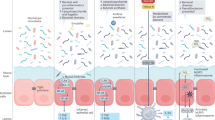
Abstract
Small peptide formulas versus standard polymeric formulas for enteral nutrition in critically ill patients with acute gastrointestinal injury (AGI) have been a topic of debate. A systematic review and meta-analysis were conducted to compare their clinical and nutritional outcomes. Relevant studies from January 1980 to June 2022 were searched in PubMed, Cochrane, and Embase databases. Randomized controlled trials involving AGI grade I-IV patients were included, while children, non-AGI patients, and non-critically ill patients were excluded. Results indicated no significant difference in all-cause mortality. Patients receiving small peptide formulas showed higher daily protein intake, greater albumin growth, and higher prealbumin levels. They also had shorter lengths of stay in the intensive care unit and hospital. Conversely, patients receiving standard polymeric formulas had a higher daily calorie intake. In conclusion, the choice of formula may not affect mortality in critically ill patients with AGI. Small peptide formulas were more conducive to increase daily protein intake, decrease intensive care unit and hospital length of stay. Further large-scale randomized controlled trials evaluating the effects of these two nutritional formulas on clinical and nutritional outcomes in critically ill patients with AGI are needed to confirm these results.
Similar content being viewed by others
Introduction
The gastrointestinal tract is a vital organ that plays a key role in nutrient digestion, absorption, and assimilation1. It is vulnerable for critically ill patients since intestinal integrity is impaired by reduced epithelial cell proliferation, mucous integrity, increased epithelial cell apoptosis, and permeability2. The Working Group on Abdominal Problems of the European Society of Intensive Care Medicine proposed a set of definitions and different grades for gastrointestinal dysfunction. They defined acute gastrointestinal injury (AGI) as a malfunction of the gastrointestinal tract in critically ill patients due to acute illness3. Studies have shown that the prevalence of AGI in critically ill patients was 40%, and the mortality of critical patients with AGI is higher than that of patients without AGI4,5,6, what’s more, the mortality rate increases with AGI grades7. However, the monitoring of gastrointestinal function is limited and overlooked. To improve clinical outcomes, we should pay sufficient attention to reducing gastrointestinal injury in critically ill patients1.
Adequate nutritional support is crucial for restoring cells, organ function, and wound healing in critically ill patients8, especially those with AGI. EN can reduce the incidence of infection, postoperative complications, and the length of hospital stay9,10, maintain small intestinal structure and function11, and promote the restoration of enterocyte mass and create a more optimal microbiota profile compared with parenteral nutrition12,13,14. Nevertheless, AGI patients have digestive and absorption disorders, impaired mucosal barrier function, intestinal flora migration, and increased intestinal vascular permeability3,15,16,17. Therefore, feeding intolerance syndrome often happens in patients with AGI, which may lead to a reduction or even interruption of EN feeding, resulting in malnutrition, and may even affect clinical outcomes18,19,20,21. EN feeding is a double-edged sword for patients with AGI, and it is particularly important to develop a reasonable EN feeding strategy.
Choosing appropriate nutrition formulas is an important part of the EN strategy for AGI patients, which can fully use the advantages of EN. In theory, small peptide formulas have advantages over standard polymer formulas, including increased gastrointestinal tolerance, accelerated gastric emptying, and reduced incidence of diarrhea. Studies have shown that the transport of the bacterial product N-formyl-methionyl-leucyl-phenylalanine (fMLP) in rat colon increases the expression of oligopeptide transporter (PepT1)22,23, which may lead to colonic mucosa damage, the small peptide has competitive inhibition of fMLP transport or greater efficiency of transportation to reduce the expression of PepT1, thus playing a role in intestinal protection24. The advantage of the standard polymer formulas are that they are closer to the physiological conditions of the human body, which can promote the secretion of digestive enzymes, and the slow rate of absorption can also promote protein deposition after meals25. Society of Critical Care Medicine (SCCM) and the American Society for Parenteral and EN suggest considering the use of small peptide formulations in patients with persistent diarrhea, suspected malabsorption, ischemia, or lack of response to fiber26. However, evidence for this recommendation is of low quality and highly subjective, and randomized controlled trials (RCTs) have been documented inconsistently and are very controversial. Therefore, we conducted a meta-analysis, which extracted results from published RCTs to compare the nutritional and clinical outcomes of small peptide formulas and standard polymeric formulas for critically ill patients with AGI.
Methods
Protocol and registration
This systematic review and meta-analysis are reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines27 (Additional file 1: Table S1). The protocol for this meta-analysis is available in PROSPERO (CRD42022332185. Registered 10 May 2022).
Eligibility criteria
Only RCTs were included in this review, and participants were critically ill patients with AGI grade I-IV. Inclusion and exclusion criteria are outlined in Table 1.
AGI criteria
The 2012 ESICM guidelines3 proposed the definition of AGI (the malfunctioning of the gastrointestinal tract in critically ill patients due to their acute illness, additional file: Table S2). They proposed the concepts of primary AGI (associated with a primary disease or direct injury to organs of the gastrointestinal system) and secondary AGI (the consequence of a host response in critical illness without primary pathology in the gastrointestinal system) depending on the cause of AGI. The scale of RCTs using AGI to define patient populations is modest (possible reasons include: trials conducted before 2012, patients without AGI, patients with AGI but without gastrointestinal function assessment, etc.). The full text was screened to assess the study participants’ primary disease, occurrence, and risk of gastrointestinal adverse events. Studies that did not meet AGI criteria were excluded. Considering that the different sources of gastrointestinal injury (first or second hit3) may have different impacts on clinical outcomes, nutritional outcomes, and gastrointestinal adverse events in critically ill patients, we conducted subgroup analysis as follows:
Only secondary AGI: All patients included in the study only had secondary AGI.
Primary and secondary AGI: Some enrolled patients had primary AGI, and the rest had secondary AGI.
Data sources and search strategies
We comprehensively searched articles and references in the PubMed, Embase, and Cochrane Library databases. The literature search was carried out, without language restrictions, from 01 January 1980 to 30 May 2022. The search was slightly adjusted according to the requirements of the different databases. The search strategy (Additional file 1: Table S3) was designed and formulated by the librarians of our college. Two researchers (YQ Wang and YH Li) selected the studies independently; they identified relevant articles by browsing titles and abstracts and then read the full text to decide whether to include them. Any dispute was solved through discussion by a team of other researchers.
Types of outcome measures
The primary outcome was all-cause mortality (28-day mortality, 90-day mortality, and hospital mortality).
The secondary outcomes were daily calorie and protein intake, serum levels of albumin and prealbumin, nitrogen balance, gastrointestinal adverse events (including diarrhea, gastric retention > 500 ml, and vomiting), ICU length of stay, hospital length of stay, infections, and mechanical ventilation duration. Weighted means were calculated based on the number of patients in each study.
Quality assessment
Two reviewers (YQ Wang and YH Li) independently used the Cochrane risk assessment tool to assess the methodological quality of the included trials28. The specific elements were adequacy of the methods used to minimize bias through (1) randomization sequence (selection bias), (2) allocation concealment (selection bias), (3) blinding of study personnel and participants (performance bias), (4) blinding of outcome assessors (performance bias), (5) complete reporting of data without arbitrarily excluded patients and with low to minimal loss to follow-up (attrition bias), (6) selective reporting bias, and (7) other sources of bias. Satisfactory performance, unclear performance, and unsatisfactory performance of each domain from the tool are denoted by green, yellow, and red colours, respectively. Disagreements were solved by a discussion with a third author (Hongxiang Li or D Zhang). The risk of bias summary and graph are presented in Fig. 1.
Statistical analysis
Articles that met the inclusion criteria and did not meet the exclusion criteria for meta-analysis were exported to Review Manager Version 5.3 (RevMan, The Cochrane Collaboration, Oxford, UK) for data analysis. Relative risk (RR) with 95% confidence intervals (CI) was calculated for dichotomous variables. As to the continuous variables, mean difference (MD) and 95% CI was estimated as the effect result. A random-effects model was used to pool studies with significant heterogeneity, as determined by the chi-squared test (P < 0.10) and inconsistency index (I2 ≥ 50%)29. Some of the selected continuous variables were represented by the median (interquartile range). We calculated their mean and standard deviation according to the sample size with a calculator30 and then performed a meta-analysis. A sensitivity analysis was conducted to test the robustness and reliability of all outcomes. Engage Digitizer was used to extract data points from images of graphs. Funnel plot and Egger’s or Begg’s weighted regression tests were applied to identify any potential publication bias and a P value of < 0.05 determined statistical significance for the overall effect of the intervention.
Ethical approval and consent to participate
The protocol for this meta-analysis is available in PROSPERO (CRD42022332185).
Results
Study selection process
The search identified 158 potential trials. Ten additional studies were found during cross-referencing and from the authors’ own reference collections. After removing 37 duplicates, 131 manuscripts underwent title and abstract screening, and 27 trials underwent full-text screening. Details of the study selection process are shown in Fig. 2. Ten RCTs met the inclusion criteria of our review and underwent data extraction. Among these studies, three were conducted in the USA, two were conducted in France, one was conducted in Brazil, one was conducted in Switzerland, one was conducted in the UK, one was conducted in the USA and UK, and one study was conducted in the USA and Canada. Six of these studies were single-centre studies, and four were multicentre studies. The characteristics of trials and their participants are presented in Table 2 and additional file 1: Table S4, respectively31,32,33,34,35,36,37,38,39,40. In addition, we explained how to include studies that met AGI criteria in additional file 1: Table S5. A comparison of the formulas used in trials (Small peptide formulas vs. standard polymeric formulas) is shown in additional file: Table S6.
Primary outcome
Seven trials enrolling 468 patients reported all-cause mortality in small peptide formulas and standard polymeric formulas groups and were included in the meta-analysis. The pooled data showed that all-cause mortality was comparable between small peptide and standard polymeric formula groups (RR = 0.90; 95% CI, 0.64–1.27; P = 0.55; Chi2 = 1.71; I2 = 0%). In our subgroup analysis, there was no difference in the treatment effect between patients with only secondary AGI and those with primary and secondary AGI (subgroup difference test, P = 0.72) (Fig. 3).
Secondary outcomes
Nutritional outcomes
Daily calorie intake
Six trials enrolling 461 patients reported the daily calorie intake in small peptide formulas and standard polymeric formulas groups and were included in the meta-analysis. The pooled data showed that standard polymeric formulas could significantly increase the daily calorie intake compared with small peptide formulas (MD = − 2.65; 95% CI, − 3.66 to − 1.64; P < 0.00001; Chi2 = 16.65; I2 = 70%). In our subgroup analysis, there was no difference in the daily calorie intake between patients with only secondary AGI and those with primary and secondary AGI (subgroup difference test, P = 0.40) (Fig. 4a).
Daily protein intake
Five trials enrolling 435 patients reported the daily calorie intake in small peptide formulas and standard polymeric formulas groups and were included in the meta-analysis. The pooled data showed a higher daily protein intake in the small peptide group compared with the standard polymeric formulas group (MD = 0.09; 95% CI, 0.03 to 0.15; P = 0.002; Chi2 = 25.64; I2 = 84%). In our subgroup analysis, there was no difference in the daily protein intake between patients with only secondary AGI and those with primary and secondary AGI (subgroup difference test, P = 0.87) (Fig. 4b).
Serum levels of albumin
Five trials reported the serum levels of albumin (albumin on the 5th day: three trials enrolling 261 patients; albumin on the 10th day: two trials enrolling 236 patients; albumin variation within 7 days: two trials enrolling 48 patients, respectively) in small peptide formulas and standard polymeric formulas groups, and were included in the meta-analysis. The pooled data showed that there was no statistically significant difference in the albumin on the 5th day and 10th day between small peptide formulas and standard polymeric formulas groups (MD = − 0.03; 95% CI, − 0.12 to 0.07; P = 0.58; Chi2 = 17.12; I2 = 88% and MD = − 0.04; 95% CI, − 0.14 to 0.07; P = 0.47; Chi2 = 0.72; I2 = 0%, respectively). However, the albumin variation within 7 days of the small peptide formulas group was higher than that of the standard polymeric formulas group (MD = 0.28; 95% CI, 0.10 to 0.45; P = 0.003; Chi2 = 0.47; I2 = 0%) (Additional file 1: Figure S1a).
Serum levels of prealbumin
Two trials enrolling 236 patients reported the serum levels of prealbumin in small peptide formulas and standard polymeric formulas groups and were included in the meta-analysis. The pooled data showed that the prealbumin on the 5th day was comparable between small peptide formulas and standard polymeric formulas groups (MD = − 0.05; 95% CI, − 0.17 to 0.08; P = 0.47; Chi2 = 0.01; I2 = 0%). However, the prealbumin on the 10th day of the small peptide formulas group was higher than that of the standard polymeric formulas group (MD = 0.23; 95% CI, 0.06 to 0.40; P = 0.007; Chi2 = 2.73; I2 = 63%) (Additional file 1: Figure S1b).
Nitrogen balance
Three trials enrolling 56 patients reported the nitrogen balance in small peptide formulas and standard polymeric formulas groups and were included in the meta-analysis. The pooled data showed no significant difference between the small peptide formulas and standard polymeric formulas groups in nitrogen balance (MD = − 0.15; 95% CI, − 1.21 to 0.90; P = 0.78; Chi2 = 1.53; I2 = 0%) (Additional file 1: Figure S1c).
Gastrointestinal adverse events
Diarrhea
Seven trials enrolling 431 patients reported the incidence of diarrhea in small peptide formulas and standard polymeric formulas groups and were included in the meta-analysis. The pooled data showed that the incidence of diarrhea was comparable between small peptide formulas and standard polymeric formulas groups (RR = 1.09; 95% CI, 0.83–1.41; P = 0.54; Chi2 = 10.78; I2 = 44%). In our subgroup analysis, there was no difference in the incidence of diarrhea between patients with only secondary AGI and those with primary and secondary AGI (subgroup difference test, P = 0.06) (Additional file 1: Figure S2a).
Gastric retention > 500 ml
Four trials enrolling 352 patients reported the incidence of gastric retention > 500 ml in small peptide formulas and standard polymeric formulas groups and were included in the meta-analysis. The pooled data showed that the incidence of gastric retention > 500 ml was comparable between small peptide formulas and standard polymeric formulas groups (RR = 1.49; 95% CI, 0.93–2.39; P = 0.09; Chi2 = 0.85; I2 = 0%). In our subgroup analysis, there was no difference in the incidence of gastric retention > 500 ml between patients with secondary AGI and those with primary or secondary AGI (subgroup difference test, P = 0.78) (Additional file 1: Figure S2b).
Vomiting
Two trials enrolling 116 patients reported the vomiting rate in small peptide formulas and standard polymeric formulas groups and were included in the meta-analysis. The pooled data showed no significant difference between small peptide formulas and standard polymeric formulas groups in vomiting rate (RR = 1.61; 95% CI, 0.65–3.98; P = 0.31; Chi2 = 0.07; I2 = 0%) (Additional file 1: Figure S2c).
Other clinical outcomes
ICU length of stay
Two trials enrolling 336 patients reported the ICU length of stay in small peptide formulas and standard polymeric formulas groups and were included in the meta-analysis. The pooled data showed that the small peptide formulas were associated with shorter ICU length of stay compared with standard polymeric formulas (MD = − 2.23; 95% CI, − 3.56 to – 0.90; P = 0.001; Chi2 = 3.66; I2 = 18%) (Additional file 1: Figure S3a).
Hospital length of stay
Five trials enrolling 266 patients reported the hospital length of stay in small peptide formulas and standard polymeric formulas groups and were included in the meta-analysis. The pooled data showed that the small peptide formulas were associated with shorter ICU length of stay compared with standard polymeric formulas (MD = − 1.85; 95% CI, − 2.54 to – 1.16; P < 0.0001; Chi2 = 50.60; I2 = 92%). In our subgroup analysis, there was a statistically significant difference in the hospital length of stay between patients with only secondary AGI and those with primary and secondary AGI (subgroup difference test, P < 0.00001). In the only secondary AGI subgroup, there was no statistically significant difference in the hospital length of stay between small peptide formulas and standard polymeric formulas groups (MD = 0.02; 95% CI, − 0.89 to 0.92; P = 0.97; Chi2 = 2.10; I2 = 52%). However, in the primary and secondary AGI subgroup, the small peptide formulas were associated with shorter hospital length of stay compared with standard polymeric formulas (MD = − 4.37; 95% CI, − 5.43 to – 3.32; P < 0.0001; Chi2 = 10.12; I2 = 80%) (Additional file 1: Figure S3b).
Infections
Four trials enrolling 365 patients reported the incidence of infections in small peptide formulas and standard polymeric formulas groups and were included in the meta-analysis. The pooled data showed that the incidence of infections was comparable between small peptide formulas and standard polymeric formulas groups (RR = 0.97; 95% CI, 0.63–1.48; P = 0.87; Chi2 = 1.91; I2 = 0%) (Additional file 1: Figure S3c).
Mechanical ventilation duration
Two trials enrolling 285 patients reported the mechanical ventilation duration in small peptide formulas and standard polymeric formulas groups and were included in the meta-analysis. The pooled data showed that the mechanical ventilation duration was comparable between small peptide formulas and standard polymeric formulas groups (MD = − 0.47; 95% CI, − 1.78 to 0.30; P = 0.16; Chi2 = 0.01; I2 = 0%) (Additional file 1: Figure S3d).
Risk of bias and sensitivity analyses within outcomes
Funnel plots were used to assess publication bias for all outcomes (Additional file 1: Figure S4). According to the one-study-out method, the result of the sensitivity analysis of albumin variation within 7 days was the opposite after excluding the study by Tiengou et al.35, which might be influenced by the small number of included studies and the large difference in sample size between studies. Sensitivity analyses showed that the results of the primary outcome and other secondary outcomes were stable (Additional file 1: Figure S5).
Discussion
This systematic review and meta-analysis of ten trials, including 589 patients, compared clinical outcomes of small peptide formulas with standard polymeric formulas in critically ill patients with AGI. We did not find significant differences in all-cause mortality between the small peptide formulas group and the standard polymer formulas group. On the other hand, while the daily calorie intake of the small peptide formulas group was lower than that of the standard polymeric formulas group, compared with the standard polymer formulas group, the small peptide formulas group had a higher daily protein intake, higher serum levels of albumin elevation within 7 days, higher serum levels of prealbumin on the 10th day, and shorter ICU and hospital length of stay. However, the two groups found no differences in nitrogen balance, the incidence of gastrointestinal adverse events, infection, and mechanical ventilation duration.
The findings of this review and the 7 trials that reported the primary clinical outcomes are consistent with other similar clinical studies, none of which found a difference in the impact of small peptide formulas and standard polymer formulas on mortality in critically ill patients with AGI41,42,43. David et al.42 found that all deaths in their study seem to be related to the patients’ underlying clinical condition but not feeding. Rational selection of EN formulas and optimization of EN feeding strategies may reduce the incidence of gastrointestinal adverse events, improve nutritional status, and increase muscle protein synthesis, but the effect on short-term mortality (< 90 days) is ignorable, and this primary outcome meets our expectations. However, due to the small sample size and heterogeneity among studies (such as differences in feeding regimen, characteristics of study subjects, and study design), the reliability of the results will be reduced, and a large sample size of RCTs is still needed to further verify the impact of nutrition formulas on mortality. Whether there is a long-term clinical outcome measure to evaluate the impact of nutritional formulas on critically ill patients with AGI needs to be further explored.
We did not find a significant difference in calorie density between the two formulas. However, this study found that standard polymer formulas provided higher daily calorie intake for critically ill patients with AGI, which is consistent with Rice et al.38. The possible reasons were as follows: first, the protein density of the standard polymer formulas was lower in these studies37,38,39, so the standard polymer formulas group required higher doses of enteral feeding to achieve the same protein goals. This resulted in higher daily calorie intake in the standard polymer formulas group. Secondly, the higher osmolality of small peptide formulas could promote osmotic diarrhea. Although the study by Carteron et al.39 reported no difference in the incidence of feeding intolerance between the small peptide formulas and standard polymer formulas groups, significantly more patients in the small peptide formulas group required dilution or reduction of the formulas due to severe diarrhea compared with the standard polymer formulas. These two factors may have resulted in a relative reduction in the amount of enteral feeding in the small peptide formulas group compared with the standard polymer formulas group, resulting in lower daily calorie intake. Although higher calorie intake may meet the nutritional needs of critically ill patients, several trials have demonstrated that meeting short-term caloric goals is of little or no significant clinical benefit44,45,46,47. A higher calorie intake in the acute phase of critical illness may increase the burden of mitochondrial oxidative metabolism, cause autophagy disorders, which can lead to persistent cell damage and dysfunction, and Higher mortality48,49,50.
Annika et al.51 showed that three or more gastrointestinal symptoms on the first day in the ICU were independently associated with a threefold increased risk of mortality. Hu et al.19 showed that the persistence of gastrointestinal adverse events during the first week of ICU stay is an independent determinant of mortality. Therefore, reducing the incidence of gastrointestinal adverse events is related to the clinical outcomes of critically ill patients with AGI. Carteron et al.39 reported more patients requiring clinical intervention (Requiring the addition of saline or loperamide) for diarrhea in the small peptide formulas group. The opposite argument had also been made; a RCT performed in multiple ICUs showed42 that pre-digested small peptide formulas reduced the incidence of gastrointestinal adverse events. However, in this study, despite the small peptide formula having higher osmolality, especially in the study by Meredith et al. (490 VS 310 mOsm/L)32, there were no differences in the incidence of diarrhea, gastric retention > 500 ml, and vomiting between the two groups, which was consistent with some studies37,40. High-quality RCTs are still needed to validate the difference between using the two formulas in the incidence of gastrointestinal adverse events.
Small peptide formulas can accelerate protein digestion and absorption from the gut, augment postprandial amino acid availability and have a tendency to increase the incorporation rate of dietary amino acids into skeletal muscle protein52. In addition, the intestinal protective effect of the small peptide formulas has been demonstrated in animal experiments22,23,24. As mentioned above, small peptide formulas may be more suitable for critically ill patients with AGI. Daily protein intake was higher in the small peptide group compared to the standard polymer group, which may be related to the additional whey protein in the formula. Protein reach goal is more critical than calorie requirements for critically ill patients with AGI, considering protein is the most important macronutrient for supporting immune function, healing wounds, and reducing muscle loss53. Studies have shown (Allingstrup et al., 2012) that a higher provision of protein and amino acids is associated with lower mortality compared to higher calories. This study also shows that the small peptide formulas group had a higher elevation in serum levels of albumin during 7 days and higher serum levels of prealbumin on the 10th day. This may be explained that the small peptide formulation can be absorbed without trypsin digestion and has a higher utilization rate52. In addition, small peptide formulation benefits in reduced ICU and hospital length of stay since adequate protein supplementation and absorption can lead to better nutritional status and higher muscle mass (respiratory muscle, skeletal muscle, etc.) in critically ill patients with AGI54. We found an interesting problem, the difference in the hospital length of stay was found in the primary and secondary AGI subgroup, but it was not found in the only secondary AGI subgroup. This outcome was associated with more severe intestinal damage in patients with primary AGI and thus may be more reliant on small peptide formulas. For serum albumin and prealbumin levels, only a few studies have been fully reported, so the results are volatile and further high-quality studies are needed to confirm the results. In the case of critically ill patients with AGI, it appears that standard polymer formulas may not fully exploit the advantages of physiological relevance, while small peptide formulas more suitable for critically ill patients with AGI.
Based on the findings of this study, it is evident that there is a considerable degree of heterogeneity in the meta-analysis, with I2 values exceeding 50% and even surpassing 75% for multiple secondary outcomes. Despite our efforts to minimize heterogeneity through subgroup analyses, significant heterogeneity persisted within subgroups, particularly for the three secondary outcomes: daily calorie intake, daily protein intake, and length of hospital stay. The observed high heterogeneity can be attributed to variations in formula composition across studies, differences in feeding management, variations in the characteristics of study subjects, and disparities in ICU types. These factors contribute to divergent outcomes among the studies analyzed, thereby undermining the robustness of the meta-analysis results. Additionally, there is a potential for publication bias, as certain studies reported positive outcomes despite having inadequate sample sizes to validate clinical findings. To address the issue of heterogeneity, sensitivity analysis was conducted. However, the results of this analysis did not yield significant findings for the majority of the secondary outcomes, suggesting that it did not effectively identify the studies with the highest levels of heterogeneity. In summary, the presence of high heterogeneity undermines the confidence in most of the secondary clinical outcomes of this study. Further research of superior quality is necessary to validate and reinforce the findings presented herein.
There is a degree of controversy about which EN formula should be used in critically ill patients with AGI. The results of some high-quality clinical studies were inconsistent, which will limit the rationalization of EN strategies. We conducted a meta-analysis of the data collected from these high-quality clinical trials to answer this controversial question in clinical practice. Therefore, this study is very necessary and meaningful.
There are several limitations in our meta-analysis. Firstly, the number of included trials was small, and most RCTs included a small number of patients. Further large-scale clinical trials should be conducted to confirm these results. Secondly, three trials did not describe the primary outcome of this study; we reached out to the corresponding author but did not receive a reply. Thirdly, due to the limitation of enrolled trials, our primary outcome of all-cause mortality included 28-day mortality, 90-day mortality, and hospital mortality. It is well known that these mortality rates are not interchangeable, and they depend on the mortality provided in each included trial. Fourthly, the data pertaining to secondary outcomes in certain reports exhibit a significant degree of heterogeneity, with only a limited number of studies providing descriptions for some of these secondary outcomes. As a result, the reliability of the outcomes may be compromised. Therefore, our results should be interpreted with caution. Fifthly, trials with different EN feeding strategies have been excluded from this study. However, there are still differences in feeding strategies among trials, which may result in a certain degree of bias.
Conclusions
The choice of formula may not affect mortality in critically ill patients with AGI. Small peptide formulas were more conducive to increase daily protein intake, decrease intensive care unit and hospital length of stay. Further large-scale randomized controlled trials evaluating the effects of these two nutritional formulas on clinical and nutritional outcomes in critically ill patients with AGI are needed to confirm these results.
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- AGI:
-
Acute gastrointestinal injury
- ICU:
-
Intensive care unit
- EN:
-
Enteral nutrition
- RCTs:
-
Randomized controlled trials
- RR:
-
Relative risk
- CI:
-
Confidence interval
- APACHE II:
-
Acute Physiology and Chronic Health Evaluation II
References
Thompson, A. J. et al. Understanding the role of the gut in undernutrition: What can technology tell us?. Gut https://doi.org/10.1136/gutjnl-2020-323609 (2021).
Klingensmith, N. J. & Coopersmith, C. M. The gut as the motor of multiple organ dysfunction in critical illness. Crit. Care Clin. 32, 203–212. https://doi.org/10.1016/j.ccc.2015.11.004 (2016).
Reintam Blaser, A. et al. Gastrointestinal function in intensive care patients: terminology, definitions and management. Recommendations of the ESICM working group on abdominal problems. Intensive Care Med. 38, 384–394. https://doi.org/10.1007/s00134-011-2459-y (2012).
Li, H., Zhang, D., Wang, Y. & Zhao, S. Association between acute gastrointestinal injury grading system and disease severity and prognosis in critically ill patients: A multicenter, prospective, observational study in China. J. Crit. Care 36, 24–28. https://doi.org/10.1016/j.jcrc.2016.05.001 (2016).
Reintam, A., Parm, P., Kitus, R., Starkopf, J. & Kern, H. Gastrointestinal failure score in critically ill patients: A prospective observational study. Crit. Care 12, R90. https://doi.org/10.1186/cc6958 (2008).
Reintam, A. et al. Gastrointestinal failure in intensive care: A retrospective clinical study in three different intensive care units in Germany and Estonia. BMC Gastroenterol. 6, 19. https://doi.org/10.1186/1471-230X-6-19 (2006).
Zhang, D. et al. Prevalence and outcome of acute gastrointestinal injury in critically ill patients: A systematic review and meta-analysis. Medicine 97, e12970. https://doi.org/10.1097/MD.0000000000012970 (2018).
Casaer, M. P. & Ziegler, T. R. Nutritional support in critical illness and recovery. Lancet Diabetes Endocrinol. 3, 734–745. https://doi.org/10.1016/s2213-8587(15)00222-3 (2015).
Marik, P. E. & Zaloga, G. P. Meta-analysis of parenteral nutrition versus enteral nutrition in patients with acute pancreatitis. Bmj 328, 1407. https://doi.org/10.1136/bmj.38118.593900.55 (2004).
Boelens, P. G. et al. Reduction of postoperative ileus by early enteral nutrition in patients undergoing major rectal surgery: prospective, randomized, controlled trial. Ann. Surg. 259, 649–655. https://doi.org/10.1097/sla.0000000000000288 (2014).
Tappenden, K. A. Mechanisms of enteral nutrient-enhanced intestinal adaptation. Gastroenterology 130, S93-99. https://doi.org/10.1053/j.gastro.2005.11.051 (2006).
Piton, G. et al. Impact of the route of nutrition on gut mucosa in ventilated adults with shock: An ancillary of the NUTRIREA-2 trial. Intensive Care Med. 45, 948–956. https://doi.org/10.1007/s00134-019-05649-3 (2019).
Andersen, S., Banks, M. & Bauer, J. Nutrition support and the gastrointestinal microbiota: A systematic review. J. Acad. Nutr. Diet 120, 1498–1516. https://doi.org/10.1016/j.jand.2020.04.024 (2020).
Andersen, S. et al. Pilot study investigating the effect of enteral and parenteral nutrition on the gastrointestinal microbiome post-allogeneic transplantation. Br. J. Haematol. 188, 570–581. https://doi.org/10.1111/bjh.16218 (2020).
Li, H., Chen, Y., Huo, F., Wang, Y. & Zhang, D. Association between acute gastrointestinal injury and biomarkers of intestinal barrier function in critically ill patients. BMC Gastroenterol. 17, 45. https://doi.org/10.1186/s12876-017-0603-z (2017).
Zhang, Y. et al. Acute cold water-immersion restraint stress induces intestinal injury and reduces the diversity of gut microbiota in mice. Front. Cell Infect. Microbiol. 11, 706849. https://doi.org/10.3389/fcimb.2021.706849 (2021).
Herrera, J., Bockhorst, K., Bhattarai, D. & Uray, K. Gastrointestinal vascular permeability changes following spinal cord injury. Neurogastroenterol. Motil. 32, e13834. https://doi.org/10.1111/nmo.13834 (2020).
Zhang, D., Li, H., Li, Y. & Qu, L. Gut rest strategy and trophic feeding in the acute phase of critical illness with acute gastrointestinal injury. Nutr. Res. Rev. 32, 176–182. https://doi.org/10.1017/S0954422419000027 (2019).
Hu, B. et al. Severity of acute gastrointestinal injury grade is a predictor of all-cause mortality in critically ill patients: A multicenter, prospective, observational study. Crit. Care 21, 188. https://doi.org/10.1186/s13054-017-1780-4 (2017).
Wang, W. N. et al. Optimal time and target for evaluating energy delivery after adjuvant feeding with small bowel enteral nutrition in critically Ill patients at high nutrition risk. Nutrients https://doi.org/10.3390/nu11030645 (2019).
Haines, K. L. et al. Evaluation of malnutrition via modified GLIM criteria for in patients undergoing emergent gastrointestinal surgery. Clin Nutr 40, 1367–1375. https://doi.org/10.1016/j.clnu.2020.08.026 (2021).
Shi, B. et al. Abnormal expression of the peptide transporter PepT1 in the colon of massive bowel resection rat: A potential route for colonic mucosa damage by transport of fMLP. Dig. Dis. Sci. 51, 2087–2093. https://doi.org/10.1007/s10620-005-9067-z (2006).
Ziegler, T. R. et al. Distribution of the H+/peptide transporter PepT1 in human intestine: Up-regulated expression in the colonic mucosa of patients with short-bowel syndrome. Am. J. Clin. Nutr. 75, 922–930. https://doi.org/10.1093/ajcn/75.5.922 (2002).
Nosworthy, M. G. & Brunton, J. A. Cysteinyl-glycine reduces mucosal proinflammatory cytokine response to fMLP in a parenterally-fed piglet model. Pediatr. Res. 80, 293–298. https://doi.org/10.1038/pr.2016.69 (2016).
Boirie, Y. et al. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 94, 14930–14935. https://doi.org/10.1073/pnas.94.26.14930 (1997).
Taylor, B. E. et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically Ill patient: Society of critical care medicine (SCCM) and American society for parenteral and enteral nutrition (ASPEN). Crit. Care Med. 44, 390–438. https://doi.org/10.1097/CCM.0000000000001525 (2016).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. Bmj 339, b2700. https://doi.org/10.1136/bmj.b2700 (2009).
Cumpston, M. et al. Updated guidance for trusted systematic reviews: A new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst. Rev., 10, ED000142. https://doi.org/10.1002/14651858.Ed000142 (2019).
Biggerstaff, B. J. & Jackson, D. The exact distribution of Cochran’s heterogeneity statistic in one-way random effects meta-analysis. Stat. Med. 27, 6093–6110. https://doi.org/10.1002/sim.3428 (2008).
Wan, X., Wang, W., Liu, J. & Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 14, 135. https://doi.org/10.1186/1471-2288-14-135 (2014).
Brinson, R. R. & Kolts, B. E. Diarrhea associated with severe hypoalbuminemia: A comparison of a peptide-based chemically defined diet and standard enteral alimentation. Crit. Care Med. 16, 130–136 (1988).
Meredith, J. W., Ditesheim, J. A. & Zaloga, G. P. Visceral protein levels in trauma patients are greater with peptide diet than with intact protein diet. J. Trauma 30, 825–828. https://doi.org/10.1097/00005373-199007000-00011 (1990).
Mowatt-Larssen, C. A., Brown, R. O., Wojtysiak, S. L. & Kudsk, K. A. Comparison of tolerance and nutritional outcome between a peptide and a standard enteral formula in critically ill, hypoalbuminemic patients. JPEN J. Parenter Enteral. Nutr. 16, 20–24. https://doi.org/10.1177/014860719201600120 (1992).
Heimburger, D. C., Geels, V. J., Bilbrey, J., Redden, D. T. & Keeney, C. Effects of small-peptide and whole-protein enteral feedings on serum proteins and diarrhea in critically ill patients: A randomized trial. JPEN J. Parenter Enteral. Nutr. 21, 162–167. https://doi.org/10.1177/0148607197021003162 (1997).
Tiengou, L. E. et al. Semi-elemental formula or polymeric formula: Is there a better choice for enteral nutrition in acute pancreatitis? Randomized comparative study. J. Parenteral Enteral Nutr. 30, 1–5. https://doi.org/10.1177/014860710603000101 (2006).
de Aguilar-Nascimento, J. E., Prado Silveira, B. R. & Dock-Nascimento, D. B. Early enteral nutrition with whey protein or casein in elderly patients with acute ischemic stroke: a double-blind randomized trial. Nutrition 27, 440–444. https://doi.org/10.1016/j.nut.2010.02.013 (2011).
Jakob, S. M., Bütikofer, L., Berger, D., Coslovsky, M. & Takala, J. A randomized controlled pilot study to evaluate the effect of an enteral formulation designed to improve gastrointestinal tolerance in the critically ill patient-the SPIRIT trial. Crit. Care 21, 2441. https://doi.org/10.1186/s13054-017-1730-1 (2017).
Rice, T. W. et al. Dietary management of blood glucose in medical critically Ill overweight and obese patients: An open-label randomized trial. JPEN J. Parenter Enteral. Nutr. 43, 471–480. https://doi.org/10.1002/jpen.1447 (2019).
Carteron, L. et al. Semi-elemental versus polymeric formula for enteral nutrition in brain-injured critically ill patients: A randomized trial. Crit Care 25, 31. https://doi.org/10.1186/s13054-020-03456-7 (2021).
de Brito-Ashurst, I. et al. Gastrointestinal tolerance and protein absorption markers with a new peptide enteral formula compared to a standard intact protein enteral formula in critically ill patients. Nutrients https://doi.org/10.3390/nu13072362 (2021).
Wang, Y.-Q., Li, Y.-H., Li, Y.-T., Li, H.-X. & Zhang, D. Comparisons between short-peptide formula and intact-protein formula for early enteral nutrition initiation in patients with acute gastrointestinal injury: A single-center retrospective cohort study. Ann. Transl. Med. 10, 573 (2022).
Seres, D. S. & Ippolito, P. R. Pilot study evaluating the efficacy, tolerance and safety of a peptide-based enteral formula versus a high protein enteral formula in multiple ICU settings (medical, surgical, cardiothoracic). Clin. Nutr. 36, 706–709. https://doi.org/10.1016/j.clnu.2016.04.016 (2017).
Endo, A., Shiraishi, A., Fushimi, K., Murata, K. & Otomo, Y. Comparative effectiveness of elemental formula in the early enteral nutrition management of acute pancreatitis: A retrospective cohort study. Ann. Intensive Care 8, 69. https://doi.org/10.1186/s13613-018-0414-6 (2018).
Ochoa Gautier, J. B. & Machado, F. R. Early nutrition in critically ill patients: Feed carefully and in moderation. Jama 309, 2165–2166. https://doi.org/10.1001/jama.2013.4867 (2013).
Rice, T. W. et al. Initial trophic vs full enteral feeding in patients with acute lung injury: The EDEN randomized trial. Jama 307, 795–803. https://doi.org/10.1001/jama.2012.137 (2012).
Arabi, Y. M. et al. Permissive underfeeding or standard enteral feeding in critically Ill adults. N. Engl. J. Med. 372, 2398–2408. https://doi.org/10.1056/NEJMoa1502826 (2015).
Petros, S., Horbach, M., Seidel, F. & Weidhase, L. Hypocaloric vs normocaloric nutrition in critically Ill patients: A prospective randomized pilot trial. JPEN J. Parenter Enteral. Nutr. 40, 242–249. https://doi.org/10.1177/0148607114528980 (2016).
Casaer, M. P. et al. Role of disease and macronutrient dose in the randomized controlled EPaNIC trial: A post hoc analysis. Am. J. Respir. Crit. Care Med. 187, 247–255. https://doi.org/10.1164/rccm.201206-0999OC (2013).
Greig, P. D., Elwyn, D. H., Askanazi, J. & Kinney, J. M. Parenteral nutrition in septic patients: Effect of increasing nitrogen intake. Am. J. Clin. Nutr. 46, 1040–1047. https://doi.org/10.1093/ajcn/46.6.1040 (1987).
Puthucheary, Z. A. et al. Acute skeletal muscle wasting in critical illness. Jama 310, 1591–1600. https://doi.org/10.1001/jama.2013.278481 (2013).
Reintam Blaser, A. et al. Gastrointestinal symptoms during the first week of intensive care are associated with poor outcome: A prospective multicentre study. Intensive Care Med. 39, 899–909. https://doi.org/10.1007/s00134-013-2831-1 (2013).
Koopman, R. et al. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am. J. Clin. Nutr. 90, 106–115. https://doi.org/10.3945/ajcn.2009.27474 (2009).
Taylor, B. E. et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically Ill patient: Society of critical care medicine (SCCM) and American society for parenteral and enteral nutrition (A.S.P.E.N.). Crit. Care Med. 44, 390–438. https://doi.org/10.1097/ccm.0000000000001525 (2016).
Ferrie, S., Allman-Farinelli, M., Daley, M. & Smith, K. Protein requirements in the critically Ill: A randomized controlled trial using parenteral nutrition. JPEN J. Parenter Enteral. Nutr. 40, 795–805. https://doi.org/10.1177/0148607115618449 (2016).
Funding
This work was supported by the Project of Jilin Provincial Department of Finance (Standardized implementation and promotion and application of enteral nutrition feeding process for critically ill patients) and Wu Jieping Medical Foundation (320.6750.2022-13-2).
Author information
Authors and Affiliations
Contributions
Y.Q. Wang searched the scientific literature and drafted the manuscript. Y.H. Li and Y.T. Li contributed to conception, design and data interpretation. Y.Q. Wang and H.X. Li helped to collect the data and performed statistical analyses. D Zhang contributed to conception, design, data interpretation, manuscript revision for critical intellectual content and supervision of the study. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Y., Li, Y., Li, H. et al. Small peptide formulas versus standard polymeric formulas in critically ill patients with acute gastrointestinal injury: a systematic review and meta-analysis. Sci Rep 13, 20469 (2023). https://doi.org/10.1038/s41598-023-47422-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-47422-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.