Abstract
Antrodia cinnamomea (AC) is a treasured Asian medicinal mushroom, which has attracted attention due to recent research on its effectiveness in targeting a variety of serious ailments such as cancer and liver diseases. Among different A. cinnamomea constituents, triterpenoids are regarded as the most therapeutically attractive components because of their anti-inflammatory and cytotoxic activities. In the present study, we proposed a mathematical and statistical extraction protocol to evaluate the concentrations of total ergostane and lanostane triterpenoid derivatives from the ethanolic extract of the wild fruiting bodies of A. cinnamomea (EEAC) by utilizing response surface methodology (RSM) and quantitative NMR (qNMR) approaches. The optimum response surface model showed that the variations of the investigated response variables reached more than 90%, suggesting that the developed model is accurate in explaining response variability. Furthermore, the EEAC major characteristic triterpenoids were quantified through the comparison of the HPLC-tandem MS results with those of the qNMR results. The precision of the used techniques was also evaluated. The experimental design of the EEAC optimum extraction procedure obtained by using RSM and qNMR enabled accurate characterization and quantitation of A. cinnamomea triterpenoids.
Similar content being viewed by others
Introduction
Antrodia cinnamomea (AC), also known as Antrodia camphorata or Taiwanofungus camphoratus, is a precious medicinal mushroom native to Taiwan and is renowned for its fascinating pharmacological activities including anti-inflammation, anti-cancer, immunomodulatory, and hepato-protective effects1,2,3,4,5. It has also been used as folk medicine by the Taiwanese aboriginal tribes for the treatment of various health-related issues such as food and alcohol detoxification, abdominal pain, diarrhea, and hypertension, and to improve liver function1. Several classes of chemical constituents were purified and identified from A. cinnamomea such as benzenoids, terpenoids, benzoquinone, lignans, polysaccharides, maleic and succinic acid derivatives2,3,4. Among them, the terpenoids, the main bioactive components of A. cinnamomea fruiting bodies, demonstrated many fascinating pharmacological effects4. A. cinnamomea contains complicated tetracyclic triterpenoids including ergostane-type and lanostane-type triterpenoids2, 4. These types of triterpenoids show similar chemical structures resulting in technical difficulties in their separation for quality control purposes or the preparation of commercial products6.
Highly efficient extraction and analytical methodologies were developed for the purification and quantification of biologically active secondary metabolites. However, few reports are available on the quantification of triterpenoids, especially ergostanes and lanostanes derivatives, and their use as chemical markers for the quality control of A. cinnamomea. Several research groups reported the use of the colorimetric method with the vanillin/acetic acid system to quantify AC total triterpenoids7, 8. However, colorimetric method protocols suffered from low sensitivity and poor accuracy in the determination of the concentrations of AC total triterpenoids. Currently, there are no reference standard protocols for the quality control of AC active constituents from different AC materials. Thus, developing proper standardization and an efficient quality control protocol to identify the concentration of bioactive components will provide necessary information on the quality and biological activity of AC commercial products. AC triterpenoids are intriguing chemical markers for AC products that are present in major quantities, which can be quantified and inspected by spectroscopic techniques.
Previously, we developed and established a feasible quantification methodology to comprehensively evaluate the quality of benzenoid components of AC wild fruiting bodies and their related commercial cultivation products9. Additionally, we developed a convenient fractionation process to concentrate the bioactive triterpenoids from AC fruiting bodies. The chemical profile of the triterpenoids-rich fraction was revealed by HPLC, HPLC–PDA/MS, and NMR strategies10. In the current study, we established and developed a mathematical and statistical protocol to evaluate the concentrations of the total ergostane and lanostane triterpenoid derivatives, from the ethanolic extract of the wild fruiting bodies of AC (EEAC) using response surface methodology (RSM) and quantitative NMR (qNMR). The total triterpenoids in EEAC were quantified using the HPLC-tandem MS methodology.
Materials and methods
Standard compounds and AC materials
The standard compounds of 25R/S-ergostane epimers triterpenoids (E1–E12) and lanostane triterpenoids (L1–L4) employed for comparative analysis experiments were separated from the triterpenoid-rich fraction (FEA) of EEAC. They were 25S/R-antcin K (E1/E2), 25S/R-antcin C (E3/E4), 25R/S-zhankuic acid C (E5/E6), 25R/S-zhankuic acid B (E7/E8), 25S/R-zhankuic acid A (E9/E10) and 25S/R-antcin A (E11/E12) of ergostane epimers triterpenoids and dehydrosulphurenic acid (L1), sulphurenic acid (L2), dehydroeburicoic acid (L3) and eburicoic acid (L4) of lanostane triterpenoids (Fig. 1). The structures of sixteen isolates were confirmed and elucidated based on NMR and MS data as illustrated in our previous study and the results were compared with those reported in the literature (Fig. S1)4, 10. The used internal standard (IS), ganoderic acid A, for tandem MS experiment was isolated in our laboratory. The internal standard, pyrazine, used for quantitative NMR analysis was purchased from Sigma-Aldrich (St. Louis, MO, USA). The purity of these analytes was higher than 97%, which was evaluated with a three-point peak purity method and was determined using Shimadzu “Class VP” software.
The wild fruiting bodies of A. cinnamomea were collected from the forest in Taiwan in 2020 and was further identified on the basis of the morphological characteristics and nucleotide sequence of the internal transcribed spacers (ITS) analysis using universal primers by Professor Tun-Tschu Chang (Taiwan Forestry Research Institute). The obtained sequences of ribosomal RNA/ITS was further blasted with the National Center for Biotechnology Information (NCBI) database. The blasting results showed that the sample shared the 99.5% sequence identity with A. cinnamomea (GenBank accession number: AY378095). The cutting wood culture (CWC) and dish culture (DC) samples were purchased from different Taiwan Biotech Co., Ltd in 2022. The voucher specimens were deposited in the authors' laboratory.
Determination and quantification of the EEAC total ergostane and lanostane triterpenoids with 1H NMR spectrum
The determination and quantification of the total ergostane and lanostane triterpenoids in EEAC were performed using the NMR spectrum analysis method. The NMR apparatus used was Varian UNITY plus 400 MHz spectrometers, and each sample was examined 10 times on the basis of the following parameters: the spectra width was established to 6002.4 Hz, and the width of the impulse intensity was established to 6.3 µs. The EEAC was added to the internal standard solution (0.132 mg pyrazine dissloved in 0.6 mL DMSO-d6). In the detection analysis of the total ergostane and lanostane triterpenoids, the starting and ending points of the characteristic proton signals were manually selected to calculate the integration for every peak, which were the terminal olefinic proton signals (H-28) at δH 4.82 (2H, br d) of ergostane triterpenoid and signals at δH 4.63 (1H, s) and 4.70 (1H, s) of lanostane triterpenoid, respectively. The ratios of the characteristic proton integration area to its corresponding integration area of the added internal standard were calculated. In the quantitative analysis, the standard compounds of ergostane and lanostane triterpenoids with different concentrations were prepared to establish a specific calibration plot under investigation, which were zhankuic acid A (E9/E10) of the ergostane triterpenoid in the range of 2.02 to 6.06 mg and dehydroeburicoic acid (L3) of the lanostane triterpenoid in the range of 1.15 to 5.01 mg, respectively. All experiments were accomplished in triplicate, and the relative standard deviation was calculated. The absolute concentrations of the total ergostane and lanostane triterpenoids in EEAC were obtained by introducing the obtained ratio to the calibration curve equation below:
where \({Y}_{E}\) and \({Y}_{L}\) are the ergostane and lanostane triterpenoids ratios of the characteristic proton integration area of the standard compounds to its corresponding integration area of the additional internal standard in EEAC. \({X}_{E}\) and \({X}_{L}\) are the absolute concentrations of the total ergostane and lanostane triterpenoids in EEAC.
EEAC preparation procedures
The ethanolic extract of AC wild fruiting bodies (EEAC) was prepared as described previously11. In brief, the mushroom samples were refluxed with ethanol in a 1:10 (w/v) ratio at 75 °C for 2 h, and the ethanolic extracts were then cooled and enabled to precipitate at 4 °C overnight. The supernatant of the extracts was filtrated to remove the precipitate, yielding EEAC that was stored at 0–4 °C before analysis. Following the establishment and development of "Green Chemistry" principles over the past few decades, environmentally friendly methods are becoming more attractive. Therefore, developing an efficient extraction and eco-friendly protocol that reduces the use of large quantities of the slow growing and expensive AC will be highly desirable. To compare the extraction efficiency of different protocols, the ultrasonic-assisted extraction (UAE) method was selected to evaluate the concentrations of the total ergostane and lanostane triterpenoids from EEAC in the preliminary test. The UAE experiment was carried out using an ultrasonic apparatus with a working frequency of 40 kHz and the power of 150 W (Delta DC150H, Taiwan). The bath was a temperature-controlled rectangular container (300 × 160 × 150 mm, 7.2 L). A set of 50 ml Erlenmeyer flasks was filled with 2.5 g of ground AC samples of 3 mm particle size (controlled with a ratio of powder (g): ethanol (ml) of 1:10) and ultrasonicated in an ultrasonic bath with a working frequency of 40 kHz at 75 °C for 2 h.
The extraction conditions of UAE were the same as those mentioned above and the concentrations of the total ergostane and lanostane triterpenoids were obtained by calculating their integrations area of the characteristic proton signals to its corresponding integrations area of the added internal standard in the 1H NMR spectra. The results indicated that the integrations of the total ergostane and lanostane triterpenoids from EEAC obtained using the UAE method (39.98 and 12.03) were similar to those obtained by the reflux mode (40.01 and 12.11). Furthermore, in our preliminary test, the EEAC yield of the UAE extraction method was similar to those measured by the reflux mode in the range of 29–31% at 75 ºC for 2 h. Therefore, the UAE method can be used in the present study as an extraction procedure for the quantitative analysis of EEAC because of its high reproducibility, shorter time for extraction, simple procedures, reduced solvent consumption, lower temperature, and lower energy input.
Experimental design of EEAC extraction
The optimization parameters of the UAE extraction of the total ergostane and lanostane triterpenoids from EEAC were conducted using an on face experimental strategy based on a three factor/three level design known as a rotatable central composite design. The application of a CCD design is a convenient tool for optimizing a procedure with three levels (−1, 0, and + 1) for every factor. The purpose of this design was performed to evaluate the effects and interactions of three independent variables, namely temperature (°C, \({\rm X}1\)), time (minutes, \({\rm X}2\)) and ethanol concentrations (EtOH%, ethanol–water ratio, v/v, \({\rm X}3\)). Three levels of values for the independent variables were selected and demonstrated in their coded and uncoded forms based on the preliminary test (Table 1). A set of designs of experiments consisting of sixteen independent runs, which include six replicates of a central point, each designated by the coded value 0, were selected in randomized order based on a CCD-On face configuration for three factors.
Statistical and mathematical analysis
Statistical analysis was performed using Design Expert software JMP (Version 9.0.0, 2010 SAS Institute Inc.). The response of the UAE extraction procedure, the ergostane and lanostane triterpenoids ratios of the characteristic proton integration area of the standard compounds to the corresponding integration area of the added internal standard (\(Y\)) was modeled as a second-order polynomial equation. This equation enclosed a function of the entire primary variables and their interactions as displayed in the following equation.
where \({X}_{i}\) and \({X}_{j}\) are the coded variables, \({\beta }_{0}\), \({\beta }_{i}\), \({\beta }_{ii}\) and \({\beta }_{ij}\) are the model coefficient for intercept, first-order, quadratic, and interaction between \({X}_{i}\) and \({X}_{j}\), respectively. The model obtained from the regression analysis was used to generate the response surface and contour plots. The adequacy of the model was determined by evaluating the lack of fit, coefficient of determination (R2), and the Fisher test value (F-value) acquired from the analysis of variance (ANOVA) which was obtained from the software. Significant variables were those with a confidence level higher than 95% (p < 0.05). The validation of the model illustrated that the optimal conditions for the extraction of the total ergostane and lanostane triterpenoids from EEAC, depending on the extraction temperature, time and ethanol–water ratio were obtained using the predictive RSM equations.
To generate response surfaces, the software used the quadratic model equation shown above. Three-dimensional response surface plots and contour plots were created by retaining one response variable at its optimum levels and plotting it against two factors (independent variables). For each solvent, response surface plots were generated. Additionally, the coded values of the experimental factors and factor levels utilized for the response surface analysis are illustrated in Table 1. The entire design using aqueous EtOH and water as extraction solvents consisted of 16 experimental points including six replications of the center point. The coded values for the experimental designs are shown in Table 1.
HPLC and HPLC-tandem MS conditions for EEAC analysis
The analysis of EEAC was performed using an LC-20A VP HPLC system (Shimadzu Inc.) equipped with a quaternary solvent pump (LC-20AT), an autosampler (SIL-20AD), an online degasser (DGU-14A), a photodiode-array detector (SPD-M20A) and the Class VP software for data collection. An Agilent Poroshell 120 EC-C18 column (150 mm × 4.6 mm, i.d., 2.7 µm, Agilent Technologies, CA, USA) was used for liquid chromatography. The injection volume of the sample was 10 µL. The optimum mobile phase is composed of H2O containing 0.1% formic acid and 3 mM ammonium acetate (A) and ACN (B). A gradient program was used as follows, the preliminary elution condition was A–B (61:39, v/v), which was linearly changed to A–B (56:44, v/v) at 15 min, A–B (55:45, v/v) at 17.5 min, A–B (53:47, v/v) at 22.5 min, A–B (50:50, v/v) at 27.5 min, A–B (47:53, v/v) at 30 min, A–B (45:55, v/v) at 35 min, A–B (35:65, v/v) at 45 min, A–B (2:98, v/v) at 55 min, then increased to 100% B in 5 min, and eventually decreased to 39% B in 1 min and held for 9 min, for regeneration. The mobile phase was filtered through a 0.22 µm Millipore filter and was degassed before use. The flow rate was adjusted to 1.3 mL/min, the column temperature was maintained at ambient temperature, and the detection wavelength was performed at 254 nm. Before loading to the HPLC column, 1 mg of the EEAC dry extract was dissolved in 1 mL of methanol and was further filtered through the 0.45 µm membrane filter.
The quantitative HPLC-tandem mass experiment was carried out using an Agilent 1200 HPLC system (Agilent Technologies) equipped with an API 4000 triple quadrupole mass spectrometer (Applied Biosystem, CA, USA). The ion source for the detection of electrospray ionization was selected as the negative ion mode and the analytical conditions were similar to those described previously. The quantification was accomplished in multiple reactions monitoring (MRM) mode using the precursor-to-product ion transitions of 487 to 443, 469 to 425, 485 to 441, 469 to 425, 467 to 423 and 453 to 409, corresponding to 25R/S-ergostane epimers triterpenoids (E1–E12) and 483 to 83, 485 to 355, 467 to 337, 469 to 339 and 515 to 497, corresponding to lanostane triterpenoid (L1–L4) and IS, respectively. The optimized ESI source parameters were as follows, the ion spray voltage is −4500 V, the nitrogen nebulizer gas pressure is 60 psi, the nitrogen auxiliary gas pressure is 55 psi, the nitrogen curtain gas pressure is 12 psi, the heater temperature is 600 °C, and collisionally activated dissociation (CAD) gas is 12. To maximize the intensity of the transition ions, the cone voltage and collision energy were optimized for each tested sample. The detailed information of specific declustering potential (DP), collision energies (CE), collision cell exit potential (CXP), and MRM transitions of E1–E12, L1–L4, and IS are given in Table 2. All data acquisition and processing were accomplished using Analyst 1.5.1 software (AB SCIEX, Concord, ON, Canada).
HPLC-tandem MS calibration
To create a calibration plot for each compound under the current study, a stock solution of each standard compound (1 mg/mL in methanol) was prepared and serially diluted to yield the reference solution at various concentrations (10 to 1000 ng/mL). Each reference standard solution received an adequate amount of the internal standard (IS) (ganoderic acid A) to achieve a final concentration of 100 ng/mL. Calibration curves were generated by comparing the peak area of each compound to the IS peak area in triplicate for every concentration.
Results and discussion
NMR analysis of the EEAC ergostane and lanostane triterpenoids
Developing an accurate and efficient method to determine the concentrations of target active ingredients in natural products and nutritional supplements became an attractive point to researchers, particularly for the simultaneous determination of various target components with diversified physicochemical characteristics against extremely complicated matrices12. Thus, the identification of specific chemical markers in natural products and nutritional supplements has emerged as a straightforward quality control strategy. In general, NMR is applied to elucidate the structure of chemical compounds and biological substances. Moreover, it can be utilized to provide rapid and accurate information on the concentrations of specific constituent in a mixture. This technique is referred to quantitative NMR (qNMR), and it has found distinctive applications in biomedical, pharmaceutical, and phytochemical research13, 14. While compared to those other instrumental analysis techniques, qNMR is a primary ratio method because the acquired peak areas are proportional to the number of corresponding nuclei15. Therefore, qNMR was selected for the quantitative analysis of the EEAC total ergostane and lanostane triterpenoids due to its being considered superior to other conventional chromatographic methods for the determination of the concentrations of certain herbal mixture constituents. In our previous studies, we successfully developed efficient analytical procedures using 1H NMR to evaluate AC fractions10. The 1H NMR spectra of FNH (the benzenoid-rich fraction), FEA (the triterpenoid-rich fraction), FET (the saccharide-rich fraction) and EEAC under identical conditions (10 mg/0.75 ml, pyridine-d5, 400 MHz) were plotted for comparison. EEAC 1H NMR spectrum showed signals at δH 0.70 and 1.61, corresponding to the two tertiary methyl signals and three secondary methyl signals at δH 0.89, 1.03 and 1.52. Three methine signals at δH 3.01, 3.17 and 3.48 as well as the terminal olefinic protons (2H-28) in a 24-exo-methylene-26-oic acid sidechain at δH 4.89, 4.94, 5.08 and 5.24 were also detected. These distinguishable signals are the characteristic signals of AC triterpenoids, which were also observed in the 1H NMR spectra of FEA but were absent from the 1H NMR spectra of FNH and FET.
To provide an accurate method to evaluate the concentrations of EEAC total ergostane and lanostane triterpenoids in the present study, an efficient determination procedure was proposed utilizing 1H NMR. While compared to pyridine-d5, the polar properties of EEAC suggested that DMSO-d6 would be the best deuterated solvent for ensuring the solubility of all components and that its signals were not interfering with the characteristic signals of the analyte. On the other hand, the characteristic signals must be designed by integrating a well-separated specific proton signal and the signal not interfering with the deuterated solvent. For the present experiment, the terminal olefinic proton signals (H-28) of ergostane triterpenoid (2H, br d, δH 4.82) and the signals of lanostane triterpenoid (1H, s, δH 4.63) and (1H, s, δH 4.70) were selected. These signals were well-separated without any interference from other signals and hence their integration values would provide characterized information on the concentration of the total ergostane and lanostane triterpenoids in EEAC (Fig. 2). These distinguishable signals were also detected in the 1H NMR spectra of the standard compounds of zhankuic acid A (E9/E10) and dehydroeburicoic acid (L3) under identical conditions (10 mg dissolved in 0.6 mL DMSO-d6, 400 MHz). Additionally, a highly stable pure internal standard with signals that do not interfere with the characteristic signals of the analyte must be employed in the qNMR experiment. For this purpose, pyrazine, with a proton signal at δH 8.60, was selected as an internal standard. Moreover, zhankuic acid A and dehydroeburicoic acid as the standard compounds proved that the process developed in the present study could determine the concentrations of EEAC total ergostane and lanostane triterpenoids in the 1H NMR spectrum. The developed quantitative method combined the ratios of the characteristic proton integration area to its corresponding integration area of the added internal standard and the constructional calibration curves of the standard compounds can be utilized to evaluate the absolute concentrations of the total ergostane and lanostane triterpenoids in EEAC.
Selection of extraction parameters in preliminary experiments
The extraction efficiency varies with the process conditions. For instance, some of the most important factors affecting the extraction efficiency in terms of quality and yield are temperature, contact time, and material/solvent ratio. However, the role of each factor in the mass transfer of the procedure is unpredictable because of the chemical characteristics of the solvent and the diversity of its structure as well as the composition of the natural products, which cause each material–solvent system to behave differently.
Preliminary studies were carried out to establish the optimal extraction parameters for the extraction of the total ergostane and lanostane triterpenoids from AC. The three independent variables of the solid-to-liquid (material/EtOH, w/v) ratio, time, temperature, and ethanol concentrations (EtOH%, ethanol–water ratio, v/v) were determined. The response was evaluated by calculating the total ergostane and lanostane triterpenoids integration ratios of the characteristic proton integration area of the standard compounds to its corresponding integration area of the added internal standard using the constructed calibration curves of the standard compounds in 1H NMR analysis. To determine the required parameters of the solid-to-liquid ratio (material/EtOH, w/v) for the extraction of the total ergostane and lanostane triterpenoids from AC, three parameters of the solid-to-liquid ratio (1:10, 1:20, and 1:30) were assessed. The results indicated that the extraction of the AC ergostane and lanostane triterpenoids was dependent on the solid-to-liquid ratio. The integration ratios of the total ergostane and lanostane triterpenoids increased with an increasing solid-to-liquid ratio, reaching a plateau at a solid-to-liquid ratio of 1:20. Thus, the solid-to-liquid ratio of 1:20 was selected. This ratio was suggested to enhance the extraction efficiency in comparison with the solid-to-liquid ratio of 1:10. The selection of an appropriate extraction parameter including time, temperature and ethanol concentrations (EtOH%) depended on our previously reported extraction condition10. The extraction procedure was carried out at time intervals of 30, 60, 90, and 120 min. The other extraction parameters were as follows including an ultrasonic temperature of 25 °C and a 1:20 solid-to-liquid ratio. While the extraction time varied from 30 to 90 min, the variance of the integration ratios of the total ergostane and lanostane triterpenoids were not significantly increased and reached a maximum at 60 min. This suggested that the extraction time of 30–90 min was sufficient to obtain AC ergostane and lanostane triterpenoids. Further, we tested the effect of changing the extraction temperature by experimenting at 25, 50, and 75 ºC. The other extraction parameters were as follows including an extraction time of 30 min and a 1:20 solid-to-liquid ratio. As expected, the integration ratios of the total ergostane and lanostane triterpenoids increased with the temperature and reached their maximum at 75 °C. The increase in temperature may improve the extraction efficiency by enhancing the solubility of triterpenoids, increasing the diffusion coefficient, and decreasing the viscosity coefficient. By increasing the temperature from 25 to 75 °C, the integration ratios of the total ergostane and lanostane triterpenoids increased 5.7 and 4.9%, respectively. Therefore, the extraction temperature range of 25–75 °C was optimal in the present experiment. The other parameter of ethanol concentrations (EtOH%) was suggested to be crucial in determining the efficiency of extracting AC triterpenoids due to their lipophilic properties. The extraction procedure was performed with various ethanol concentrations of 15, 35, 65, and 95% (v/v), as well as following extraction parameters: an ultrasonic temperature of 25 °C, extraction time of 30 min, and a solid-to-liquid ratio of 1:20. The results showed that the integration ratios of the total ergostane and lanostane triterpenoids increased with the increase in the ethanol concentrations. When ethanol concentrations varied from 35 to 95%, the variance of the integration ratios of the total ergostane and lanostane triterpenoids was significantly increased and reached a plateau of 95%. While the ethanol concentration increased from 35 to 95%, the integration ratios of the total ergostane and lanostane triterpenoids increased by 25.8% and 18.4%, respectively. Thus, the ethanol concentration of 35%-95% was favorable for extracting AC triterpenoids. The designed extraction parameters of the three independent variables with three levels of values were evaluated using predictive RSM equations.
Optimization of the extraction condition of AC total ergostane and lanostane triterpenoids by RSM analysis
The experimental design matrix and the corresponding results are illustrated according to their design parameters (Table 1). The response of the integration ratios of the total ergostane and lanostane triterpenoids were recorded in the range of 24.22–40.33 and 9.63–13.59, respectively. The adequacy of the second-order quadratic equation model developed from multiple regression analysis for this experiment could be validated using the analysis of variance (ANOVA) (Table 3). For the design model, the coefficient of determination, R2 was recorded at 0.9710 of ergostane and 0.9350 of lanostane which explained the good fit of the model to the data input. For a 95% confidence interval, P > F values must be smaller than 0.05 to be considered statistically significant toward the effect of the response. In the design model, the P > F values of ergostane and lanostane were less than 0.05 suggesting that this model was significant. The significance of the model was also evaluated by the lack-of-fit test. The P > F values were more than 0.05 (insignificant) in both ergostane and lanostane implying that the model was sufficiently accurate for predicting those response variations. Using mathematical models, the significance of the investigated factors and their interactions were evaluated. The quadratic model showed that the linear variable of EtOH% (\({\rm X}3\)) was significant (P < 0.01) in both of ergostane and lanostane. The one quadratic term of temperature (\({\rm X}_{1}^{2}\)) was significant (P < 0.05) of lanostane triterpenoids (Table 4).
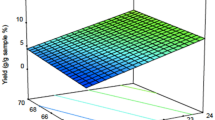
The three-dimensional representation of the response surfaces model demonstrated the relationship between the independent and dependent variables (Fig. 3). The suitability of the model equation in predicting the optimum response values of three independent variables was evaluated using these three-dimensional plots and their respective contour plots. The results indicated that the optimized conditions were the extraction temperature of 54.6 °C, the extraction time of 58.9 min, and the ethanol proportion of 95%. These conditions resulted in corresponding maximum integration ratios of the total ergostane and lanostane triterpenoids of 40.53 and 13.50, respectively (Fig. 4). However, the variation in the extraction temperature (significant, P < 0.05) may play a key role for the extraction AC ergostane and lanostane triterpenoids in this designed model. The discovery of an optimal extraction temperature of 54.6 °C was kind of surprising as we had anticipated that higher temperatures would enhance extraction efficiency. However, higher temperatures than this specific point had the opposite effect and reduced the yield of compounds of the lanostane type. Therefore, to obtain the highest yield of AC total triterpenoids, it was suggested to apply the optimum extraction conditions in any future experiment.
Using a combination of the developed extraction protocol and constructional calibration curves of the standard compounds, the concentrations of the EEAC total ergostane and lanostane triterpenoids were determined to be 513 ± 0.18 and 187 ± 0.25 µg/mg, respectively. The optimum response surface extraction protocol and qNMR approaches were applied to the analysis of AC total triterpenoids in different cultivation products. The developed protocol results in the concentrations of the EEAC total ergostane and lanostane triterpenoids were determined to be 292 ± 0.45 and 344 ± 0.35 µg/mg of the CWC sample and 37 ± 0.01 and 54 ± 0.03 of the DC sample, respectively. The results indicated that the media conditions and culture techniques have an important effect on fungal metabolites, resulting in products with inconsistent biological activity and concentration of major compounds.
Characterization of the EEAC major ergostane and lanostane triterpenoids with HPLC-tandem MS
Over the last few years, several research groups reported the application of HPLC/UPLC-UV or PDA (photodiode array) detector and SFC (supercritical fluid chromatography) coupled with MS detector to analyze and quantify the components of AC fruiting bodies and mycelia16, 17. Despite the success of the developed methods in identifying or separating certain complicated ingredients in AC extracts, they were inefficient in quantifying AC triterpenoids. Owning to the poor sensitivity and poor accuracy of the reported methods, the desired peaks could not be analyzed simultaneously. To address this issue, a highly sensitive and selective method based on HPLC coupled with triple quadrupole mass spectrometry in multiple reactions monitoring (MRM) mode was developed to quantify the EEAC characteristic triterpenoids (E1–E12 and L1–L4). The quantification of EEAC total triterpenoids obtained using RSM and qNMR was evaluated by comparing the obtained results with those collected from HPLC-tandem MS.
In our previous study, we tried to separate AC triterpenoids by evaluating the use of three different 0.1% organic acids, including trifluoroacetic, acetic or formic acids as an additive to the mobile phase. The addition of organic acids aimed to facilitate the separation of AC triterpenoids due to their acidic nature. The results suggested that the optimum mobile phase was acetonitrile containing 0.1% aqueous acetic acid, however, the major 25R/S-epimers of ergostane triterpenoids were not completely separated in the chromatogram10. To efficiently separate these epimers, the use of ammonium acetate at different concentrations was evaluated as an additive to the mobile phase (containing 0.1% formic acid). The results in the HPLC–UV chromatogram showed that the best resolution to separate the major ergostane epimers was a mobile phase containing 0.1% formic acid, 3 mM ammonium acetate, and acetonitrile at a detection wavelength of 254 nm (Fig. S2). Although lanostane triterpenoids (L1/L2 and L3/L4) contain Δ7,9 orΔ8 double bonds, they could not be separated under these optimum conditions. To identify and quantify their concentrations, a triple quadrupole mass spectrometer was selected due to the difference in molecular weights of these analytes. An internal standard was required to accurately determine of the concentrations of triterpenoids. Particularly, an internal standard should be a stable isotope-labeled compound or a structurally related analog. However, stable isotope-labeled substances are difficult to obtain and must be handled under special precautions18, 19. Therefore, a structurally similar analog such as ganoderic acid A, which was isolated in our laboratory, was used in the proposed method. This analog possesses comparable solubility, chromatographic and spectrometric characteristics to the target analyte and thus can be employed as an internal standard. Initially, E1–E12, L1–L4, and the internal standard were ionized using both positive and negative ion modes through mass spectrometry. The results indicated that the negative mode was suitable and was selected for the subsequent analysis. To monitor the effect of the ionization source temperature on the MS peak intensity, three different temperatures (450 °C, 550 °C, and 600 °C) was tested. The results suggested that the optimum temperature for the ionization source was 600 °C.
The negative ESI–MS/MS spectra for the EEAC characteristic triterpenoids (E1–E12 and L1–L4) are shown in Fig. S3. All investigated ergostanes in the present study possessed a COOH group at C-25, which could generate [M-44]- ions from [M − H]− in their MS/MS spectra. The loss of the fragment ion was assigned to the CO2 cleavage of the side chain. The fragment ions of 4-OH ergostanes of 25S/R-antcin K (E1/E2) were m/z 443, 425, and 407, which appeared due to the loss of CO2 and H2O. The abundant fragments of 7-OH ergostanes of 25S/R-antcin C (E3/E4) were m/z 301, 259, and 247 after the collision, and the collision energy can trigger B-ring RDA fragmentation and can lead to B/C-ring cleavage (Fig. S3). Therefore, the fragments at m/z 301, 259, and 247 could be regarded as diagnostic ions for 7-OH ergostanes, and these unique fragments were also found in other 7-OH compounds 25S/R-antcin K (E1/E2). The structure in ergostanes of 25R/S-zhankuic acid C (E5/E6) was substituted for the hydroxyl group at the C-12 position. Their MS/MS spectra revealed three major product ions at m/z 423, 413, and 383 fragments implying the lost ions were [M-CO2-H2O]−, [M-CO2-CO]− and [M-CO2-CO-CH2O]−, respectively. On the other hand, the MS/MS spectra of 25R/S-zhankuic acid B (E7/E8), 25S/R-zhankuic acid A (E9/E10) and 25S/R-antcin A (E11/E12) were dominated by [M-60]− ions. The loss of the fragment ion was assigned to C2H4O2 cleavage from the side chain (Fig. S3). The abundant [M-60]− ion could be a diagnostic signal for 7-ΔO/7-H ergostanes. For the analysis of lanostanes (L1/L2 and L3/L4), we combined Q1 and Q3 scans to increase their sensitivity and selected the reliable fragment ions to quantify in EEAC because of their low level of oxygenation. These fragment ions contained m/z 83 (C6H11) or m/z 97 (C7H13) from the side chain. The calibration curves were generated by plotting the peak area ratios of E1–E12, L1–L4, and the internal standard against the known concentration of each compound. The plot was revealed to be linear towards the range of 10–1000 ng/mL with r2 values of > 0.99 (Table 2).
HPLC-tandem MS method validation and quantification of the EEAC ergostane and lanostane triterpenoids
The developed quantitative method based on the characteristic fragmentation reactions of triterpenoid compounds was highly specific with no interfering peaks at the retention times of the marker compounds (E1–E12 and L1–L4). The intra-day accuracy and the precision were calculated by analyzing of characteristic triterpenoids (E1–E12 and L1–L4) in five replicates at three different concentrations (50, 500 and 1000 ng/ml) on the same day. The inter-day accuracy and precision were obtained by repeating the measurements on five consecutive days. The relative standard deviation and relative error were less than 8.84, 8.14% and 8.22, 8.02% of ergostane and lanostane triterpenoids, respectively (Tables S1 and S2). The quantification of the sixteen triterpenoids was accomplished using HPLC-tandem MS protocol, the EEAC extraction method was prepared with our previous extraction procedure (refluxed with 95% ethanol at 75 °C in a 1:10 (w/v) ratio for 2 h) aiming to evaluate the accuracy of evaluating EEAC total triterpenoids concentrations using the optimum extraction conditions. The quantification results of EEAC total triterpenoids in two different extraction procedures were also compared. Therefore, the total concentrations of the ergostane and lanostane triterpenoids in the EEAC were found to be 567.83 and 113.86 µg/mg, respectively. The concentrations of each of the sixteen triterpenoids were found to be 16.739 ± 0.38, 34.539 ± 0.31 µg/mg (E1/E2), 5.997 ± 0.30, 10.751 ± 0.32 μg/mg (E3/E4), 40.206 ± 0.35, 54.288 ± 0.30 μg/mg (E5/E6), 48.102 ± 0.45, 39.012 ± 0.40 μg/mg (E7/E8), 31.509 ± 0.25, 22.251 ± 0.21 μg/mg (E9/E10), 120.418 ± 0.53, and 144.023 ± 0.57 μg/mg (E11/E12) for ergostanes and 45.685 ± 0.40, 38.561 ± 0.35, 26.724 ± 0.38, and 2.897 ± 0.41 μg/mg (L1–L4) for lanostane, respectively.
The results demonstrated that the optimum extraction procedures resulted in high yields of the total triterpenoids in EEAC. The concentration of the total ergostane triterpenoids in EEAC under the expected temperature (54.6 °C) achieved a plateau point. The concentration can slowly increase with increasing temperature. On the contrary, the concentration of the total lanostane triterpenoids in EEAC obtained using the optimum extraction temperature (54.6 °C) was 1.65 folds higher than those obtained by the original extraction temperature (75 °C). Consequently, the extraction temperature was an important variable for extracting the EEAC ergostane and lanostane triterpenoids in addition to the main parameter of EtOH concentration.
Conclusions
An efficient methodology was developed for the characterization and quantification of AC triterpenoids using experimental design. The wild fruiting body of AC (EEAC) was analyzed in an accurate protocol using RSM and qNMR. The quantification of the total triterpenoids was conducted by comparing the HPLC-tandem MS and qNMR results. The lack of standard protocols to ensure AC product quality highlighted the importance of developing and establishing a quantification method to provide AC components. This method will have practical applications for the production of highly specialized AC supplements and related commercial products.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- AC:
-
Antrodia cinnamomea
- EEAC:
-
Ethanolic extract from A. cinnamomea
- FEA:
-
The ethyl acetate fraction from EEAC (the triterpenoid-rich fraction)
- UAE:
-
Ultrasonic-assisted extraction
- HPLC-PDA:
-
High-performance liquid chromatography equipped with a photodiode array detector
- HPLC-tandem MS:
-
High-performance liquid chromatography equipped to triple quadrupole mass spectrometry
- qNMR:
-
Quantitative nuclear magnetic resonance
References
Lu, M. C. et al. Recent research and development of Antrodia cinnamomea. Pharmacol. Ther. 139, 124–156. https://doi.org/10.1016/j.pharmthera.2013.04.001 (2013).
Qiao, X. et al. Comprehensive chemical analysis of triterpenoids and polysaccharides in the medicinal mushroom Antrodia cinnamomea. RSC Adv. 5, 47040–47052. https://doi.org/10.1039/c5ra04327a (2015).
Ganesan, N., Baskaran, R., Velmurugan, B. K. & Thanh, N. C. Antrodia cinnamomea—An updated minireview of its bioactive components and biological activity. J. Food Biochem. 43, e12936. https://doi.org/10.1111/jfbc.12936 (2019).
Kuang, Y., Li, B., Wang, Z., Qiao, X. & Ye, M. Terpenoids from the medicinal mushroom Antrodia camphorata: Chemistry and medicinal potential. Nat. Prod. Rep. 38, 83–102. https://doi.org/10.1039/d0np00023j (2021).
Zhang, B. B. et al. Production of bioactive metabolites by submerged fermentation of the medicinal mushroom Antrodia cinnamomea: Recent advances and future development. Crit. Rev. Biotechnol. 39, 541–554. https://doi.org/10.1080/07388551.2019.1577798 (2019).
Xu, C., Wang, B., Pu, Y., Tao, J. & Zhang, T. Techniques for the analysis of pentacyclic triterpenoids in medicinal plants. J. Sep. Sci. 41, 6–19. https://doi.org/10.1002/jssc.201700201 (2018).
He, Y. C., He, K. Z., Pu, Q., Li, J. & Zhao, Z. J. Optimization of cultivating conditions for triterpenoids production from Antrodia cinnmomea. Indian J. Microbiol. 52, 648–653. https://doi.org/10.1007/s12088-012-0290-y (2012).
Ma, T. W., Lai, Y. & Yang, F. C. Enhanced production of triterpenoid in submerged cultures of Antrodia cinnamomea with the addition of citrus peel extract. Bioprocess Biosyst. Eng. 37, 2251–2261. https://doi.org/10.1007/s00449-014-1203-8 (2014).
Wu, T. Y. et al. New approach to the characterization and quantification of Antrodia cinnamomea benzenoid components utilizing HPLC-PDA, qNMR and HPLC-tandem MS: Comparing the wild fruiting bodies and its artificial cultivated commercial products. Food Res. Int. 51, 23–31. https://doi.org/10.1016/j.foodres.2012.11.014 (2013).
Du, Y. C. et al. Chemical profiling of the cytotoxic triterpenoid-concentrating fraction and characterization of ergostane stereo-isomer ingredients from Antrodia camphorata. J. Pharm. Biomed. Anal. 58, 182–192. https://doi.org/10.1016/j.jpba.2011.09.007 (2012).
Lu, M. C. et al. Active extracts of wild fruiting bodies of Antrodia camphorata (EEAC) induce leukemia HL 60 cells apoptosis partially through histone hypoacetylation and synergistically promote anticancer effect of trichostatin A. Arch. Toxicol. 83, 121–129. https://doi.org/10.1007/s00204-008-0337-3 (2009).
Li, Y. G., Song, L., Liu, M., Hu, Z. B. & Wang, Z. T. Advancement in analysis of Salviae miltiorrhizae Radix et Rhizoma (Danshen). J. Chromatogr. A 1216, 1941–1953. https://doi.org/10.1016/j.chroma.2008.12.032 (2009).
Lynch, M. J. et al. Ultra high field NMR spectroscopic studies on human seminal fluid, seminal vesicle and prostatic secretions. J. Pharm. Biomed. Anal. 12, 5–19. https://doi.org/10.1016/0731-7085(94)80004-9 (1994).
Bollard, M. E., Stanley, E. G., Lindon, J. C., Nicholson, J. K. & Holmes, E. NMR-based metabonomic approaches for evaluating physiological influences on biofluid composition. NMR Biomed. 18, 143–162. https://doi.org/10.1002/nbm.935 (2005).
Rundlof, T. et al. Survey and qualification of internal standards for quantification by 1H NMR spectroscopy. J. Pharm. Biomed. Anal. 52, 645–651. https://doi.org/10.1016/j.jpba.2010.02.007 (2010).
Huang, C. H. et al. Fruiting body of Niuchangchih (Antrodia camphorata) protects livers against chronic alcohol consumption damage. J. Agric. Food Chem. 58, 3859–3866. https://doi.org/10.1021/jf100530c (2010).
Lin, T. Y. et al. Metabolite profiles for Antrodia cinnamomea fruiting bodies harvested at different culture ages and from different wood substrates. J. Agric. Food Chem. 59, 7626–7635. https://doi.org/10.1021/jf201632w (2011).
Schwarz, C. V. et al. Developing a learning progression for scientific modeling: Making scientific modeling accessible and meaningful for learners. J. Res. Sci. Teach. 46, 632–654. https://doi.org/10.1002/tea.20311 (2009).
Puiggros, F., Sola, R., Blade, C., Salvado, M. J. & Arola, L. Nutritional biomarkers and foodomic methodologies for qualitative and quantitative analysis of bioactive ingredients in dietary intervention studies. J. Chromatogr. A 1218, 7399–7414. https://doi.org/10.1016/j.chroma.2011.08.051 (2011).
Acknowledgements
We appreciate the grants supported from the Ministry of Science and Technology, Taiwan awarded to Tung-Ying Wu (Grant number: MOST 109-2320-B-276-001-MY2). We are grateful to Prof. Tun-Tschu Chang for kind assistance with species identification.
Funding
This work was supported by grants from the Ministry of Science and Technology, Taiwan awarded to Tung-Ying Wu (Grant number: MOST 109-2320-B-276-001-MY2).
Author information
Authors and Affiliations
Contributions
C.-Y.L.: Writing-original draft, data curation, and formal analysis. Y.-C.W.: Resources, data curation, and supervision. F.-R.C.: Conceptualization, formal analysis, and supervision. M.E.-S.: Formal analysis, writing-reviewing & editing. Y.-C.D.: Methodology, formal analysis, and data curation. C.-Y.L.: Methodology, and data curation. T.-H.D.: Data curation and Supervision. T.-Y.W.: Conceptualization, methodology, investigation, data curation, funding acquisition, resources, writing-reviewing & editing and supervision. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, CY., Wu, YC., Chang, FR. et al. Application of response surface methodology and quantitative NMR for the optimum extraction, characterization, and quantitation of Antrodia cinnamomea triterpenoids. Sci Rep 13, 20265 (2023). https://doi.org/10.1038/s41598-023-47615-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-47615-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.