 7.88 (d, J = 8.0 Hz, 1H), 7.85–7.81 (m, 1H), 7.51–7.45 (m, 2H), 6.63 (s, 1H), 6.48 (d, J = 4.0 Hz, 1H), 6.20 (d, J = 14.7 Hz, 1H), 6.00 (d, J = 16.0 Hz, 1H), 5.93 (d, J = 3.0 Hz, 1H), 5.87 (t, J = 4.0 Hz, 1H), 5.78 (dd, J = 9.0 Hz, 4.0 Hz, 1H), 5.29 (t, J = 8.0 Hz, 1H), 4.31 (dd, J = 12.0, 5.0 Hz, 1H), 3.99 (dd, J = 13.0 Hz, 3.0 Hz, 1H), 3.79–3.76 (m, 1H), 2.08 (s, 3H), 2.02 (s, 3H), 2.00 (s, 3H), 1.99 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 170.5, 169.6, 169.5, 169.3, 162.2, 158.2, 153.8, 141.8, 139.7, 135.0, 134.2, 133.2, 129.0, 128.4, 127.7, 127.3, 127.0, 126.4, 124.9, 124.4, 100.1, 98.8, 83.6, 72.4, 68.6, 68.0, 66.0, 61.4, 47.1, 20.6, 20.5. HRMS (ESI-TOF), m/z calcd. C33H33N6O10 [M + H]+ 673.2253; Found: 673.2241.
(2R,3R,4S,5S,6S)-2-(acetoxymethyl)-6-(4-(((5-(benzo[d][1,3]dioxol-5-yl)pyrazolo[1,5-a]pyrimidin-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (11i): yellow colored sticky solid; yield: 125.36 mg (96%), Rf = 0.31 (EtOAc); 1H NMR (500 MHz, CDCl3) δ 7.94 (s, 1H), 7.89 (d, J = 4.0 Hz, 1H), 7.47 (d, J = 8.0 Hz, 1H), 7.43 (s, 1H), 6.83 (d, J = 8.0 Hz, 1H), 6.40 (d, J = 4.0 Hz, 1H), 6.37 (s, 1H), 6.17 (d, J = 16.0 Hz, 1H), 5.97 (br s, 3H), 5.92 (d, J = 3.0 Hz, 1H), 5.86 (t, J = 4.0 Hz, 1H), 5.77 (dd, J = 9.0 Hz, 4.0 Hz, 1H), 5.28 (t, J = 9.0 Hz, 1H), 4.30 (dd, J = 13.0, 5.5 Hz, 1H), 3.98 (dd, J = 12.0 Hz, 3.0 Hz, 1H), 3.78–3.75 (m, 1H), 2.08 (s, 3H), 2.02 (s, 3H), 2.01 (s, 3H), 2.00 (s, 3H). 13C NMR (125 MHz, CDCl3) δ 170.5, 169.6, 169.5, 169.3, 161.8, 158.2, 153.6, 149.4, 148.2, 141.8, 139.6, 132.0, 124.9, 121.7, 108.4, 107.6, 101.5, 100.0, 97.7, 83.6, 72.4, 68.7, 68.0, 66.0, 61.4, 47.1, 20.6, 20.6. HRMS (ESI-TOF), m/z calcd. C30H31N6O12 [M + H]+ 667.1994; Found: 667.1921.
Cell culture
MDA-MB-231, a human breast cancer cell line, was cultured under standard conditions. Specifically, they were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% heat-inactivated Fetal Bovine Serum (FBS) and 1% penicillin–streptomycin. The cells were maintained as a monolayer in a 100 mm culture plate and were used for experiments before reaching their 8th passage. Subculturing was performed every third-day using trypsin EDTA treatment. All incubation and maintenance procedures were carried out in a humidified CO2 incubator at 37 °C.
Cell viability assay
To evaluate cell viability, the MTT assay was conducted following standard procedures. After 72 h of cell incubation with or without each compound, cell viability was assessed using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), which is a colorimetric method for determining the number of viable cells in various assays including proliferation, cytotoxicity, or chemosensitivity. The MTT reagent was added to the cells after removal of the medium and incubated for 3 h at 37 °C in the CO2 incubator. The formazan product, which is soluble in the tissue culture medium, was dissolved in DMSO, and the absorbance of the formazan product was directly measured at 595 nm using a multimode plate reader without additional processing. The absorbance values are directly proportional to the number of viable cells in culture. The percentage of viable cells in each group was determined relative to the untreated control cells.
Docking analysis
The docking studies were carried out using various derived triazole-linked pyrazolo[1,5-a]pyrimidine-based glycohybrids with proposed binding pocket of X-ray crystallographic structure (Protein Data Bank ID: 3PP0, resolution: 2.4 Å). Docking was performed using Autodock Vina 4.0, and the interaction between the ligands and protein after docking was visualized and analyzed using PyMol software. The Biovia Discovery Studio Visualizer v20.1.0.19295 was used for 2D visualization and detailed ligand interaction visualization. The Schrödinger Maestro tool was utilized for QSAR and SAR studies.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Schenone, S., Radi, M., Musumeci, F., Brullo, C. & Botta, M. Biologically driven synthesis of pyrazolo[3,4-d]pyrimidines as protein kinase inhibitors: An old scaffold as a new tool for medicinal chemistry and chemical biology studies. Chem. Rev. 114, 7189–7238 (2014).
Khanna, A., Dubey, P. & Sagar, R. Exploiting microwave-assisted organic synthesis (MAOS) for accessing bioactive scaffolds. Curr. Org. Chem. 25, 2378–2456 (2021).
Chaurasiya, A. et al. Pathogen induced subversion of NAD+ metabolism mediating host cell death: A target for development of chemotherapeutics. Cell Death Discov. 7, 10 (2021).
Mishra, V. K., Tiwari, G., Khanna, A., Tyagi, R. & Sagar, R. Efficient synthesis of chirally enriched 1H-Imidazo[1,2-b]pyrazole- and 4H-Imidazo[1,2-b][1,2,4]triazole-based bioactive glycohybrids. Synthesis https://doi.org/10.1055/a-2157-9100 (2023).
Ahmed, O. M., Mohamed, M. A., Ahmed, R. R. & Ahmed, S. A. Synthesis and anti-tumor activities of some new pyridines and pyrazolo[1,5-a]pyrimidines. Eur. J. Med. Chem. 44, 3519–3523 (2009).
Hassan, A. S., Morsy, N. M., Awad, H. M. & Ragab, A. Synthesis, molecular docking, and in silico ADME prediction of some fused pyrazolo[1,5-a]pyrimidine and pyrazole derivatives as potential antimicrobial agents. J. Iran. Chem. Soc. 19, 521–545 (2022).
Pesci, E. et al. Novel hits in the correction of ΔF508-cystic fibrosis transmembrane conductance regulator (CFTR) protein: Synthesis, pharmacological, and ADME evaluation of tetrahydropyrido[4,3-d]pyrimidines for the potential treatment of cystic fibrosis. J. Med. Chem. 58, 9697–9711 (2015).
Al-Adiwish, W. M. et al. Synthesis, antibacterial activity and cytotoxicity of new fused pyrazolo[1,5-a]pyrimidine and pyrazolo[5,1-c][1,2,4]triazine derivatives from new 5-aminopyrazoles. Eur. J. Med. Chem. 64, 464–476 (2013).
Alsaedi, A. M., Farghaly, T. A. & Shaaban, M. R. Synthesis and antimicrobial evaluation of novel pyrazolopyrimidines incorporated with mono-and diphenylsulfonyl groups. Molecules 24, 4009 (2019).
Ballesteros-Casallas, A. et al. Synthesis of 2,7-diarylpyrazolo [1,5-a] pyrimidine derivatives with antitumor activity. Theoretical identification of targets. Eur. J. Med. Chem. Rep. 4, 100028 (2022).
Ismail, N. S. M., Ali, G. M. E., Ibrahim, D. A. & Elmetwali, A. M. Medicinal attributes of pyrazolo[1,5-a]pyrimidine based scaffold derivatives targeting kinases as anticancer agents. Future J. Pharm. Sci. 2, 60–70 (2016).
Ren, J., Ding, S., Li, X., Bi, R. & Zhao, Q. An approach for the synthesis of pyrazolo[1,5-a]pyrimidines via Cu(II)-catalyzed [3+3] annulation of saturated ketones with aminopyrazoles. J. Org. Chem. 86, 12762–12771 (2021).
Almansa, C. et al. Synthesis and SAR of a new series of COX-2-selective inhibitors: pyrazolo[1,5-a]pyrimidines. J. Med. Chem. 44, 350–361 (2001).
Dooley, M. & Plosker, G. L. Zaleplon. Drugs 60, 413–445 (2000).
Neubauer, D. N. Indiplon: The development of a new hypnotic. Exp. Opin. Investig. Drugs 14, 1269–1276 (2005).
Cherukupalli, S. et al. An insight on synthetic and medicinal aspects of pyrazolo[1,5-a]pyrimidine scaffold. Eur. J. Med. Chem. 126, 298–352 (2017).
Barbas, R., Llinas, A. & Prohens, R. The solid state landscape of the sildenafil drug. J. Pharm. Sci. 111, 1104–1109 (2022).
Varki, A. Biological roles of oligosaccharides: All of the theories are correct. Glycobiology 3, 97–130 (1993).
Lis, H. & Sharon, N. Lectins: Carbohydrate-specific proteins that mediate cellular recognition. Chem. Rev. 98, 637–674 (1998).
Bertozzi, C. R. & Kiessling, L. L. Chemical glycobiology. Science 291, 2357–2364 (2001).
Singh, K., Tyagi, R., Mishra, V. K., Tiwari, G. & Sagar, R. Recent advances in the synthesis of bioactive glycohybrids via click-chemistry. SynOpen 07, 322–352 (2023).
Narayana, C., Kumari, P. & Sagar, R. Regioselective synthesis of chirally enriched tetrahydrocarbazolones and tetrahydrocarbazoles. Org. Lett. 20, 4240–4244 (2018).
Sampathkumar, S.-G., Campbell, C. T., Weier, C. & Yarema, K. J. Short-chain fatty acid-hexosamine cancer prodrugs: The sugar matters. Drug Future 31, 1099–1116 (2006).
Pouillart, P., Cerutti, I., Ronco, G., Villa, P. & Chany, C. Butyric monosaccharide ester-induced cell differentiation and anti-tumor activity in mice. Importance of their prolonged biological effect for clinical applications in cancer therapy. Int. J. Cancer 49, 89–95 (1991).
Khanna, A., Tiwari, G., Mishra, V. K., Singh, K. & Sagar, R. An efficient synthesis of natural product-inspired naphthoquinone fused glycohybrids and their in-silico docking studies. Synthesis https://doi.org/10.1055/a-2181-9709 (2023).
Kumari, P., Narayana, C., Dubey, S., Gupta, A. & Sagar, R. Stereoselective synthesis of natural product inspired carbohydrate fused pyrano[3,2-c]quinolones as antiproliferative agents. Org. Biomol. Chem. 16, 2049–2059 (2018).
Kumari, P., Narayana, C., Tiwari, G. & Sagar, R. In Carbohydrates in Drug Discovery and Development 451–479 (Elsevier, 2020).
Cao, X. et al. Carbohydrate-based drugs launched during 2000–2021. Acta Pharm. Sin. B 12, 3783–3821 (2022).
Kumari, P. et al. Synthesis of new triazole linked carbohybrids with ROS-mediated toxicity in breast cancer. New J. Chem. 43, 18590–18600 (2019).
Kumari, P. et al. Stereoselective synthesis of carbohydrate fused pyrano[3,2-c]pyranones as anticancer agents. New J. Chem. 42, 13985 (2018).
Kumari, P. et al. Design and efficient synthesis of pyrazoline and isoxazole bridged indole C-glycoside hybrids as potential anticancer agents. Sci. Rep. 10, 6660 (2020).
Gantt, R. W., Peltier-Pain, P., Singh, S., Zhou, M. & Thorson, J. S. Broadening the scope of glycosyltransferase-catalyzed sugar nucleotide synthesis. Proc. Natl. Acad. Sci. 110, 7648–7653 (2013).
Lin, Y.-S. et al. Targeting the delivery of glycan-based paclitaxel prodrugs to cancer cells via glucose transporters. J. Med. Chem. 51, 7428–7441 (2008).
Liu, D.-Z., Sinchaikul, S., Reddy, P. V. G., Chang, M.-Y. & Chen, S.-T. Synthesis of 2′-paclitaxel methyl 2-glucopyranosyl succinate for specific targeted delivery to cancer cells. Bioorg. Med. Chem. Lett. 17, 617–620 (2007).
Hande, K. R. Etoposide: Four decades of development of a topoisomerase II inhibitor. Eur. J. Cancer 34, 1514–1521 (1998).
Stähelin, H. & Von Wartburg, A. From podophyllotoxin glucoside to etoposide. Prog. Drug Res. 33, 169–266 (1989).
Gui, M. et al. D11, a novel glycosylated diphyllin derivative, exhibits potent anticancer activity by targeting topoisomerase IIα. Investig. New Drugs 29, 800–810 (2011).
Kluza, J., Mazinghien, R., Irwin, H., Hartley, J. A. & Bailly, C. Relationships between DNA strand breakage and apoptotic progression upon treatment of HL-60 leukemia cells with tafluposide or etoposide. Anticancer Drugs 17, 155 (2006).
Calvaresi, E. C. & Hergenrother, P. J. Glucose conjugation for the specific targeting and treatment of cancer. Chem. Sci. 4, 2319–2333 (2013).
Shivappagowdar, A. et al. A small bioactive glycoside inhibits epsilon toxin and prevents cell death. Dis. Models Mech. 12, dmm040410 (2019).
Tiwari, G., Khanna, A., Mishra, V. K. & Sagar, R. Recent developments on microwave-assisted organic synthesis of nitrogen- and oxygen-containing preferred heterocyclic scaffolds. RSC Adv. 13, 32858–32892 (2023).
Shelton, J. et al. Metabolism, biochemical actions, and chemical synthesis of anticancer nucleosides, nucleotides, and base analogs. Chem. Rev. 116, 14379–14455 (2016).
Wu, P. et al. Efficiency and fidelity in a click-chemistry route to triazole dendrimers by the copper(I)-catalyzed ligation of azides and alkynes. Angew. Chem. 116, 4018–4022 (2004).
Agrahari, A. K. et al. Cu(I)-catalyzed click chemistry in glycoscience and their diverse applications. Chem. Rev. 121, 7638–7956 (2021).
Narayana, C., Kumari, P., Tiwari, G. & Sagar, R. Triazole linked N-acetylglucosamine based gelators for crude oil separation and dye removal. Langmuir 35, 16803–16812 (2019).
Gu, G. et al. Enantioselective and diastereoselective Ir-catalyzed hydrogenation of α-substituted β-ketoesters via dynamic kinetic resolution. Org. Lett. 20, 1888–1892 (2018).
Lansu, K. et al. In silico design of novel probes for the atypical opioid receptor MRGPRX2. Nat. Chem. Biol. 13, 529–536 (2017).
Acknowledgements
The authors are thankful to Banaras Hindu University (BHU) and Jawaharlal Nehru University (JNU) for providing research facilities to carry out this work. GT and AK are thankful to Banaras Hindu University (BHU) for the Fellowships. VKM and RT is thankful to UGC New Delhi for the Senior Research Fellowship.
Author information
Authors and Affiliations
Contributions
V.K.M., G.T., A.K., R.T., C.N. and R.S. envisioned and designed the molecules. V.K.M., G.T. and A.K. synthesized the molecules in supervision of R.S. V.K.M. and R.T. performed and analysed anticancer activity data. A.K. performed docking studies. R.S. conceived and planned the project and wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tiwari, G., Khanna, A., Tyagi, R. et al. Copper-catalyzed synthesis of pyrazolo[1,5-a]pyrimidine based triazole-linked glycohybrids: mechanistic insights and bio-applications. Sci Rep 14, 529 (2024). https://doi.org/10.1038/s41598-023-50202-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-50202-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.