Abstract
The distribution of tick-borne encephalitis virus (TBEV) is expanding to Western European countries, including the Netherlands, but the contribution of different rodent species to the transmission of TBEV is poorly understood. We investigated whether two species of wild rodents native to the Netherlands, the wood mouse Apodemus sylvaticus and the yellow-necked mouse Apodemus flavicollis, differ in their relative susceptibility to experimental infection with TBEV. Wild-caught individuals were inoculated subcutaneously with the classical European subtype of TBEV (Neudoerfl) or with TBEV-NL, a genetically divergent TBEV strain from the Netherlands. Mice were euthanised and necropsied between 3 and 21 days post-inoculation. None of the mice showed clinical signs or died during the experimental period. Nevertheless, TBEV RNA was detected up to 21 days in the blood of both mouse species and TBEV was also isolated from the brain of some mice. Moreover, no differences in infection rates between virus strains and mouse species were found in blood, spleen, or liver samples. Our results suggest that the wood mouse and the yellow-necked mouse may equally contribute to the transmission cycle of TBEV in the Netherlands. Future experimental infection studies that include feeding ticks will help elucidate the relative importance of viraemic transmission in the epidemiology of TBEV.
Similar content being viewed by others
Introduction
Tick-borne encephalitis virus (TBEV) is endemic in large parts of Europe and Asia, where it causes between 4000 and 9000 human cases of tick-borne encephalitis (TBE) each year1. In Europe, the virus is predominantly transmitted between Ixodes ricinus ticks and small mammals, particularly rodents. Ticks can acquire the virus while feeding on a viraemic host or via co-feeding (also called non-viraemic transmission) with an infected tick on a naïve or immune host2,3. The non-viraemic transmission route is considered the most important transmission pathway and principally occurs between infected nymphs and uninfected larvae that feed simultaneously on a rodent host3,4.
Rodents, such as the wood mouse Apodemus sylvaticus, the yellow-necked mouse Apodemus flavicollis and the bank vole Myodes glareolus, are the primary hosts of I. ricinus larvae and, to a lesser extent, nymphs. The relative contribution to the eco-epidemiology of TBEV likely differs between rodent species. For example, Apodemus mice have higher tick burdens5, higher tick-feeding success, and more efficiently support TBEV transmission via co-feeding compared to bank voles2. Whether Apodemus mice also develop higher or prolonged viraemia than bank voles remain to be determined. Likewise, it remains unclear if differences in infection rates and viraemia also exist among species within the genus Apodemus. These questions are important to address, because given that the community composition of rodents differs across the geographic distribution of TBEV6,7,8, any difference in reservoir competence among rodent species is likely to affect TBEV transmission dynamics.
In recent years, TBEV has been detected in new regions in Europe, including several Western-European countries such as the Netherlands, the United Kingdom and Belgium9,10,11. In the Netherlands, TBEV was first detected in ticks in 2015, followed by the first human cases in 201610,12. After that, multiple TBEV strains have been detected in the Netherlands13. Some of these strains are closely related to the classical European subtype that is found in central parts of the Netherlands whereas another strain (TBEV-NL) forms a subclade of the previously known Western-European strains and has only been detected in National Park Sallandse Heuvelrug, The Netherlands, and in the United Kingdom14.
Apodemus flavicollis is widespread in Central Europe and is considered one of the most important hosts for TBEV transmission2. In the Netherlands, however, this species is relatively rare and its distribution is confined to the eastern parts of the Netherlands, where TBEV was first detected15,16. In contrast, A. sylvaticus is widespread in the Netherlands15,17. The geographic overlap between A. flavicollis and the first cases of TBEV raised the question whether A. sylvaticus and A. flavicollis may differentially contribute to TBEV transmission. Moreover, we hypothesized that the restricted distribution of TBEV-NL compared to classical European strains such as TBEV-Neudoerfl may be the result of reduced susceptibility of wild rodents to TBEV-NL.
Compared to co-feeding transmission, direct transmission of TBEV from viraemic hosts to ticks was previously suggested to play only a minor role in the TBEV transmission cycle, as studies showed that viraemia in rodents only lasts between 2 and 4 days18,19. However, recent studies showed that TBEV RNA is present in the blood of experimentally infected bank voles (Myodes glareolus) and common voles (Microtus arvalis) for over 30 days and that infectious virus can be isolated from different organs after more than 90 days7,20. Furthermore, the virus has been isolated from leukocytes of bank voles 14 days after inoculation21. This suggests that viraemic transmission of TBEV by rodents may be more important than basic reproduction models currently estimate22. To what degree the viraemic period, and hence the potential contribution to TBEV transmission, differs between rodent species is also largely unknown.
Here, we tested the susceptibility of two rodent hosts, A. sylvaticus and A. flavicollis, for two different TBEV strains. Animals were live trapped and inoculated with TBEV-NL or TBEV-Neudoerfl after which both systemic and local infection was followed in time for up to three weeks.
Results
Clinical signs and weight loss in mice after TBEV inoculation
A total of 112 (51 male and 49 female) wild-caught A. flavicollis (n = 56) and A. sylvaticus (n = 56) mice were used for the TBEV infection experiments; 100 animals were subcutaneously inoculated with 105 TCID50 of either TBEV-Neudoerfl (n = 50) or TBEV-NL (n = 50) on day 0, while 12 animals were mock infected and served as controls. All mice tested seronegative for TBEV IgG at the start of the experiment. Mice were necropsied on 3, 5, 7, 14, or 21 days post inoculation (dpi, Fig. 1A). For each timepoint, five animals were necropsied per species and per virus strain. Three A. flavicollis and three A. sylvaticus mice were necropsied at 7 and 21 dpi as negative control animals.
(A) Schematic representation of the study design. Mice were inoculated with 105 TCID50 at day 0. Five animals per species (A. flavicollis and A. sylvaticus) per virus (TBEV-NL or TBEV-Neudoerfl) were necropsied per timepoint (3, 5, 7, 14-and 21 days post infection). Three negative control mice were necropsied at 7 and 21 dpi. (B) Mesocosm cage in which the animals were housed before transport to the BSL-3 facility. (C) An A. flavicollis mouse in a Makrolon IIL cage in the BSL-3 facility.
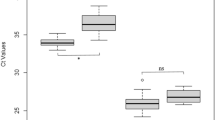
Mice were weighed prior to inoculation with TBEV. Median weight for A. flavicollis males and females was 27.2 g and 30.2 g, respectively. Median weight for A. sylvaticus males and females was 24.9 g and 22.3 g, respectively. Apodemus flavicollis males and females were significantly heavier than A. sylvaticus males and females, respectively (Fig. 2A, Supplementary Table S1). Furthermore, A. sylvaticus males were significantly heavier than female A. sylvaticus (Supplementary Table S1).
(A) Weight (g) of mice used for the study at the start of the infection experiment. ns not significant, **p < 0.01, ***p < 0.001. (B) Weight difference of animals relative to the start of the experiment per species from 3 to 21 days post inoculation (dpi). (C) Weight difference of A. flavicollis and A. sylvaticus relative to the start of the experiment of (mock) infected animals per treatment from 3 to 21 dpi.
Of importance, none of the A. sylvaticus and A. flavicollis mice showed signs of distress or clinical signs during the observation period. Furthermore, no apparent weight loss was observed in the majority of TBEV inoculated animals and only seven animals showed weight loss with a maximum loss of 10% during the experiment (Fig. 2B, C and Supplementary Table S2). In fact, most mice gained weight between 3 and 21 dpi, most likely due to ad libitum feeding and controlled climate conditions. The animal with the 10% weight loss was infected with TBEV-NL, and only had detectable TBEV RNA in the blood and not in the organs. None of the mock-infected control mice experienced weight loss, as determined by measuring body weights at either 7 or 21 dpi.
TBEV RNA detection in blood, spleen, liver and brain tissue
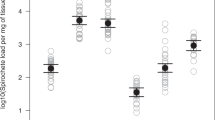
To assess TBEV infection in different mouse tissues following inoculation, we used an RT-PCR able to detect both TBEV strains in whole blood, spleen, liver and brain samples of the mice (Fig. 3A–D, Supplementary Table S3). TBEV RNA was detected in blood throughout the study period of 21 days and the presence or absence of TBEV RNA was not influenced by time after inoculation, TBEV strain, mouse species, the sex of the mice or the weight of the mice (GLMM, P > 0.05 for all variables, Fig. 3A, Supplementary Table S2). Nevertheless, we observed a decrease in TBEV RNA copy numbers from the start of inoculation until the end of the experiment (GLMM, P < 0.001).
TBEV RNA copy numbers in mouse (A) whole blood, (B) spleen, (C) liver, (D) and brain tissue at different timepoints (days) after inoculation. RNA copy numbers were expressed as the number of TBEV RNA copy numbers per microgram (µg) of total RNA. The dashed lines indicate the limit of detection. Samples below this line are negative for TBEV RNA.
TBEV RNA was detected in tissues throughout the study period, with viral RNA levels being lower in liver tissue compared to spleen tissue (Fig. 3B, C, Supplementary Table S2). Furthermore, the proportion of mice with detectable TBEV RNA in the liver and spleen tissue decreased over time (GLMM, P < 0.05, Supplementary Table S2). No effects of virus strain, mouse species or mouse weight on the likelihood of detecting TBEV RNA were found, however, TBEV RNA in the liver was more often found in females compared to males (GLMM, P < 0.05). In line with the blood samples, both the TBEV RNA copy numbers in liver and spleen samples decreased over time (GLMM, P < 0.05 and GLMM, P < 0.001, Fig. 3B,C and Supplementary Table S2). No differences in TBEV RNA copy numbers between the TBEV strains were observed in liver tissue (Supplementary Table S2). In contrast, in spleen tissue, viral RNA copy numbers for TBEV-Neudoerfl were significantly higher compared to TBEV-NL (GLMM, P < 0.001) and were negatively related to the weight of the mice (GLMM, P < 0.01). There was no effect of mouse species on the TBEV RNA copy numbers.
The likelihood of TBEV RNA detection in the brain did not depend on the time after inoculation, or the sex, weight, or species of the mice. In contrast, the TBEV strain affected the presence of detectable TBEV RNA in mice brains. TBEV-Neudoerfl was more often detected in the brain compared to TBEV-NL (GLM, P < 0.01). Furthermore, TBEV RNA copy numbers in the brain increased over time (GLM, P < 0.01) and higher copy numbers were observed in the brains of A. flavicollis compared to A. sylvaticus (GLMM, P < 0.05, Fig. 3D) and in male mice compared to female mice (GLMM, P < 0.01, Supplementary Fig. S2). Overall, TBEV RNA could be detected in all tissue samples from 3 up to 21 dpi. Blood and spleen samples were the most likely to contain TBEV RNA compared to liver and brain tissue.
Virus isolation from brain tissue and blood of mice
Virus isolation was attempted for 29 TBEV RNA-positive brain tissue samples. Interestingly, only male mice had high enough viral loads to successfully isolate the virus, roughly ≥ 5 × 103 TBEV RNA copies/µg RNA and virus isolation attempts below this RNA level were unsuccessful. TBEV was successfully isolated from 7 out of 29 brain samples of mice inoculated with TBEV between 3 and 21 dpi (Supplementary Table S4). The majority of successful virus isolations (5) were from A. flavicollis males inoculated with TBEV-Neudoerfl. The other two successful isolations were from a male A. flavicollis and a male A. sylvaticus, both inoculated with TBEV-NL (Supplementary Table S4). We attempted virus isolation from 20 TBEV RNA-positive blood samples of mice euthanised between 3 and 21 days post infection but we were unsuccessful, due to either issues in sensitivity of the assay or potential interference of neutralizing antibodies (see next section).
Antibody detection
We used virus neutralization tests (VNT) as well as an adapted commercial TBEV IgG ELISA to test for the presence of antibodies against TBEV. Neutralizing antibodies were detected as early as 3 dpi for both TBEV-NL and TBEV-Neudoerfl in both Apodemus species (Fig. 4A,B). The measured titers ranged from 20 to 640 ND50 between 3 and 21 dpi. All animals had neutralizing antibodies at 7 dpi except for one A. sylvaticus male infected with TBEV-Neudoerfl that was necropsied at 21 dpi. We were unable to collect serum from two A. flavicollis mice necropsied at 21 dpi because of insufficient blood draw. IgG ELISA suggested that all animals except one individual (male A. sylvaticus infected with TBEV-NL and necropsied at 21 dpi) had seroconverted after 14 dpi (Fig. 4C, D and Supplementary Table S4). The mock-inoculated mice were not positive for TBEV IgG nor did they have neutralizing antibodies against TBEV (Fig 4A-D, Supplementary Table S4).
Neutralizing antibodies and TBEV IgG detection in TBEV infected Apodemus mice over time (days). A virus neutralization test was used to determine the neutralizing antibodies against TBEV in A. flavicollis (A) or A. sylvaticus (B). ND50: 50% neutralizing dose. The dashed line indicates the lower limit of detection of the neutralization assay at 20 ND50. IgG antibody levels against TBEV were determined using a TBEV IgG ELISA in (C) A. flavicollis or (D) A. sylvaticus. (E,F) The TBEV IgG ELISA result of mice before TBEV inoculation. An optical density (OD) of the mean plus three times the standard deviation of the negative controls was used as cut-off. The cut-off value is indicated by the shaded area. Dots represent individual mice for mock (grey), TBEV-Neudoerfl (blue) or TBEV-NL (red) inoculations for all panels.
Histopathology, immunohistochemistry and in situ hybridization of brain samples
We conducted histopathology, immunohistochemistry (IHC) and in situ hybridization (ISH) on selected mice with high levels of TBEV RNA copy numbers in the brain. All brain tissue of TBEV infected mice showed signs of a (meningo)-encephalitis, mononuclear perivascular cuffing and occasionally glial nodules (Fig. 5B–E). No viral proteins could be detected with IHC, but ISH showed localized granules of TBEV RNA in four out of 11 tested mice, sometimes coinciding with microglial nodules (Fig. 5F). However, in the four ISH positive mouse brains, the localized TBEV granules were only sporadically observed indicating low systemic viral replication in the brain. The mock infected mice did not show any histopathological alterations in the brain (Fig. 5A).
Histopathology of the brain of TBEV infected Apodemus mice. (A) Normal aspect of the meninges in a control mouse (arrow). (B) Meningitis in a TBEV-infected mouse showing increased number of mononuclear cells in the meninges (arrows). (C) Perivascular cuffing by mononuclear cells in the brain of TBEV-infected mouse (arrows). (D,E) Microglial nodule in TBEV-infected brain shown with hematoxylin–eosin (HE) staining (D, arrows) and anti-iba1 staining for microglia (E, arrows). (F)In situ hybridization showing the presence of TBEV RNA in TBEV infected mouse brain (arrows). Bar = 50 μm (A,B) or 20 μm (C–F).
Discussion
Rodents are important hosts in the transmission of TBEV, however, the relative contribution of different rodent species in the circulation and spread of different TBEV strains is not well understood. Here, we studied the infection dynamics of two TBEV strains in two wild mouse species, A. sylvaticus and A. flavicollis. The two species of mice were clinically unaffected after inoculation with either TBEV-Neudoerfl or TBEV-NL. Nevertheless, viral RNA was detected in whole blood and brain samples over a period of 21 days, showing the persistence of viral RNA in both mouse species.
TBEV RNA was detected throughout the study period of 21 days in all types of samples, i.e. blood, spleen, liver and brain tissue. Interestingly, an apparent peak in viraemia was not observed, in contrast to previous studies on A.sylvaticus, A. flavicollis and M. glareolus19,23. Most mice had TBEV neutralizing antibodies after 7 dpi and IgG antibodies against TBEV around 14 dpi, though some mice already presented with TBEV neutralizing antibodies from 3 dpi onwards. This early response is in line with a previous report in which A. sylvaticus had neutralizing antibodies against TBEV after 3 dpi19, but we cannot exclude these animals were primed in the field, despite that we did not detect IgG antibodies prior to TBEV inoculation. Previous work showed that TBEV RNA could be detected up to 28 days in whole blood samples of the bank vole Myodes glareolus21 and up to 120 days post TBEV inoculation in the northern red-backed vole M. rutilus and the striped wood mouse A. agrarius24. Furthermore, virus was successfully isolated from whole blood of M. rutilus after 60 dpi and from leukocytes of M. glareolus at 14 dpi21,24, both in the presence of neutralizing antibodies.
The presence of TBEV RNA in blood, liver and spleen tissue did not differ between mouse species and TBEV strains, however, differences in neuro-invasion were recorded. TBEV-Neudoerfl showed more efficient infection of the brain compared to TBEV-NL. Both viruses were detected for up to 21 days in the brain of inoculated mice. This is in contrast with a recent TBEV infection study with Myodes glareolus in which TBEV-Neudoerfl was not detected in the brain of voles after more than 7 days post inoculation, while other TBEV strains were detected in the brain of voles up to 28 days post inoculation using a similar inoculation dose and route21,25. Differences in the reservoir competence of hosts for different viral lineages have previously been shown for Powassan virus (POWV)26, a virus closely related to TBEV, thereby influencing the eco-epidemiology of the viral lineages.
Besides differences in the susceptibility of the host species, differences in neuroinvasive properties between TBEV-NL and TBEV-Neudoerfl could also be related to genetic variance of the virus strains, in particular in the 3’ untranslated regions (UTRs). The 3’UTR of flaviviruses, including TBEV, is known to influence virulence and neuroinvasion27,28. TBEV-NL has a truncated 3’UTR compared to TBEV-Neudoerfl29, which has a poly-A domain in the 3’UTR30. Insertion of poly-A in the 3’UTR of TBEV is known to increase virulence in mice31, possibly by increased evasion of viral sensing and type I interferon response32.
In addition to host and virus strain-specific effects, we also identified a sex-specific difference in susceptibility to infection. Male mice had significantly higher TBEV RNA loads in the brain compared to female mice. Furthermore, virus isolation from brain tissue was only successful for male mice. While we did not measure any immune parameters, the higher TBEV RNA loads in the males could reflect a difference in immune response to virus infection of male compared to female mice. Indeed, female mice often mount stronger immune responses and have a reduced susceptibility to virus infections compared to male mice33,34. Interestingly, male rodents may contribute more to the transmission of tick-borne pathogens than female rodents35, as a result of their higher activity and large home range36. As a consequence, male mice have higher tick burdens than females37. Furthermore, high testosterone levels are associated with decreased resistance to ticks38 and increased parasite loads in rodents39, thereby even further enhancing the contribution of male rodents to tick-borne pathogen transmission.
As both Apodemus species had similar TBEV infection rates in the blood for the two TBEV strains, this may indicate that A. sylvaticus and A. flavicollis are equally important for the transmission of TBEV. Nevertheless, host specific factors such as host activity and resistance to ticks could also influence their relative contribution to TBEV transmission since they influence the capacity of ticks to feed successfully on their host3. For example, yellow-necked mice have a higher tick burden compared to wood mice40, most likely the result of the higher body mass of the yellow-necked mice as observed in the current and other studies40,41. The higher tick burden of the yellow-necked mice could consequently result in a higher contribution to the transmission of TBEV either via viremic or non-viremic transmission.
The current work is a starting point to improve our understanding of the relative importance of viraemic versus non-viraemic transmission of TBEV from wild mice to ticks. In contrast to viraemic transmission, non-viraemic transmission is a recognized phenomenon experimentally tested in voles and mice2. However, the exact role of viraemic transmission of TBEV in natural hosts has gained little attention. The detection of persistent virus infections in leukocytes and the liver shows long-term presence of TBEV in experimentally infected rodents7,21. Furthermore, we detected viral RNA up to three weeks after infection in Apodemus mice. These studies may hint to the long-term presence of infectious TBEV in rodents. Nonetheless, the detected RNA levels in this study were low and may not reflect actual viraemia in the rodents as we were unsuccessful in isolating virus from a subset of TBEV RNA positive whole blood samples of mice. However, the level of viraemia needed for mouse-to-tick transmission is unknown and could even be below the detection limit of our assay. Therefore, transmission studies with ticks are needed to understand whether infectious virus still circulates in these rodents either as viraemia or cell-associated virus in leukocytes, and to test whether feeding ticks can become infected even after 2–4 dpi, as currently assumed19,42,43. During tick feeding, TBEV is recruited to the feeding site via migratory cells such as leukocytes44. This can result in the infection of ticks in the absence of detectable viremia in the blood, indicating that viremia is not a necessary condition for virus transmission44. Besides host-to-tick transmission, the absence of a natural tick-to-host transmission route could have influenced the infection success and virulence in the rodents. First, the inoculated dose of TBEV in the current study may not reflect the amount of virus particles transmitted by ticks. Second, the absence of tick saliva at the inoculation site may have altered the host response to virus infection as tick saliva promotes infection success and virulence in the vertebrate host44,45,46.
Laboratory mice are frequently used as model species in TBEV transmission experiments47,48,49, however, the biology of tick-borne viruses in their natural hosts is different compared to laboratory mice50. Laboratory mice show weight loss and hind limb paralysis after TBEV infection with often a lethal outcome51,52. In contrast, wild rodents do not show clear clinical signs, although sub-clinical encephalitis is observed20,21. In addition, we and others show meningoencephalitis in infected animals using immunohistochemical analyses20,21, as a result of TBEV infection in the brains. The differences in clinical responses between laboratory and wild mice are most likely caused by the activated state of the cellular immune system of wild rodents53. These differences observed between laboratory and wild mice underline the importance of using natural host species in viraemic and non-viraemic TBEV transmission studies.
Conclusion
Apodemus sylavticus and A. flavicollis mice inoculated with either TBEV-NL or TBEV-Neudoerfl were TBEV RNA positive throughout the study period of 21 days, after which infectious virus could still be isolated from the brain. The results presented in the current study demonstrate that A. flavicollis and A. sylvaticus have a similar infection pattern to TBEV in the different tissues studied and that the presence of TBEV RNA in the blood after 21 days may indicate a longer viraemic period than previously shown. Future studies are however needed to determine the role of the induced neutralizing antibody response in the transmission potential of the virus and should assess if the low levels of TBEV RNA after 21 days reflects active viraemia. Finally, host-tick transmission experiments should be welcomed to assess the relative importance of viraemic and non-viraemic transmission in the eco-epidemiology of TBEV.
Materials and methods
Virus and cells
Human lung epithelial A549 cells were used for the preparation of virus stocks and end-point dilution assays (EPDA) and cultured in HEPES-buffered Dulbecco’s modified Eagle medium (DMEM; Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco) and 1% penicillin/streptomycin (Gibco) at 37 °C with 5% CO2. A549 cells used for virus isolation from brain samples (see below) were cultured as described but with the addition of Fungizone (2.5 μg/ml of amphotericin B and 2.1 μg/ml of sodium deoxycholate; Gibco) and Gentamycin (50 μg/ml; Gibco), subsequently named DMEM+. The absence of Mycoplasma infection in the A549 cell culture was verified using the MycoStrip™ detection kit (InvivoGen).
A P2 stock of TBEV-NL (GenBank accession no. ON502378.1) and a P4 stock of TBEV-Neudoerfl (GenBank accession no. U27495.1), kindly provided by the RIVM (Dutch National Health Institute), were used for subcutaneous inoculation of A. sylvaticus and A. flavicollis. Virus stocks were generated by infecting A549 cells at a multiplicity of infection of 0.01. Stocks were titrated on A549 cells in triplicate using end-point dilution assays according to Fros et al.54.
Animal trapping and housing
Mice were trapped using Heslinga live traps (Heslinga, Groningen, the Netherlands). Apodemus sylvaticus were trapped in a forest plot near Wageningen and in two forest plots in the Twente region (Wageningen: 51°59′52.0″ N 5°42′43.4″ E, Twente region 52°27′04.2″ N 6°50′43.3″ E and 52°26′34.4″ N 6°52′57.6″ E), whereas A. flavicollis were trapped in the two forest plots in the Twente region. Traps were pre-baited for three consecutive nights with carrots, mealworms, grains and hay. Traps were armed after sunset and emptied 8–10 h later. Apodemus species were identified to species level based on the hind foot length together with the presence or absence of a yellow band on the neck of the animal. The hind foot length of A. flavicollis is generally larger than 22 mm whereas the hind foot length of A. sylvaticus ranges from 17-21 mm (41 and Supplementary Fig. S1).
After transport from the field to the facilities at Wageningen University, animals were individually housed in large mesocosms before inoculation with TBEV. Mesocosms (70 × 90 × 40 cm, glass fiber) consisted of a layer (~ 5 to 10 cm) of sand and wood chips to mimic a natural situation together with hay and wooden branches (Fig. 1B). The mesocosms were closed with a 0.5 × 0.5 cm metal gauze lid and placed in a large farm shed exposed to the natural day-night light cycle between June and September. Mice were collected up to 1.5 months before the start of the experiment and housed for a maximum of four months in these mesocosms before transport to a BSL3 facility. They were individually transferred from the mesocosms into Makrolon type IIL cages three weeks before transport to the BSL3 facility. To rule out pre-exposure to TBEV, the mice were tested for TBEV IgG before inoculation as described below.
TBEV infection in Apodemus species
Mice were individually housed in Makrolon type IIL cages in a BSL3 laboratory at 21 °C and ~ 50% RH. Animals were anaesthetised by placing a cage in a large induction box gassed with Sevoflurane (AbbVie, the Netherlands). Mice were weighed and subcutaneously inoculated with 105 TCID50 in 100 µL of either TBEV-NL, TBEV-Neudoerfl or DMEM (mock inoculation).
Infected mice were monitored for up to 21 days, and necropsied at 3, 5, 7, 14 or 21 dpi. A total of 112 animals were used for the experiment; five mice per virus strain (TBEV-NL or TBEV-Neudoerfl) and per mouse species (A. sylvaticus or A. flavicollis) were necropsied at each of the five time points. Furthermore, three control animals per mouse species were necropsied at 7 and 21 dpi. Both male and female animals were used in the experiment and the fraction of males and females was similar for each virus strain or mouse species within one time point. This was either two males and three females or vice versa (Supplementary Table S4).
For blood and tissue sample collection at necropsy, animals were anaesthetised using Sevoflurane inhalation and ketamine/xylazine injected intra-peritoneally followed by exsanguination. Whole blood and serum were collected using MiniCollect EDTA tubes and MiniCollect serum tubes (Greiner, Austria), respectively. Tissue samples were collected from the animals and divided in two pieces (spleen and brain) or three pieces (liver) and frozen at −80 °C or fixed in 10% neutral buffered formalin for at least 48 h.
Ethics statement
Animal experiments were conducted in accordance with European regulations (EU directive 2010/63/EU) and the Dutch Law on Animal Experiments (Wet op de dierproeven, ID number BWBR0003081). The animal experiments were approved by the Dutch Central Authority for Scientific Procedures on Animals (Permit Number: AVD1040020209209). All procedures were approved by the Animal Ethics Committees of Wageningen Research. The study was conducted in compliance with the ARRIVE guidelines for the reporting of animal studies55.
RNA isolation
Total RNA from whole blood samples was isolated using the Mag-Bind Blood RNA kit (Omega Bio-tek) according to the manufacturer’s instructions: 200 µl whole blood was used per sample. Total RNA from mouse brain, spleen and liver samples was extracted using Trizol reagent (Invitrogen) according to the manufacturer’s instructions. Samples were first homogenized in DMEM+ with a Bullet Blender Storm (Next Advance) using 0.9–1.6 mm stainless steel beads at speed 10 for 2 min. For brain and liver samples, 500 µl of DMEM+ was added. For the spleen samples, 300 µl of DMEM+ was added. Samples were spun down for 1 min after homogenization and 100 µl homogenate was added to 1 ml Trizol. Total RNA yield was measured using a DS-11 spectrophotometer (DeNovix).
RT-PCR
TBEV RNA was quantified using a real-time RT-PCR as described previously by Schwaiger and Cassinotti56. The reaction was performed in a CFX96 Real-Time PCR machine (Bio-Rad) with a 20 μl reaction system using the TaqMan RNA-to-CT 1 step Kit (Applied Biosystems).
An RNA standard to quantify TBEV RNA copy number was made based on a 83 bp amplicon of the 3’UTR of TBEV-Neudoerfl. cDNA was used as template to generate a specific PCR product using T7 primers (Supplementary Table S5) and the PCR product was verified by Sanger sequencing. The target sequence was transcribed in vitro using T7 polymerase with the T7 MEGAscript kit (Ambion) according to the manufacturer’s instruction. In vitro RNA was cleaned using conventional phenol/chloroform/isoamyl alcohol RNA extraction. RNA was quantified using a DS-11 spectrophotometer (DeNovix) in order to prepare a 10-time dilution series.
Virus neutralization tests and TBEV IgG ELISA
A virus neutralization test (VNT) was performed on all serum samples using TBEV-Neudoerfl. Briefly, two-fold serial dilutions of sera (50 µl) were mixed with a fixed amount of virus (~ 100 TCID50 in 50 µl) in 96-well plates. After 2 h of incubation, 50,000 A549 cells (in 50 µl) were added to each well and plates were incubated for 5 days at 37 °C and 5% CO2, and scored based on the presence of cytopathic effect (CPE). Neutralizing titres were determined using the Spearman–Kärber method57,58. Serum samples were considered positive at ND50 ≥ 1/20.
Sera from mice before inoculation as well as from TBEV-infected mice were incubated at 56 °C for 30 min in a heating block prior to serological analysis. EIA TBE Virus IgG (Testline, Brno, Czech Republic) was used according to the manufacturer’s instruction with the following modifications: mouse sera were diluted 1:50, and goat anti-mouse IgG antibody HRP conjugate (1:10,000) was used (Sigma-Aldrich, 2584AP181P). As a positive control, a serum found positive before was used. The cut-off value was calculated by adding three standard deviations (SD) to the mean optical density (OD) of the negative sera59.
Virus isolation from brain and blood samples
Virus isolation was attempted from brain tissue using A549 cells. Briefly, A549 cells with a confluency of 70–80% were incubated with 100 µl brain homogenate or 50 µl of whole blood for 1.5 h. Cells were washed once with 1× PBS and checked for CPE after 4 days. When no CPE was visible, the cell supernatant was passaged for a maximum of three times.
Histopathology and immunohistochemistry
Formalin-fixed tissues were routinely processed into paraffin blocks, cut into 4 µm sections, placed on positively charged glass slides (SuperfrostPlus®, Thermo Scientific) and dried for at least 48 h at 37 °C. After deparaffinization and rehydration in graded alcohols, sections were stained with hematoxylin–eosin (HE) or immunostained. For TBEV immunostaining, endogenous peroxidase was blocked for 30 min in methanol/H2O2, followed by antigen retrieval with 0.1% trypsin in Tris-buffered saline for 30 min at 37 °C. The monoclonal antibody 12292 (Native Antigen Company, Oxford, UK) directed against the NS1 protein of TBEV was used as the primary antibody at a dilution of 1:100 and incubated for 1 h. HRP conjugated anti-mouse IgG polymer (Invitrogen, Carlsbad, USA) was used as secondary antibody and incubated for 30 min followed by incubation with DAB + substrate (Agilent, Santa Clara, USA) for 5 min.
For immunostaining of microglia, sections were pre-treated by autoclaving at 121 °C in citrate buffer of pH 6 (Antigen unmasking solution, Vector Laboratories, Peterborough, UK)) for 5 min. The primary antibody used was the rabbit polyclonal anti-IBA-1 (Fujifilm Cellular Dynamics, Madison, WI, USA) diluted 1:500 and incubated for 1 h. Alkaline phosphatase conjugated anti-rabbit IgG polymer (Vector laboratories) was used as secondary antibody and incubated for 30 min followed by incubation with ImmPACT VectorRed substrate (Vector Laboratories) for 20 min at 37 °C. Sections were briefly counterstained with hematoxylin, dehydrated, and mounted permanently.
In situ hybridization
For detection of TBEV RNA by RNAscope® (Bio-Techne Ltd. Abingdon, UK) sections were deparaffinized in xylene and rehydrated in graded alcohols. After pre-treatment by boiling in RNAscope® target retrieval reagent for 15 min followed by 30 min incubation with RNAscope® Protease plus at 40 °C, sections were hybridized with the RNAscope® probe pair V-TBEV-NS3. The probes were further hybridized to a cascade of signal amplification molecules according to the manufacturer’s instructions and signal was developed using the Fast Red substrate. Sections were briefly counterstained with hematoxylin, dried and mounted permanently.
Statistical analyses
A linear model (LM) with a log-link function was used to test the associations of sex and mouse species with the weight of the mouse, including an interaction term between mouse and sex. A generalized linear model (GLM) with a log-link function was used to test for an effect of sex and mouse species on hindfoot length of the mouse. GLMs with a binomial distribution and logit-link function were used to evaluate the effects of virus strain, mouse species, time after infection, mouse sex and mouse weight on the presence/absence of TBEV RNA. GLMs with a negative binomial distribution and log-link function were used to test the effect of virus strain, mouse species, time after infection, mouse sex and mouse weight on TBEV RNA copy numbers. Model diagnostics were performed using the DHARMa package60. (G)LMs were constructed using the glmmTMB package61. Estimated marginal mean infection rates and TBEV RNA copy numbers were calculated using the emmeans package62. Pairwise contrasts of significant effects were performed with a Tukey HSD. All statistical analyses were carried out with the statistical software package R version 4.2.0 using Rstudio63.
Data availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
Erber, W., Schmitt, H.-J. & Janković, T. V. TBE-Epidemiology by Country—An Overview. (Global Health Press Singapore, 2020).
Labuda, M. et al. Tick-borne encephalitis virus transmission between ticks cofeeding on specific immune natural rodent hosts. Virology 235, 138–143 (1997).
Labuda, M. et al. Non-viraemic transmission of tick-borne encephalitis virus: A mechanism for arbovirus survival in nature. Experientia 49, 802–805 (1993).
Labuda, M., Jones, L. D., Williams, T., Danielova, V. & Nuttall, P. A. Efficient transmission of tick-borne encephalitis-virus between cofeeding ticks. J. Med. Entomol. 30, 295–299 (1993).
Kiffner, C., Vor, T., Hagedorn, P., Niedrig, M. & Rühe, F. Factors affecting patterns of tick parasitism on forest rodents in tick-borne encephalitis risk areas, Germany. Parasitol. Res. 108, 323–335 (2011).
Rosà, R. et al. Changes in host densities and co-feeding pattern efficiently predict tick-borne encephalitis hazard in an endemic focus in northern Italy. Int. J. Parasitol. 49, 779–787 (2019).
Achazi, K. et al. Rodents as sentinels for the prevalence of tick-borne encephalitis virus. Vector-Borne Zoonotic Dis. 11, 641–647 (2011).
Gassner, F. et al. Rodent species as natural reservoirs of Borrelia burgdorferi sensu lato in different habitats of Ixodes ricinus in the Netherlands. Ticks Tick-Borne Dis. 4, 452–458 (2013).
Holding, M., Dowall, S. & Hewson, R. Detection of tick-borne encephalitis virus in the UK. Lancet 395, 411 (2020).
Jahfari, S. et al. Tick-borne encephalitis virus in ticks and roe deer, the Netherlands. Emerg. Infect. Dis. 23, 1028–1030 (2017).
Stoefs, A. et al. Autochthonous cases of tick-borne encephalitis, Belgium, 2020. Emerg. Infect. Dis. 27, 217–2182 (2021).
de Graaf, J. A. et al. First human case of tick-borne encephalitis virus infection acquired in the Netherlands, July 2016. Euro Surveill. 21, 4–6 (2016).
Esser, H. J. et al. Continued circulation of tick-borne encephalitis virus variants and detection of novel transmission foci, the Netherlands. Emerg. Infect. Dis. 28, 2416–2424 (2022).
Holding, M. et al. Tick-borne encephalitis virus, United Kingdom. Emerg. Infect. Dis. 26, 90–96 (2020).
Michaux, J. R., Libois, R. & Filippucci, M. G. So close and so different: Comparative phylogeography of two small mammal species, the yellow-necked fieldmouse (Apodemus flavicollis) and the woodmouse (Apodemus sylvaticus) in the Western Palearctic region. Heredity 94, 52–63 (2005).
Zoogdiervereniging. Grote bosmuis—Apodemus flavicollis. Verspreidingsatlas. https://www.verspreidingsatlas.nl/8496029 (2022).
Zoogdiervereniging. Bosmuis—Apodemus sylvaticus. Verspreidingsatlas. https://www.verspreidingsatlas.nl/8496030 (2022).
Chunikhin, S. P. & Kurenkov, V. B. Viraemia in Clethrionomys glareolus, a new ecological marker of tick-borne encephalitis virus. Acta Virol. 23, 257–260 (1979).
Kopecký, J., Tomková, E. & Vlcek, M. Immune response of the long-tailed field mouse (Apodemus sylvaticus) to tick-borne encephalitis virus infection. Folia Parasitol. 38, 275–282 (1991).
Tonteri, E. et al. The three subtypes of tick-borne encephalitis virus induce encephalitis in a natural host, the bank vole (Myodes glareolus). PLoS ONE 8, 15–19 (2013).
Michelitsch, A. et al. Long-term presence of tick-borne encephalitis virus in experimentally infected bank voles (Myodes glareolus). Ticks Tick-Borne Dis. 12, 101693 (2021).
Hartemink, N. A., Randolph, S. E., Davis, S. A. & Heesterbeek, J. A. P. The basic reproduction number for complex disease systems: Defining R0 for tick-borne infections. Am. Nat. 171, 743–754 (2008).
Heigl, Z. & von Zeipel, G. Experimental infection with tick-borne encephalitis virus in Clethrionomys glareolus, Apodemus flavicollis, Apodemus sylvaticus and Mus musculus. 1. Virological studies. Acta Pathol. Microbiol. Scand. 66, 489–509 (1966).
Morozova, O. V., Panov, V. V. & Bakhvalova, V. N. Innate and adaptive immunity in wild rodents spontaneously and experimentally infected with the tick-borne encephalitis virus. Infect. Genet. Evolut. 80, 104187 (2020).
Michelitsch, A. et al. In vivo characterization of tick-borne encephalitis virus in bank voles (Myodes glareolus). Viruses 11, 1–17 (2019).
Nemeth, N. M., Root, J. J., Hartwig, A. E., Bowen, R. A. & Bosco-Lauth, A. M. Powassan virus experimental infections in three wild mammal species. Am. J. Trop. Med. Hyg. 104, 1048–1054 (2021).
Sakai, M. et al. Variable region of the 3’ UTR is a critical virulence factor in the Far-Eastern subtype of tick-borne encephalitis virus in a mouse model. J. Gen. Virol. 95, 823–835 (2014).
Muto, M. et al. Identification and analysis of host proteins that interact with the 3′-untranslated region of tick-borne encephalitis virus genomic RNA. Virus Res. 249, 52–56 (2018).
Kutschera, L. S. & Wolfinger, M. T. Evolutionary traits of tick-borne encephalitis virus: Pervasive non-coding RNA structure conservation and molecular epidemiology. Virus Evolut. 8, 1–11 (2022).
Ternovoi, V. A. et al. Variability in the 3′ untranslated regions of the genomes of the different tick-borne encephalitis virus subtypes. Virus Genes 55, 448–457 (2019).
Sakai, M., Muto, M., Hirano, M., Kariwa, H. & Yoshii, K. Virulence of tick-borne encephalitis virus is associated with intact conformational viral RNA structures in the variable region of the 3’-UTR. Virus Res. 203, 36–40 (2015).
Asghar, N. et al. The role of the poly(A) tract in the replication and virulence of tick-borne encephalitis virus. Sci. Rep. 6, 39265 (2016).
Klein, S. L. & Huber, S. Sex differences in susceptibility to viral infection. In Sex Hormones and Immunity to Infection (Eds. Klein, S. L. & Roberts, C.). 93–122 (Springer, 2010). https://doi.org/10.1007/978-3-642-02155-8_4.
Olsson, G. E. et al. Demographic factors associated with hantavirus infection in bank voles (Clethrionomys glareolus). Emerg. Infect. Dis. 8, 924–929 (2002).
Perkins, S. E., Cattadori, I. M., Tagliapietra, V., Rizzoli, A. P. & Hudson, P. J. Empirical evidence for key hosts in persistence of a tick-borne disease. Int. J. Parasitol. 33, 909–917 (2003).
Stradiotto, A. et al. Spatial organization of the yellow-necked mouse: Effects of density and resource availability. J. Mammal. 90, 704–714 (2009).
van Duijvendijk, G. et al. Seasonal dynamics of tick burden and associated Borrelia burgdorferi s.l. and Borrelia miyamotoi infections in rodents in a Dutch forest ecosystem. Exp. Appl. Acarol. 17 (2022).
Hughes, V. L. & Randolph, S. E. Testosterone depresses innate and acquired resistance to ticks in natural rodent hosts: A force for aggregated distributions of parasites. J. Parasitol. 87, 49–54 (2001).
Hughes, V. L. & Randolph, S. E. Testosterone increases the transmission potential of tick-borne parasites. Parasitology 123, 365–371 (2001).
Cull, B., Vaux, A. G. C., Ottowell, L. J., Gillingham, E. L. & Medlock, J. M. Tick infestation of small mammals in an English woodland. J. Vector Ecol. 42, 74–83 (2017).
Bartolommei, P. et al. Field identification of Apodemus flavicollis and Apodemus sylvaticus: A quantitative comparison of different biometric measurements. Mammalia 80, 541–547 (2015).
Randolph, S. E. The shifting landscape of tick-borne zoonoses: Tick-borne encephalitis and Lyme borreliosis in Europe. Philos. Trans. R. Soc. B Biol. Sci. 356, 1045–1056 (2001).
Kozuch, O. et al. Experimental characteristics of viraemia caused by two strains of tick-borne encephalitis virus in small rodents. Acta Virol. 25, 219–224 (1981).
Labuda, M. et al. Importance of localized skin infection in tick-borne encephalitis virus transmission. Virology 219, 357–366 (1996).
Santos, R. I., Hermance, M. E., Reynolds, E. S. & Thangamani, S. Salivary gland extract from the deer tick, Ixodes scapularis, facilitates neuroinvasion by Powassan virus in BALB/c mice. Sci. Rep. 11, 20873 (2021).
Nuttall, P. A. & Labuda, M. Tick–host interactions: Saliva-activated transmission. Parasitology 129, S177–S189 (2004).
Khasnatinov, M. A. et al. Tick-borne encephalitis virus structural proteins are the primary viral determinants of non-viraemic transmission between ticks whereas non-structural proteins affect cytotoxicity. PLoS ONE 11, e0158105 (2016).
Ličková, M. et al. Dermacentor reticulatus is a vector of tick-borne encephalitis virus. Ticks Tick-Borne Dis. 11, 101414 (2020).
Ebel, G. D. & Kramer, L. D. Short report: Duration of tick attachment required for transmission of Powassan virus by deer ticks. Am. J. Trop. Med. Hyg. 71, 268–271 (2004).
Mlera, L., Meade-White, K., Saturday, G., Scott, D. & Bloom, M. E. Modeling Powassan virus infection in Peromyscus leucopus, a natural host. PLOS Neglect. Trop. Dis. 11, e0005346 (2017).
Kurhade, C. et al. Correlation of severity of human tick-borne encephalitis virus disease and pathogenicity in mice. Emerg. Infect. Dis. 24, 1709–1712 (2018).
Růžek, D., Dobler, G. & Mantke, O. D. Tick-borne encephalitis: Pathogenesis and clinical implications. Travel Med. Infect. Dis. 8, 223–232 (2010).
Abolins, S. et al. The comparative immunology of wild and laboratory mice, Mus musculus domesticus. Nat. Commun. 8, 14811 (2017).
Fros, J. J. et al. The dinucleotide composition of the Zika virus genome is shaped by conflicting evolutionary pressures in mammalian hosts and mosquito vectors. PLOS Biol. 19, e3001201 (2021).
Percie Du Sert, N. et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 18, e3000410 (2020).
Schwaiger, M. & Cassinotti, P. Development of a quantitative real-time RT-PCR assay with internal control for the laboratory detection of tick borne encephalitis virus (TBEV) RNA. J. Clin. Virol. 27, 136–145 (2003).
Spearman, C. The method of right and wrong cases (constant stimuli) without Gauss’s formulae. Br. J. Psychol. 2, 227 (1908).
Kärber, G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 162, 480–483 (1931).
Saraswati, K., Phanichkrivalkosil, M., Day, N. P. & Blacksell, S. D. The validity of diagnostic cut-offs for commercial and in-house scrub typhus IgM and IgG ELISAs: A review of the evidence. PLoS Neglect. Trop. Dis. 13, e0007158 (2019).
Hartig, F.F. Maintainer Florian Hartig. Package ‘DHARMa’ (2017).
Brooks, M. E. et al. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378–400 (2017).
Lenth, R. V. emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.6.0. (2021).
Allaire, J. RStudio: Integrated development environment for R. Boston MA 770, 165–171 (2012).
Acknowledgements
We would like to thank Overijssels landschap and Staatsbosbeheer for access to their lands, Wilma Blauw for help with serum collection, Dennis Bente for advice on experimental set-up, Johan Meijer, Albertjan ter Heide, Arnold van Zoelen and Antonique Spitshoven for their help in the rodent infection experiments.
Funding
This work was supported by the Production Ecology & Resource Conservation graduate school of Wageningen University & Research, the NWO Ideeëngenerator project No. NWA.1228.191.350, Fonds de Vos-Thijssen, and partly supported with funding from the European Union’s Horizon 2020 research and innovation program under LEAP-Agri grant agreement No. 727715.
Author information
Authors and Affiliations
Contributions
Conceptualization: J.W.B., C.J.M.K., J.K., P.J.W.S., H.E.J., W.F.B., H.S. Methodology: J.W.B., C.J.M.K., J.K., P.J.W.S., H.E.J., W.F.B., E.L.P., S.W., A.V., L.C.W. Formal analysis: J.W.B., C.J.M.K., H.E.J., W.F.B., L.K. Writing-original draft preparation: J.W.B. and C.J.M.K. Writing-reviewing and editing: J.W.B., E.L.P., S.W., L.K., A.V., H.E.J., G.P.P., W.F.B., H.S., J.K., P.J.W.S., C.J.M.K. Supervision: C.J.M.K., H.E.J., W.F.B., P.J.W.S. Funding acquisition: J.W.B., C.J.M.K., H.E.J., P.W.J.S. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bakker, J.W., Pascoe, E.L., van de Water, S. et al. Infection of wild-caught wood mice (Apodemus sylvaticus) and yellow-necked mice (A. flavicollis) with tick-borne encephalitis virus. Sci Rep 13, 21627 (2023). https://doi.org/10.1038/s41598-023-47697-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-47697-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.