Abstract
Gynura procumbens is a medicinal herb that contains bioactive compounds that can relieve coughs and prevent liver cancer. Supercritical fluid extraction (SFE) was suggested as one of the techniques that can be used to extract the valuable compounds from the G. procumbens. SFE was widely applied in extracting medicinal ingredients from herbs. However, most of them were performed only at the laboratory scale. Moreover, study to increase the yield performance, economic studies and safety assessments of the SFE process were also performed; however, these tests were conducted individually. Moreover, to date, there is no integration study between all the factors stated for determining the overall performance of SFE with herbs specifically G. procumbens. The integration between all the factors is beneficial because the data on the overall performance can assist in developing the SFE process with G. procumbens at the pilot or industrial scale. Therefore, this study incorporated a multifactor approach to measure the overall performance of the SFE process towards G. procumbens by using a rating and index approach. A summary of factors, such as the solubility of G. procumbens in CO2, operational cost and safety assessment elements, were taken into consideration as the main influences that determine the overall performance index of this study. Iperformance or overall performance of SFE from G. procumbens was successfully assessed and compared with response surface methodology (RSM). Overall, the results from Iperformance exhibit satisfactory solubility values when compared to the optimized value from RSM when considering the lowest operational costs in the safest SFE environment.
Similar content being viewed by others
Introduction
Supercritical CO2 extraction is usually applied to extract valuable compounds, including bioactive compounds from plant structures such as leaves, seeds, fruits and roots1,2,3. In Malaysia, the process of extracting herbs is rapidly developing. This was highly initiated in 2011 during the NKEA agriculture, and in one of the EPP projects, 18 types of herbs were chosen for further development. According to Dionysia 4, Gynura procumbens was the substance least used by traditional medicinal practitioners. G. procumbens, which is easily found in the tropical forests of Malaysia, Thailand and Indonesia, is an herb that contains useful compounds that can be used to relieve coughs, reduce blood glucose levels and prevent and treat liver cancer5. The herb is consumed raw as a salad or ‘ulam’ and can be applied topically. Moreover, G. procumbens contains flavonoids, saponins, tannins, and steroids, which all have potential as antioxidants6. The extracts contain medicinal ingredients, such as kaempferol 3-O-rutinoside, which can treat hypertension7; kaempferol, which is an anti-inflammatory8; and quercetin 3-o glucoside, which can treat diabetes9. Clinical studies were rigorously performed on the extracts, and all of the extracts were obtained by applying conventional and traditional techniques of extraction, such as solvent extraction using ethanol, methanol and water and hot and cold maceration techniques10. SC-CO2 extraction with ethanol–water has yet to be used in extracting valuable compounds from this herb.
The supercritical solvent used in SFE, which is CO2, is efficient in extracting non-polar components, such as terpenes and alkaloids, from plant samples11. Since the targeted compounds are antioxidants that are semi-polar, a co-solvent is introduced to improve the selectivity. For example, a semi-polar co-solvent, such as ethanol, was introduced to enhance the overall quality of the yield12. To date, many researchers have incorporated water into the co-solvent to further enhance the extraction process13,14,15,16. Water can modify the structure of the matrix inside the sample due to its higher viscosity compared to that of CO2 and ethanol and its lower solubility in CO2 compared to that of ethanol17.
Simulation of the extraction curve has been performed rigorously to predict the effect of parameters on the extract yields and to determine the optimum parameter for the best yield using various mathematical model approaches18. Sovová and Stateva19 reviewed types of mathematical models for SFE kinetics. According to them, there are five types of models, including the following: mass balance for solute, extraction steps and their characteristic times, a one-stage model, a model based on the complex structure of plant particles and a model for the SFE of mixtures19. Table 1 summarizes the reported study on applying a mathematical model in fitting the experimental data for the SFE-co-solvent process. The model was then analysed to determine the best operating conditions that produce the highest yield. According to Table 1, the pure ethanol and ethanol–water mixture was the most commonly used co-solvent for SFE. The broken and intact cell model (BIC) model was the most fitted model in fitting the experimental data. The BIC model is usually applied to a mechanically damaged cell sample due to the sample preparation20. The most important parameter for the BIC model was the initial fraction of easily accessible solute, G, in which a value between 0 and 1 was obtained21.
The experiment that obtained the highest yield under optimum conditions was also considered to achieve the best performance by the SFE process. The values of the optimum conditions can be evaluated by using a statistical tool, such as response surface methodology. This tool enables the user to choose the operating conditions, which can be optimized to obtain the highest yield. Moreover, the tool offers a good package that can provide a good design and analysis of the process by applying the statistical significance of all the factors used with analysis of variance. In addition, an artificial neural network is another piece of computing system that has been fully utilized in simulating the results of SFE. The network simulates the results by following the way the human brain analyses and processes information. This software is highly used due to its advantages, including that no thermophysical understanding of the SFE process is needed to conduct the simulation. In addition, previous knowledge about the neural network is not needed35. The ANN structure consists of a multi-layer, fully connected input layer, hidden layer, and output layer. The sensory data (experimental data) fed to the network is interpreted by the machine perceptron, which labels the input data and identifies the numerical patterns.
Table 2 shows the studies that have applied techniques to achieve the highest yield for the SFE process. Several studies have reported the application of two types of extraction techniques to achieve high yields in contrast to the process with SFE alone. For example, when extracting Caryocar Brasiliense, clove buds and Dipteryx alata, cold pressing was used together with SFE to achieve higher yield than that of SFE alone36,37,38. Economic evaluation has been conducted previously, in which the cost of manufacturing (COM) was determined. The calculated COM was compared between the SFE plant at the laboratory scale, pilot scale and industrial scale for production37,38,39. There is also a study focusing on economic assessment to evaluate the feasibility of the SFE process for the purpose of scale-up17. Moreover, there were also assessments of the safety in conducting the SFE process40. Most of these assessments were done separately and independently. None of the evaluations were systematically integrated to measure the overall performance of the SFE process.
To have an idea of the overall performance of SFE, a statistical report to measure the performance is needed. This study tends to consider the incorporation of multiple factors, such as yield, economic factors, and safety, in evaluating the overall performance of the SFE process of G. procumbens during the operation of the system. The results will be represented as an index that will act as an indicator to determine the overall performance of the SFE process.
Materials and method
Sample and chemicals
Gynura procumbens was obtained from a local company, HERBagus Trading Sdn. Bhd which is located in Kepala Batas, Pulau Pinang, Malaysia. The sample received was cleaned and dried in open air for two days followed by drying using an oven at 50 °C until the total moisture content in the sample was less than 10%. The sample was then sieved to obtain a size of 2.0 mm and was stored at room temperature until use. The chemicals used were carbon dioxide (99.5% purity), which was purchased from Alpha Gas (Malaysia), and ethanol (99.8% purity), which was purchased from QRec (Malaysia).
Supercritical fluid extraction
A laboratory scale SFE unit that was designed and installed by a previous researcher was used for this study34. Chiller was first switched on to let the CO2 cool to − 4 °C to let the gas change to the liquid phase before being pumped to the SFE system. Three grams of G. procumbens ground leaves was inserted into the pressure vessel (H/D = 8). The oven was switched on, and the temperature was set to the designated operating temperature. The pressure on the back-pressure regulator was also set to its designated operating pressure. The combination factors for the SFE run for this study are shown in Table 3. The design of this experiment was performed by using a response surface method by Design Expert® (Stat-Ease, USA). Central composite design was utilized with three factors, which were the temperature (°C), pressure (MPa) and water content in ethanol (%). The response was fixed to the overall yield (g/g %) and solubility of G. procumbens in CO2 (g/g %). The α value was chosen as k > 5 with a value of 1.32. There are 20 runs, and the CCD positions of 8 factorial points, 6 axial points and 6 centre points are shown in Table 3.
Optimization using the overall performance index
Economic evaluation
To evaluate the economic element in the SFE of G. procumbens, the methodology by Turton et al. 45 was referred to when estimating the operational costs. Initially, they presented 3 categories to estimate the cost of manufacturing (COM), including the following: direct costs, fixed costs, and general expenses. Since this study focuses on calculating the operational cost, which is tabulated in Table 4, the fixed costs were not taken into consideration. The cost of waste treatment was also excluded since solid waste from SFE can be added to soil for the decomposition process. Therefore, the operational cost consists of the cost of raw materials (CRM), cost of utilities (CUT) and labour cost (COL). The economic parameter that was used to estimate the operational costs (OC) is also shown in Table 4. Therefore, the estimation of OC can be simplified from Turton et al. 45 to Eq. (1) as follows:
where OC is in units of RM/year.
Table 4 shows the description of each category in direct costs. In the raw materials costs (CRM), the price of CO2 dominates the costs. For utility costs (CUT), the use of electricity mostly originates from the equipment in the SFE system, as listed in Table 4.
Safety assessment
-
1.
First stage of the safety assessment.
Two objectives in evaluating the first stage were used to identify the hazard when conducting the SFE G. procumbens experiments and to classify the risk of hazards that can occur (light, moderate, intermediate, heavy, and severe). An analysis of the most hazardous equipment for SFE was conducted. After that, the potential of the second effect from the main scenario analysis was performed.
-
2.
Second stage of safety assessment
A methodology by a previous researcher was used when evaluating the quantitative analysis of safety from SFE of G. procumbens46,47. This study used a mixture of ethanol–water as the co-solvent for the SFE process at a ratio of 10–30% v/v water–ethanol. Therefore, the risk of using the mixed co-solvent was estimated by identifying the boiling point (tb), flash point (fp) and Hansen solubility value (δ) for each of the ratios of water in ethanol. Equation (2) was applied to estimate tb. Equation (3) was used to determine the fp.
Equation (2) was used to determine the value of tb for the mixture. x1 is the mol fraction of solvent 1, x2 is the mol fraction of solvent 2, tb1 is the boiling point for solvent 1 and dan tb2 is the boiling point for solvent 2.
To estimate the flash point of the mixture, Eq. (3) was used.
where xi is the mol fraction, \({\gamma }_{i}\) is the activity coefficient, \({P}_{i,sat}\) is the vapour pressure at T and \({P}_{i,sat FP}\) is the vapour pressure at the flash point.
Equation (4) was used to determine the Hansen solubility value, whereby x1 is the mol fraction of solvent 1, x2 is the mol fraction of solvent 2, D1 is the solubility for solvent 1 and D2 is the solubility for solvent 2.
To estimate the Chemical Safety Total Score (CSTS), several factors need to be taken into consideration, and these factors are listed in Table 5. The factors that need to be determined are flammability, toxicity, reactivity, and explosiveness parameter. The equations used are displayed in Table 6. Then, the parameter was summed up as in Eq. (9) to obtain the value of CSTS.
Overall performance index of SFE G. procumbens
Equation (10) was used to determine the performance index of SFE as follows:
All of the parameters were a function of temperature (T), pressure (P) and water content in ethanol (\(\omega\)) and can be written as \(f\left(T,P,\omega \right)\). The solubility data were taken from RSM. The data for cost can be calculated based on Eq. (1), and safety was obtained from Eq. (9).
Results and discussion
Regression using RSM
Both responses were successfully simulated using RSM, with yield regression using a 2-factor interaction (2FI) and solubility regression by a quadratic model. Table 6 shows the ANOVA by the CCD design and the details of the significant factor and coefficient for each factor for the regression equation.
From Table 6, Prob > F values are significant for both responses, and the values obtained were less than 0.05. Both responses were affected by the individual factors A and C and the interaction factor of A and C. The R2 values above 0.86 for both responses are also reasonable. Therefore, the reported research on applying CCD for the model regression for SFE was also indicated to be reasonable for use in this study48.
Effect of temperature and pressure on the yield and solubility
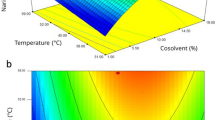
Table 7 shows the yield and solubility results from the experiment. Figures 1 and 2 show the response surface plot for the effect of temperature and pressure on the yield and solubility. Figure 1 shows that at 60 °C, the yield increased with pressure and improved at a higher temperature of 70 °C. This shows that the contact between the solute and solvent is better with pressure. The density of CO2 depends on the pressure and greatly affects the solubility of the solute in the CO249. Figure 2 clearly shows that the contour density is greatly affected by pressure compared to temperature for the solubility of G. procumbens in CO2. In other research, it was reported that when higher pressures are used for SFE, the effect of temperature on density is less noticeable, and the dominant factor is the vapour pressure50. When lower pressures are used, the change in temperature is more pronounced, and the process is dominated by the solvent density51. From here, we can see that there are variations in the solvation power of supercritical CO2 under different operating conditions.
Effect of water content in ethanol on yield and solubility
Figures 3 and 4 show the response surface plot for the effect of water content in ethanol on the yield and solubility at different pressures and water contents in ethanol. The effects of water content in ethanol can clearly be seen at the highest pressure of this experiment, which is 24 MPa. This is because of the enhancement of the solute solubility to the solvent, and this enhancement was influenced by the amount of water inside the ethanol14. When in contact with the sample, water can alter the sample matrix. A previous study reported that, compared to ethanol, water can better penetrate through the cell wall52. Water can also extract more lignin compounds in the secondary cell wall than in the layer between the cells52. This is due to the higher density of water compared to ethanol (Table 8). When this happens, the hole at the surface of the wall opens widely, causing the amount of lignin inside the sample to decrease. Moreover, a report from52 mentioned that carbon dioxide created an acidic environment when reacting with water. This triggers hemicellulose and lignin degradation on the primer cell wall. Therefore, the cell wall is no longer intact because the primer cell wall has been destroyed. Resistance towards the surface tension is also zero. Therefore, more CO2 can penetrate inside the cell to extract solute located at the secondary cell wall.
Figure 3 shows that the highest extract was obtained at 24 MPa and was greater when the water content was increased from 10 to 30% inside ethanol. At low pressure, the water content does not have any effect on the yield obtained. Figure 4 clearly shows that the solubility value does not increase with increasing water content in ethanol. However, effective extraction occurred at the highest pressure of 24 MPa.
Operational cost (OC)
Figure 5 shows the fractions of the operational costs for the SFE of G. procumbens. The highest cost was the utility cost, which was 58% of the total, followed by labour cost (22%) and raw materials cost (20%). The highest contributor to the utility cost came from the electricity cost of the chiller, which utilized 13.82 kW, whereas other equipment, such as pumps, ovens and back-pressure regulators, contributed less than 1 kW each. Attard et al. (2015) reported that the same findings that CUT is the majority of the operational cost of conducting SFE53. The COM was further analysed to determine the distribution of each element (Fig. 6). The price of CO2 dominates the cost of CRM, with nearly 80%, followed by ethanol (20%). Previous research reported that the highest distributor in CRM was the cost of the sample. This is due to the sample supplier charging a high price. Moreover, a previous study was concerned with the higher production rate; therefore, a sample with a high mass was needed for extraction, resulting in higher cost54. The total operational costs were calculated for each parameter of the SFE G. procumbens. Then, it was further ranked to give the Icost value.
Safety assessment
The main equipment in the SFE process that involve risks and hazards are listed in Table 9. Three pieces of equipment with the potential of experiencing overpressure were the CO2 pump, co-solvent pump, and pressure vessel. However, the following pieces of equipment involve a risk of boiling liquid expanding vapour exploration (BLEVE): CO2 storage tanks and pressure vessels. The types of hazards for each of the main equipment are also listed in the table. In this study, chemical hazards can occur with all the main equipment. This is because each of the equipment involves the solvent, which is the chemical in this process.
Table 10 shows the potential of secondary scenarios after the main scenario that can happen due to BLEVE. BLEVE is an explosion due to the failure of the pressure vessel, which is filled with liquid and is unable to withstand a temperature greater than the boiling point temperature at atmospheric pressure. According to Table 10, when overpressure occurs, it acts as a vector for the main scenario, after which a secondary potential scenario can form, such as a flash fire and toxic release. According to a previous study, studies on BLEVE were mostly performed on LPG and propane55. The effect of CO2 is still under review and can be further explored56.
The second safety assessment was conducted to determine the chemical safety total score (CSTS) by calculating each of the factors. According to Fig. 5, the CSTS scores were found to be the highest at 7% water content in ethanol. This is because the volume of ethanol was the largest. The highest portion for each CSTS score originated from the SFL score. This shows that the water content in ethanol does influence the flammability factor of the SFE process. SEXP and STX do not exhibit any variance at different parameters. However, SR has a small influence on CSTS. The CSTS value was then ranked to determine the safety index for this study.
Overall performance index
The overall performance index for this study was determined by using Eq. (10). Individual indices including solubility, cost and safety were summed to obtain the total index, Iperformance. Figure 6 shows the overall performance of SFE G. procumbens at each parameter of the study. In addition, the individual index value was also shown to illustrate the factor that distributed the most for each performance. To choose the best performance from the parameters listed, the aim was to obtain the highest solubility at the lowest operational cost and in the safest environment. From Fig. 6, it can be concluded that the best Iperformance was obtained at 21 MPa, 65 °C and 33% water content in ethanol (v/v).
Table 11 shows the optimum results obtained from the RSM method and the best performance from the Iperformance method. Different parameter values were obtained from both. This was because the assessment for RSM does not consider the operating cost and safety factor when determining the optimum conditions, in which Iperformance integrates multiple factors to determine the best value. Overall, the results from Iperformance exhibit satisfactory solubility values when compared to the optimized value from RSM when considering the lowest operational costs in the safest SFE environment.
Conclusion
The overall performance index method is satisfactory for evaluating the SFE operation with G. procumbens. The results show that the value of optimum solubility from RSM did not differ much from the value obtained by Iperformance. However, a different parameter was chosen, whereby the pressure and temperature were chosen at the centre point and the water content in ethanol was selected at 33% (v/v) for the method by Iperformance. The water content affected the process as well as the safety of the SFE process, especially the flammability factor, SFL. Water, when added to ethanol, altered the matrix sample and assisted the mass transfer process of solute to the solvent (CO2 and ethanol). The economic evaluation reported that the highest cost in operational costs (OC) originated from utility costs (CUT), and the highest contributor was from the chiller. Breakdown of the raw materials costs (CRM) indicates that the cost of CO2 dominates the expense. The results from the safety assessments towards the SFE process imply that there were 2 types of risk that can occur to the pressure vessel, including BLEVE and overpressure. Moreover, a secondary potential scenario can occur when BLEVE is further boosted by overpressure. The solubility results from Iperformance are satisfactory compared to those from RSM. This suggests that the index method by rating of the individual factors of solubility, economy and safety was adequate to recommend the best operating conditions for the highest solubility, as well as for obtaining minimum operational costs and the safest conditions possible.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Adeib, I. S., Norhuda, I., Roslina, R. N. & Ruzitah, M. S. Mass transfer and solubility of hibiscus cannabinus l. Seed oil in supercritical carbon dioxide. J. Appl. Sci. 10, (2010).
Idris, S. A., Markom, M., Abd Rahman, N. & Mohd Ali, J. Prediction of overall yield of Gynura procumbens from ethanol-water + supercritical CO2 extraction using artificial neural network model. Case Stud. Chem. Environ. Eng. 5, 100175 (2022).
Zainuddin, N. A., Tuah, F. & Mohd Yatim, S. R. Supercritical carbon dioxide extraction of oil from Chromolaena odorata leaves. Heal. Scope 1, 152–156 (2019).
M Dionysia, MS Abdul Hayat, M Nik Musaadah, B Intan Nurulhani, MN Madihah, Z Nurul Husna, J Fadzureena, HF Lim, AL Tan, R Rosniza, MA Nor Azah, M Mastura & H Norini, Trend Penggunaan 18 Spesies Tumbuhan Ubatan Di Bawah Program Nkea Di Kalangan Pengamal Perubatan Tradisional Melayu Di Semenanjung Malaysia, Prosiding Persidangan Industri Herba, 7, 170–174 (2015).
Zhang, T., Gu, H.-W., Gao, J.-X., Li, Y.-S. & Tang, H.-B. Ethanol supernatant extracts of Gynura procumbens could treat nanodiethylnitrosamine-induced mouse liver cancer by interfering with inflammatory factors for the tumor microenvironment. J. Ethnopharmacol. 285, 114917 (2022).
Li, J. E., Wang, W. J., Zheng, G. D. & Li, L. Y. Physicochemical properties and antioxidant activities of polysaccharides from Gynura procumbens leaves by fractional precipitation. Int. J. Biol. Macromol. 95, 719–724 (2017).
Shahlehi, S. & Petalcorin, M. I. R. Activation of cholinergic pathway induced vasodilation in rat aorta using aqueous and methanolic leaf extracts of Gynura procumbens. Biomed. Pharmacother. 143, 112066 (2021).
Wong, S. K. et al. Anti-malarial and anti-inflammatory effects of gynura procumbens are mediated by kaempferol via inhibition of glycogen synthase kinase-3β (GSK3β). Sains Malaysiana 44, 1489–1500 (2015).
Chan, C.-H., Yusoff, R., Ngoh, G. & Kung, F. W. Extraction of anti-diabetic active ingredient, quercetin from herbal plant using microwave-assisted extraction (MAE) Technique. in The International Conference on Materials for Advanced Technologies 2–5 (SUNTEC Singapore, 2011). https://doi.org/10.13140/2.1.3487.4885.
Ning, T. J. et al. Inhibitory effects of gynura procumbens ethanolic extract on nitric oxide production and inducible nitric oxide synthase (iNOS) protein expression in macrophages. Sains Malaysiana 48, 1737–1744 (2019).
Guedes, A. R. et al. Extraction of Synadenium grantii Hook f. using conventional solvents and supercritical CO2 + ethanol. J. Supercrit. Fluids 160, 8–10 (2020).
Radzali, S. A., Markom, M. & Saleh, N. M. Co-solvent selection for supercritical fluid extraction (SFE) of phenolic compounds from Labisia pumila. Molecules 25, 1–15 (2020).
Hassim, N., Markom, M., Rosli, M. I. & Harun, S. Scale-up approach for supercritical fluid extraction with ethanol–water modified carbon dioxide on Phyllanthus niruri for safe enriched herbal extracts. Sci. Rep. 11, 1–19 (2021).
Markom, M., Hassim, N., Hasan, M. & Daud, W. R. W. Modeling of supercritical fluid extraction by enhancement factor of cosolvent mixtures. Sep. Sci. Technol. 00, 1–13 (2020).
Idris, S. A. & Markom, M. Effect of water content in Co solvent on yield of supercritical fluid extraction of gynura procumbens leaves. J. Comput. Theor. Nanosci. 17, 1203–1206 (2020).
Mohamed-Mahmood, M., Daud, W. R. W., Markom, M. & Mansor, C. N. A. N. C. Cosolvent selection for supercritical fluid extraction (SFE) of bioactive compounds from Orthosiphon stamineus. Sains Malaysiana 47, 1741–1747 (2018).
Hassim, N., Markom, M., Rosli, M. I. & Harun, S. Scale-up criteria and economic analysis for supercritical fluid extraction of Phyllanthus niruri. Chem. Eng. Process. Process Intensif. 139, 14–22 (2019).
Rai, A. Modeling Techniques in Empirical Supercritical Extraction Designs: Recent Trends and Practices. Innovative Food Processing Technologies Vol. 2 (Elsevier, 2021).
Sovovà, H. & Stateva, R. P. Supercritical fluid extraction from vegetable materials. Rev. Chem. Eng. 27, 79–156 (2011).
Kupski, S. C. et al. Mathematical modeling of supercritical CO2 extraction of hops (Humulus lupulus L.). J. Supercrit. Fluids 130, 347–356 (2017).
Aydi, A. et al. Supercritical CO2 extraction of extracted oil from Pistacia lentiscus L.: Mathematical modeling, economic evaluation and scale-up. Molecules 25, (2020).
Khodaie, F. & Ghoreishi, S. M. Experimental extraction of gallic acid from brown sumac seed (Rhus coriaria) using supercritical carbon dioxide and ethanol as co-solvent: Modeling and optimization. J. Supercrit. Fluids 175, 105266 (2021).
Esquı́vel, M. M., Bernardo-Gil, M. G. & King, M. B. Mathematical models for supercritical extraction of olive husk oil. J. Supercrit. Fluids 16, 43–58 (1999).
Martínez, J., Monteiro, A. R., Rosa, P. T. V., Marques, M. O. M. & Meireles, M. A. A. Multicomponent model to describe extraction of ginger oleoresin with supercritical carbon dioxide. Ind. Eng. Chem. Res. 42, 1057–1063 (2003).
Tan, C. & Liou, D. Modeling of desorption at supercritical conditions. AIChE J. 35, 1029–1031 (1989).
Sovová, H. Rate of the vegetable oil extraction with supercritical CO2—I. Modeling of extraction curves. Chem. Eng. Sci. 49, 409–414 (1994).
Díaz-Reinoso, B., Moure, A. & Domínguez, H. Ethanol-modified supercritical Co2 extraction of chestnut burs antioxidants. Chem. Eng. Process. Process Intensif. 156, 108092 (2020).
Grijó, D. R., Vieitez Osorio, I. A. & Cardozo-Filho, L. Supercritical extraction strategies using CO2 and ethanol to obtain cannabinoid compounds from Cannabis hybrid flowers. J. CO2 Util. 28, 174–180 (2018).
Santos, K. A. et al. Candeia (Eremanthus erythroppapus) oil extraction using supercritical CO2 with ethanol and ethyl acetate cosolvents. J. Supercrit. Fluids 128, 323–330 (2017).
del Valle, J. M., Martín, Á., Cocero, M. J., de la Fuente, J. C. & de la Cruz-Quiroz, R. Supercritical CO2 extraction of solids using aqueous ethanol as static modifier is a two-step mass transfer process. J. Supercrit. Fluids 143, 179–190 (2019).
Sovová, H. Mathematical model for supercritical fluid extraction of natural products and extraction curve evaluation. J. Supercrit. Fluids 33, 35–52 (2005).
Reverchon, E. Mathematical modeling of supercritical extraction of sage oil. AIChE J. 42, 1765–1771 (1996).
Almeida, R. N. et al. Supercritical extraction of Hypericum caprifoliatum using carbon dioxide and ethanol+water as co-solvent. Chem. Eng. Process. Process Intensif. 70, 95–102 (2013).
Markom, M. High-pressure extraction and fractionation of tannins from Phyllanthus Niruri Linn. (dukung Anak). (2007).
Pavlic, B., Bera, O., Vidovic, S., Ilic, L. & Zekovic, Z. Extraction kinetics and ANN simulation of supercritical fluid extraction of sage herbal dust. J. Supercrit. Fluids 130, 327–336 (2017).
Johner, J. C. F., Hatami, T. & Meireles, M. A. A. Developing a supercritical fluid extraction method assisted by cold pressing for extraction of pequi (Caryocar brasiliense). J. Supercrit. Fluids 137, 34–39 (2018).
Hatami, T., Johner, J. C. F., Zabot, G. L. & Meireles, M. A. A. Supercritical fluid extraction assisted by cold pressing from clove buds: Extraction performance, volatile oil composition, and economic evaluation. J. Supercrit. Fluids 144, 39–47 (2019).
Chañi-Paucar, L. O., Osorio-Tobón, J. F., Johner, J. C. F. & Meireles, M. A. A. A comparative and economic study of the extraction of oil from Baru (Dipteryx alata) seeds by supercritical CO2 with and without mechanical pressing. Heliyon 7, e05971 (2021).
de Aguiar, A. C., Osorio-Tobón, J. F., Silva, L. P. S., Barbero, G. F. & Martínez, J. Economic analysis of oleoresin production from malagueta peppers (Capsicum frutescens) by supercritical fluid extraction. J. Supercrit. Fluids 133, 86–93 (2018).
Horvat, G., Aladić, K. & Jokić, S. Supercritical CO2 extraction pilot plant design—Towards IoT integration. Teh. Vjesn. 24, 925–934 (2017).
Vaeli, N., Honarvar, B., Esfandiari, N. & Aboosadi, Z. A. A laboratory study on extracting active ingredients from scrophularia striata boiss using ultrasound-assisted supercritical fluid extraction technique. S. Afr. J. Chem. Eng. 35, 111–117 (2021).
Klein, E. J. et al. Techno-economical optimization of uvaia (Eugenia pyriformis) extraction using supercritical fluid technology. J. Supercrit. Fluids 174, 105239 (2021).
Murias, M. S., del Valle, J. M. & Núñez, G. A. Mathematical simulation of heat and mass transfer during controlled depressurization of supercritical CO2 in extraction vessels. J. Supercrit. Fluids 122, 43–51 (2017).
Zeng, J. & Yang, S. X. Optimal control of supercritical fluid extraction with a hybrid model. in Proceedings of the 2003 IEEE International Symposium on Intelligent Control (2003).
Turton, R., Bailie, R. C., Whiting, W. B. & Shaeiwitz, J. A. Analysis, Synthesis and Design of Chemical Processes, 2008, Pearson Education.
Ahmad, S. I., Hashim, H. & Hassim, M. H. Numerical Descriptive Inherent Safety Technique (NuDIST) for inherent safety assessment in petrochemical industry. Process Saf. Environ. Prot. 92, 379–389 (2014).
Ahmad, S. I. et al. Solvent design and inherent safety assessment of solvent alternatives for palm oil recovery. J. Loss Prev. Process Ind. 65, 104120 (2020).
Manjare, S. D. & Dhingra, K. Supercritical fluids in separation and purification: A review. Mater. Sci. Energy Technol. 2, 463–484 (2019).
Guan, M., Xu, X., Tang, X. & Li, Y. Optimization of supercritical CO2 extraction by response surface methodology, composition analysis and economic evaluation of bamboo green wax. J. Clean. Prod. 330, 129906 (2022).
Rai, A., Bhargava, R. & Mohanty, B. Simulation of supercritical fluid extraction of essential oil from natural products. J. Appl. Res. Med. Aromat. Plants 5, 1–9 (2017).
García-Pérez, J. S. et al. Thermodynamics and statistical correlation between supercritical-CO2 fluid extraction and bioactivity profile of locally available Mexican plants extracts. J. Supercrit. Fluids 122, 27–34 (2017).
Jiang, Y., Feng, Y., Lei, B. & Zhong, H. Impact mechanisms of supercritical CO2–ethanol–water on extraction behavior and chemical structure of eucalyptus lignin. Int. J. Biol. Macromol. 161, 1506–1515 (2020).
Attard, T. M., McElroy, C. R. & Hunt, A. J. Economic assessment of supercritical CO2 extraction of waxes as part of a maize stover biorefinery. Int. J. Mol. Sci. 16, 17546–17564 (2015).
Chañi-Paucar, L. O., Johner, J. C. F., Zabot, G. L. & Meireles, M. A. A. Technical and economic evaluation of supercritical CO2 extraction of oil from sucupira branca seeds. J. Supercrit. Fluids 181, 105494 (2022).
Davidy, A. CFD simulation and mitigation with boiling liquid expanding vapor explosion (BLEVE) caused by jet fire. ChemEngineering 3, 1–22 (2019).
Shang, Z. et al. Experimental investigation of BLEVE in liquid CO2 phase-transition blasting for enhanced coalbed methane recovery. Fuel 292, 120283 (2021).
Acknowledgements
This research work is financially supported by Universiti Kebangsaan Malaysia (GUP-2019-009). The authors would also like to thank the Ministry of Higher Education (MOHE) (FRGS/1/2017/TK02/UKM/02/4) and Universiti Teknologi MARA for financial support and for providing fantastic facilities.
Author information
Authors and Affiliations
Contributions
The manuscript has been constructed and written by S.A.I. under the main supervision and funding acquisition of M.M. with co-supervision by N.A.R. and J.M.A.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Idris, S.A., Markom, M., Abd. Rahman, N. et al. Multifactor assessments to determine the overall performance of supercritical fluid extraction from Gynura procumbens essential oil. Sci Rep 12, 14293 (2022). https://doi.org/10.1038/s41598-022-16773-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-16773-4
This article is cited by
-
Antioxidant, antimicrobial and cytotoxic properties of Diospyros lotus L. essential oil with supercritical fluid extraction
Journal of Food Measurement and Characterization (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.