Abstract
The study aimed at investigating the phytochemical composition, antioxidant and antibacterial activities of essential oils (EOs) of Origanum grossii and Thymus pallidus. The selection of these plants for the study was driven by a comprehensive survey conducted in the Ribat Elkheir region of Morocco, where these plants are widely utilized. The results reflect the valorization of these plants based on the findings of the regional survey. The GC–MS phytochemical analysis revealed that the main constituents of the essential oil were carvacrol and thymol for O. grossii and T. pallidus respectively. Quantitative assays demonstrated that O. grossii exhibited higher levels of polyphenols (0.136 mg AGE/mg EO) and flavonoids (0.207 mg QE/mg EO) compared to T. pallidus. The DPPH assay indicated that O. grossii EOs possessed approximately twice the antiradical activity of T. pallidus, with IC50 values of approximately 0.073 mg/mL and 0.131 mg/mL, respectively. The antibacterial activity tests showed that both essential oils exhibited significant inhibition zones ranging from 26 to 42 mm against all tested bacterial strains. The MIC values varied among the bacteria, generally falling within the range of 0.31 to 2.44 µg/mL, demonstrating the potency of the EOs to serve as antibacterial. Molecular docking revealed that O. grossii and T. pallidus essential oils interact with antibacterial and antioxidant proteins (1AJ6 and 6QME). Key compounds in O. grossii include p-cymene, eucalyptol, and carvacrol, while T. pallidus contains potent chemicals like p-cymene, ɤ-maaliene, valencene, α-terpinene, caryophyllene, himachalene, and thymol. Notably, the most potent chemicals in Origanum grossii are p-cymene, eucalyptol, and carvacrol, while the most potent chemicals in Thymus pallidus are p-cymene, α-terpinene, and thymol. These findings suggest that these plant EOs could be used to develop new natural products with antibacterial and antioxidant activity.
Similar content being viewed by others
Introduction
Historically, naturally occurring substances have been utilized as food additives and have also been explored for their therapeutic potential. The properties which are domiciled in different parts of the plant often draw wide attention, with the essential oils (EO) of aromatic plants being a cynosure in this context1,2. These EOs often possess a surfeit of pharmacological properties including anticancer, anti-inflammatory, antioxidant, insecticidal, and antimicrobial properties among many others. Concomitant with the presence of these properties is the presence of an array of phytochemicals in EOs which confers these potentials. However, the phytochemical composition of EOs is affected by different factors including ecological (geographical origin, soil composition, climate condition), genotype (species, clone, cultivar, ecotype), and technical (elements relating to agriculture, forms of collecting, and crude material storage)3,4. Consequently, EO from similar plant species can express different features and phytochemical composition which could be dependent on or influenced by the previously mentioned factors.
The Lamiaceae family, which consists of a wide variety of herbs, plays a significant role in the Mediterranean region because of its well-known medicinal and aromatic qualities. Two notable members of this family, specifically Origanum grossii (O. grossii) and Thymus pallidus (T. pallidus), epitomize the importance of this family. The Lamiaceae family is distinguished by a common physiological and morphological structure, with a notable feature being the presence of essential oils (EOs). These oils have attracted considerable interest due to their potential therapeutic properties5. The significance of investigating the phytochemical constituents of these herbs is highlighted by this shared characteristic.
The genus Origanum is widely recognized and utilized globally due to its numerous species that hold culinary and medicinal value6. However, O. grossii is considered a distinct entity that has received less attention due to its limited distribution within the Northwest (NW) region of Morocco6,7. The unique nature of its habitat is further emphasized by its preference for high elevations and humid climates within the Western Rif Mountain range8. The species’ specific ecological preferences have resulted in its unique physical characteristics, which include compact stems covered in pubescent hair, branches of different lengths (~ 55 cm), noticeable bracts (3–5 mm), and small leaves (11 mm-5-19)9. The distinctive attributes of O. grossii set it apart from other members of the Lamiaceae family, making it an intriguing subject for further investigation into its bioactive properties.
The Thymus genus, found in the Moroccan landscape, is notable for its significant diversity, boasting around nine endemic species. These plants possess a significant pharmacological value due to their ample presence of bioactive compounds, including phenolic acids, tannins, flavonoids, and resins. These compounds, which are present in both the aerial parts and essential oils, possess unique medicinal properties6,10. Notably, thymol and carvacrol are prominent constituents found in thyme oils. These compounds are recognized for their antioxidant, antiseptic, antibacterial, and antifungal properties11.
Within the domain of traditional healing, T. pallidus, a crucial member of the Thymus genus, assumes a prominent role. The historical use of this substance has included a variety of applications, such as powders and infusions, for the purpose of alleviating gastrointestinal disorders, whooping cough, bronchitis, influenza, and oral infections. The ethnobotanical uses mentioned are in accordance with scientifically supported biological activities, including antibacterial, antifungal, antioxidant, anti-inflammatory, analgesic, and spasmolytic effects12. The distinctive characteristics of T. pallidus highlight its therapeutic potential and align with the well-established reputation of the Lamiaceae family as a source of medicinal compounds.
Antimicrobial resistance (AMR) is a noteworthy issue and poses a significant threat to public health. While several mechanisms including genetic mutations and the acquisition of resistance genes through horizontal gene transfer contribute to the development of AMR, the misuse and overuse of antimicrobial drugs, both in human healthcare and in agriculture, contribute to the development and spread of the resistance13. The effect of AMR has hampered the treatment of infectious diseases, hence leading to increased morbidity, mortality, and healthcare costs. Notably, AMR continues to rise globally, hence, the search for a newer class of antimicrobial drugs remains unabated14. The phytochemical constituents of aromatic and medicinal plants have been to possess antimicrobial properties, with their EOs also possessing the ability to regulate pathogenic bacteria15,16. Within this particular context, it is imperative to undertake a comprehensive examination of the intricate tapestry presented by the various members of the Lamiaceae family, namely O. grossii and T. pallidus. This exploration aims to unravel the unique bioactive characteristics exhibited by these species and their significance within the realms of both traditional and contemporary medicinal practices.
This study has a dual objective: to showcase the value of O. grossii and T. pallidus plants and to explore their potential in combating radicals and bacteria. Specifically, the research aims to evaluate the antimicrobial properties of essential oils (EOs) extracted from these plants. The primary focus involves testing the effectiveness of these EOs against four bacterial strains—Salmonella sp, Streptococcus sp, S. aureus, and E. coli. These strains were isolated from patients' vomit or fecal samples collected at the emergency department of the University Hospital of Fez in Morocco. Through this assessment, the researchers intend to determine the presence of significant antimicrobial and antioxidant activity within these essential oils. The study's findings could shed light on the potential of these EOs as potent agents with antimicrobial and antioxidative properties, offering insights into natural solutions for these challenges.
Materials and methods
Plant material
Fresh leaves of T. pallidus and O. grossii were systematically collected in June 2017 from the region of Ribat EL Kheir located approximately 75 km from the city of Fez, Morocco (33° 49′ north, 4° 25′ west). The identification of the plant specimens was performed by Professor Amina Bari, a botanist affiliated with the Department of Biological Sciences in the Faculty of Science at Sidi Mohammed Ben Abdellah University, Fez, Morocco. Notably, O. grossii is given voucher number A51/08/06/2018/SE, whilst T. pallidus is given A52/08/06/2018/SE. The collected leaves were air-dried under suitable shade conditions to preserve their phytochemical composition. Subsequently, the dried leaf samples were stored in a dry and cool environment at a temperature of 5 °C until they were ready for further analysis or experimentation.
Isolation of essential oils
Under the established protocols outlined in the European Pharmacopoeia17, the EOs were extracted from the air-dried plant materials using a Clevenger-type apparatus for 3 h. The EOs were collected in a sealed vial and subsequently stored at a temperature of 4 °C to ensure their preservation and maintain their quality. The yield of essential oil was determined using the formula:
where the weight of the obtained essential oil refers to the mass of essential oil extracted from the plant material and the weight of the plant material is the initial mass of the plant material used for extraction.
Phytochemical analysis
The analysis of the extracted essential oils was carried out using gas chromatography-mass spectrometry (GC–MS) techniques. The analysis employed fused silica capillary columns, specifically SPB-1 and SupelcoWax-10, as described by reference18. These column types are commonly used for the separation and identification of volatile compounds present in EO17.
Total flavonoids content (TFC) and the total phenolic content (TPC)
The aluminium chloride colorimetric assay was employed to assess the total flavonoid concentration of EOs as described by reference19. The solution was prepared, and the absorbance at 510 nm was measured using a Jasco v-530 spectrophotometer, with a blank sample for comparison. Galic acid was used as the standard to create a calibration curve. The overall quantity of flavonoid content in each sample was expressed in terms of gallic acid equivalents per gram of mg of EO (mg GAE/mg EO).
For the determination of the phenolic content in the samples, the Folin-Ciocalteu method was employed, as outlined by20. The reaction took place at room temperature in the dark for 2 h, and the absorbance at 760 nm was measured using a Jasco v-530 spectrophotometer. Gallic acid was used as the standard for comparison. The total phenol content was expressed in milligrams of gallic acid equivalents per gram of EO (mg GAE/mg EO). Each specimen was evaluated in triplicate to ensure the accuracy and reproducibility of the results.
Antioxidant activity
DPPH radical scavenging activity
The DPPH radical-scavenging ability of the essential oils was assessed using the approach described by21. Noteworthy, this technique was originally developed by Blois in 195822. After a 30-min incubation period at room temperature in the absence of light, the absorbance of the mixture was measured at 517 nm using a Jasco V-530 spectrophotometer. The percentage inhibition was calculated using the following formula:
where I (%): inhibition percentage, as sample absorbance, and A0: represents the absorbance of the blank control.
While BHT was utilized as a positive control in the experiment. The IC50 values were determined based on the concentration of the essential oils that inhibited the DPPH radical by 50%23.
Reducing power capacity
The assessment of reduction power for EO was conducted in accordance with the methodology outlined by24. Quercetin is utilized as the standard. The IC50 values, denoting the concentration at which the absorbance reached 0.5, were determined to quantitatively evaluate the reduction power. To achieve this, a graph was constructed by plotting the absorbance against the corresponding concentration, allowing for the determination of the IC50 value in milligrams per milliliter (mg/mL).
Total antioxidant capacity (TAC test)
The determination of the total antioxidant capacity of the EOs was performed as described by25. The assay was based on the conversion of Mo (VI) to Mo (V) and the subsequent formation of a green phosphate/Mo (V) complex under acidic conditions. The absorbance of the resulting complex was measured at 695 nm using a Jasco v-530 spectrophotometer. The antioxidant activity was expressed in terms of ascorbic acid equivalents (mg AAE/g DW), providing a quantitative measure of the overall antioxidant capacity.
Antibacterial activity
Bacterial strains
In this study, the antibacterial activity of oregano and thyme EO against four bacterial strains, including Gram-positive Staphylococcus aureus and Streptococcus faecalis D, as well as Gram-negative Escherichia coli and Salmonella, provided by the Regional Laboratory of Epidemiological Diagnosis and Environmental Hygiene in Fez, Morocco. The density was changed to correspond to a 0.5 McFarland Standard’s turbidity, equivalent to 1–5 108 CFU per milliliter. Agar disc diffusion analysis26.
Agar well diffusion (AWD) assay
The AWD test was run in triplicate using a modified version of the Kirby-Bauer method27 Standardized suspensions (108 CFU/mL) were used to inoculate Mueller Hinton agar plates before Whatman paper discs (3 mm) were applied to the agar's surface. After that, essential oils were infused into the discs. At 37 °C, all plates were incubated for a day. The widths of the inhibitory zones were measured after incubation using a ruler. By assessing the zones of inhibition against the examined bacterial strains, the antibacterial efficacy was evaluated.
Minimum inhibitory concentration (MIC)
With some modifications, the National Committee for Clinical Laboratory Standards' experiment28 was used to determine the MIC and 96-well plates were used for the test. The different concentrations of oregano EOs and antibiotics were prepared in a suspension containing 0.2% agar in sterile distilled water29, they were carried out using successive 1/2 dilutions of the EOs, ranging from 5000 to 9 µg/mL, and the antibiotics, ranging from 200 to 0.4 µg/mL. The corresponding well of the plate was filled with varying amounts of antibiotics or oregano EO. The concentrations obtained in the wells ranged from 1250 to 2 μg/mL. The presence of bacteria was determined by adding 20 μl of a 10% aqueous solution of 2.3.5-triphenyl tetrazolium chloride to each well. The lowest concentration that doesn’t result in a red hue was designated as MIC26,30.
Molecular docking
The molecular docking methodology utilized a virtual screening process to analyze the crystal structures of different antibacterial and antioxidant proteins from the RCSB protein data bank, specifically PDB IDs 1AJ631 and 6QME32. The decision to select the crystal structures of these proteins for molecular docking methodology was likely driven by their alignment with the study's focus on assessing the antimicrobial and antioxidant activities of essential oils. These protein targets have been chosen based on their relevance to the study’s objectives, potential roles in antibacterial33,34,35 and antioxidant36,37,38 processes as indicated by prior research or literature, structural availability in the RCSB Protein Data Bank, potential for validation and benchmarking, and the feasibility of computational analysis39,40,41,42,43,44. Such protein selection allows for investigating interactions with known proteins that are pertinent to the study’s goals, contributing to a comprehensive understanding of the essential oils’ potential effects. To perform this analysis, various software tools were employed, including MGLtools, Autodock4, Autogrid445, BIOVIA Discovery Studio Visualizer46, Chemdraw Ultra47, and Chemdraw 3D Pro48.
Initially, the protein structures were processed using BIOVIA’s Discovery Studio Visualizer, wherein heteroatoms, co-crystal ligands, and solvent molecules were eliminated. To optimize the protein structure for docking, Autodock tools were utilized, assigning appropriate polar hydrogen and Kollman charges. The resulting optimized protein structure was saved as a pdbqt file49. For the ligands, ChemDraw Ultra was used to draw their structures, followed by energy minimization in Chem 3D Pro. The ligands were then converted to the pdbqt file format using the OpenBabel GUI program50. The structure-based virtual screening was conducted using Autodock4, with each ligand drug docked independently into the active site of each protein. The interactions between the ligands and proteins were visualized using BIOVIA Discovery Studio Visualizer. To validate the results, the root-mean-square deviation (RMSD) value was calculated, and the co-crystal ligand was re-docked. Poses were accepted if both the docking and experimental ligand RMSD values were less than 2.051,52.
Statistical analysis
To analyze the obtained data, the means of the three-way analyses were calculated, and the results were presented as mean ± standard deviation (SD). The IBM SPSS Statistics software version 20.0 was utilized to perform the statistical analysis. The Fisher’s least significant difference (LSD) test and one-way analysis of variance (ANOVA) were employed to assess the statistical significance between different groups at a significance level of P ≤ 0.05.
Plant collection approval
No approval is needed to collect Origanum grossii and Thymus pallidus in Morocco for research purposes.
IUCN policy statement
The collection of plant material complies with relevant institutional, national, and international guidelines and legislation.
Results
Chemical composition of EOs
The extraction of essential oils (EOs) from the leaves of O. grossii and T. pallidus resulted in yields of 3% and 1% (v/w), respectively, on a dry weight basis. The extracted EOs were then subjected to gas chromatography-mass spectrometry (GC–MS) analysis, and the results are presented in Tables 1 and 2.
The GC–MS analysis provided in Table 1 showing detailed insights into the chemical constituents of the Eos from T. pallidus. Key compounds, including p-cymene, γ-terpinene, Sabinene hydrate, linalool oxide, Santolina triene, Borneol, Terpinen-4-ol, Thymol, Caryophyllene, Himachalene, Caryophyllene oxide, and Trans-cadinol, were identified and quantified based on their retention indices, retention times, and respective areas in the chromatogram. Thymol, in particular, dominated the composition of the T. pallidus EO, constituting a significant proportion (86.354%) of the total.
Table 2 outlines the comprehensive profile of phytochemical constituents within the O. grossii EOs. Through GC–MS analysis, specific compounds such as p-cymene, Eucalyptol, γ-terpinene, Cis-sabinene, α-campholenol, Isoborneol, Borneol, Isopulegone, Myrtenyl acetate, Carvacrol, Caryophyllene, Valencene, and γ-Mamliene are identified and quantified. Notably, Carvacrol stands out as the predominant component, constituting a substantial proportion (70.963%) of the total composition. These details enrich our understanding of the chemical composition and potential bioactivity of the O. grossii EO.
The exploration of the phytochemical constituents within the essential oils (EOs) of T. pallidus, as conducted in this study, unveiled a total of 12 compounds, collectively encompassing 94.235% of the total composition. A noteworthy revelation is the prevalence of thymol, which emerged as the principal compound, constituting a substantial majority at 86% (Table 1).
In contrast, the investigation into the phytochemical makeup of O. grossii EO disclosed the presence of 14 distinct compounds that set it apart from T. pallidus. Notably, Carvacrol stood out as the predominant constituent, accounting for a significant proportion of 70% within the EOs (Table 2).
Antioxidant activity
DPPH scavenging activity
The antioxidant activity of the EOs was evaluated using the DPPH scavenging activity assay which is a frequently utilized method for antioxidant activity evaluation. The results presented in Table 3 elucidate the DPPH radical-scavenging potential of O. grossii and T. pallidus EOs. Notably, the EOs of O. grossii exhibited the highest radical-scavenging ability, as evident by its IC50 value of 0.073 ± 0.001 mg/mL, followed by T. pallidus EO with an IC50 value of 0.131 ± 0.002 mg/mL. Interestingly, statistical analysis revealed that O. grossii EO displayed significantly stronger antioxidant activity (P ≤ 0.05) in comparison to the pure reference antioxidant BHT, with an IC50 value of 0.120 ± 0.001 mg/mL.
Ferric reducing power (FRAP)
As depicted in Table 3, the EOs of O. grossii exhibited superior reducing power against ferric ions compared to the EOs of T. pallidus, yielding values of 56.333 ± 1.778mg/mL and 79.333 ± 1.556mg/mL.
Total antioxidant activity (TAC)
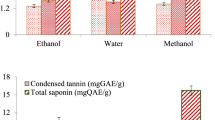
The results, presented in Fig. 1, were quantified in terms of BHT equivalents (mg BHT E/mg EO). Significantly higher TAC values were observed for the EOs pf O. grossii compared to T. pallidus, with recorded values of 0.185 ± 0.005 mg BHT E/mg EO and 0.13 ± 0.004 mg BHT E/mg EO, respectively.
Quantification of total flavonoid content (TFC) and total phenolic content (TPC)
The determination of TFC and TPC for each test sample is elaborated in Table 4. The TFC values were standardized with respect to the reference quercetin, as detailed in the same table. In a similar manner, the TPC outcomes were aligned with the benchmark gallic acid and were expressed as milligrams of gallic acid equivalents per gram of the sample (mg GAE/g of the sample). Noteworthy is the observation that among the samples, O. grossii demonstrated the most substantial TFC and TPC values, exhibiting measurements of 0.207 ± 0.007 mg QE/g EO and 136.66 ± 0.003 mg GAE/g EO, respectively.
Pearson’s correlation analysis
In order to explore the interdependence between the total phenolic and flavonoid contents and the observed antioxidant activity, Pearson’s correlation coefficient analysis was employed in this study, as delineated in Table 5. The outcome of this analysis unveiled noteworthy and statistically significant correlations among these variables.
Antibacterial activity
The outcomes of the agar disc diffusion antibacterial activity test are detailed in Table 6 and illustrated in Fig. 2. The results indicate that both essential oils (EOs) demonstrated significant antibacterial effects against all bacterial strains tested. Notably, the EOs exhibited heightened efficacy against a spectrum encompassing both Gram-positive and Gram-negative bacteria. Specifically, noteworthy antibacterial activity was observed against Salmonella sp., with O. grossii and T. pallidus EOs yielding the most substantial inhibition zone diameters of 42.6 ± 0.88 mm and 36 ± 0.5 mm, respectively. It is worth noting that the efficacy of O. grossii EO and T. pallidus EO against E. coli was comparatively lower.
When contrasting the antibacterial activity of the essential oils (EOs) with that of conventional antibiotics employed as positive controls, it becomes evident that the EOs displayed heightened potency. Through the microdilution technique, the efficacious concentrations of O. grossii and T. pallidus EOs were ascertained to fall within the range of 0.31 to 2.44 µg/mL, as outlined in Table 6. Particularly noteworthy is the concentration of 0.31 µg/mL, which emerged as the most efficacious against Salmonella sp. for both EOs.
Molecular docking
The two macromolecules i.e. antibacterial protein PDB ID: 1AJ6 and antioxidant proteins 6QME were docked with the various types of phytochemical constituents of essential oils obtained from the plants T. pallidus and O. grossii and the results of docking were tabulated in the form of tables.
The phytochemical constituents p-cymene, santolina triene, ɤ-terpinene, sabinene hydrate, linalool oxide, borneol, terpinen-4-ol, thymol, caryophyllene, himachalene, caryophyllene oxide, trans-cadinol was extracted from T. pallidus plants virtually docked with antibacterial 1AJ6 proteins and showed binding affinity of − 5.7, − 4.9, − 5.7, − 5.5, − 5.0, − 4.8, − 5.5, − 4.8, − 5.5, − 5.7, − 5.9, − 6.7, − 6.0, − 5.5 in (Kcal/mol) respectively tabulated in Table 7. The p-cymene showed hydrophobic interaction with amino acids VAL43, VAL120, VAL167, and ILE78 with distances 4.169, 3.982, 4.336, 5.287 (Å), santolina triene was bounded hydrophobically with distances 4.092, 5.232, 5.279 (Å) to amino acid residues ALA47, ILE78, VAL43, ɤ-terpinene showed two types of interaction with 1AJ6, one was electrostatic interaction to amino acid residues ASP49 with bond angle 4.092 (Å) and the other was hydrophobic interaction to amino acid ILE78 with bond distance 5.272 (Å). Sabinene hydrate hydrophobically interacted with amino acid residues ALA47, ILE78, and VAL43 with bond distances 4.092, 5.231, 5.279 (Å), linalool oxide showed two types of interactions, one with GLU50 was bounded through a hydrogen bond with bond distance 2.668 (Å) and ILE78, ILE94 interacted hydrophobically with bond distance 3.745, 3.634. In the Borneol-1AJ6 docking results, amino acid ILE78 was bounded through hydrophobic interaction with bond distance 5.026 (Å) and amino acids GLU50, and ARG76 were bounded through a hydrogen bond with bond distance 2.048, 2.7565 (Å) mentioned in Table 7. The Terpinen-4-ol showed hydrophobic interaction with distances 5.156, 3.772, 4.467(Å) to amino acid residues ILE78, VAL120, and VAL167 showed in. Thymol interacted hydrophobically with amino acids VAL167, ILE78 with bond angles 4.702, 5.038 (Å) and interacted through hydrogen bond to amino acids residues ASP73, ALA47 with bond distances 2.003, 3.473 (Å) tabulated in Table 7. Caryophyllene interacted hydrophobically with residues ARG190, and PHE41 of 1AJ6 with bond distances 5.293, 4.599 (Å); Trans-cadinol interacted hydrophobically with amino acids residues ARG190, LYS189, and ARG190, PHE41 with bond distance 4.997, 3.991, 4.076, 5.137 (Å) tabulated in Table 7. The highest top-ranked docked essential oil himachalene concerning its binding affinity -6.7 (Kcal/mol) has interacted through hydrophobic interaction with amino acids ILE78 with bond distances 4.977, 4.849 (Å) represented in Fig. 3 and Table 7. The second highest essential oil caryophyllene oxide with binding energy − 6.0 (Kcal/mole) bounded hydrophobically to ILE78 with a bond distance of 5.150 (Å) represented in Fig. 4 and Table 7. The two compounds himachalene and caryophyllene oxide have the highest binding score with antibacterial protein 1AJ6 thus they were considered good antibacterial targets.
In this study Table 8 represents interaction of antibacterial protein 1AJ6 phytochemical constituents p-cymene, ɤ-terpinene, Cis-sabinene, Eucalyptol, α-campholenal, isoborneol, borneol, isopulegone, myrtenyl acetate, carvacrol, caryophyllene, ɤ-maaliene, valencene, caryophyllene oxide of essential oils obtained from O. grossii and co-crystallized ligand novobiocin with their binding score − 5.7, − 5.7, − 4.9, − 4.9, − 4.7, − 4.7, − 4.8, − 5.3, − 5.6, − 6.1, − 5.9, − 6.4, − 7.3, − 6.0, − 7.6 (Kcal/mol) respectively showed in Table 8. Some of the constituents of O. grossii such as p-cymeme, ɤ-terpinene, borneol, caryophyllene, caryophyllene oxide were the same as with the constituents of T. pallidus already described above and tabulated in Tables 7 and 8. The remaining constituents like cis-sabinene were hydrophobically bound to amino acid residues of 1AJ6 ILE78, ILE78, and ILE94 with distances 4.349, 3.872, 3.776 (Å); the eucalyptol was bounded hydrophobically to ILE94, ALA100 with bond distance 5.397, 4.991 (Å); α-campholenal, isoborneol and caryophyllene oxide were interacted hydrophobically to ILE78 with bond distance 4.529, 4.878, 5.150 (Å) respectively tabulated in Table 8. The isopulegone has interacted hydrophobically with amino acids VAL120, and VAL167 with distances 4.112, and 4.401 (Å); myrtenyl acetate showed two types of interaction one was hydrophobic to ILE78 with a distance of 2.251 (Å), and one was hydrogen bond interaction to THR165 with distance 5.302 (Å); Carvacrol has interacted through a hydrogen bond to ASP73, ALA47 and hydrophobic bond to ILE78 with bond distance 2.126, 3.562, 5.203 respectively in Table 8.
The highest top ranked constitute valencene with a docking score of − 7.3 (Kcal/mol) has interacted through hydrophobic interaction with amino acids residues ILE78, ILE78, VAL43, and VAL167 with bond distances 4.648, 3.989, 4.628, 4.579 (Å) respectively represented in Fig. 5 and Table 8. The second highest top ranked ɤ- maaliene with a docking score of -6.4 (Kcal/mol) hydrophobically interacted with ILE78 with bond distances 4.594, 4.522 (Å) represented in Fig. 6 and Table 8.
The co-crystallized ligand novobiocin has greater binding affinity 0f. − 7.6 (Kcal/mol) with respect to all the phytochemical constituents of T. pallidus and O. grossii plants have interacted with two types of interactions; one was hydrogen bond interaction to residues THR165, LYS103, ASN46 with bond distance 2.167, 3.419, 4.016 (Å) and the second was hydrophobic interaction to ILE78, GLY77, ILE78, ILE94, PRO79 with bond distance 3.937, 3.879, 4.324, 5.034, 4.994 (Å) respectively represented in Fig. 7 and Table 8.
Table 9 represented the interactions of phytochemical constituents p-cymene, santolina triene, ɤ-terpinene, sabinene hydrate, linalool oxide, borneol, terpinen-4-ol, thymol, caryophyllene, himachalene, caryophyllene oxide, trans-cadinol were extracted from T. pallidus plants virtually docked with antioxidant 6QME proteins and showed binding score of − 5.2, − 4.7, − 5.2, − 5.8, − 6.2, − 6.1, − 5.7, − 5.9, − 7.5, − 7.1, − 7.9, − 7.0 (Kcal/mol). As we have discussed earlier some of the constituents are same for the both plants such as p-cymeme, ɤ-terpinene, borneol, caryophyllene, caryophyllene oxide, the type of interaction with residues and binding affinity were the same after docked with the protein 6QME tabulated in Tables 9 and 10. The p-cymene hydrophobically interacted with ALA366 with a distance of 3.586 (Å); ɤ-terpinene interacted hydrophobically with TYR572, TYR334, TYR334, ALA556 with a distance of 3.625, 4.655, 4.338, 5.320 (Å); Borneol was bounded hydrophobically to ALA366 with bond length 4.791 (Å) while bounded through hydrogen bond interaction to ILE559 with distance 2.177 (Å) in Tables 8 and 9. The highest top-ranked compound Caryophyllene oxide with a binding affinity of − 7.9 and the 2nd highest top-ranked compound Caryophyllene with a binding affinity of − 7.5 hydrophobically interacted with ALA366 with a distance of 4.179, 4.941 (Å) tabulated in Tables 9 and 10 and interactions represented in Figs. 8 and 9
The 3rd highest compound from T. pallidus was Himachalene with binding affinity − 7.1 (Kcal/mole) and showed hydrophobic interaction to ALA366 with bond distance 4.797 (Å) represented in Fig. 10 and Table 9.
The sabinene hydrate interacted hydrophobically with amino acids ALA366, and VAL465 with distances 3.915, and 4.314 (Å); linalool oxide was hydrophically bounded to residue ILE416 with a distance of 1.959 (Å); Terpinen-4-ol also interacted through the hydrophobic bond to ALA366, ILE559 with bond distance 5.141, 5.35 (Å); thymol was interacted by a hydrogen bond to LEU365, ILE416 with distance 2.603, 2.495 while thymol interacted by hydrophobic interaction to residues ALA556, ARG415, ARG415, ALA556 with distance 3.774, 3.968, 5.144, 5.218 (Å); Trans-cadinol was interacted by hydrophobic bond CYS368, ALA466, VAL467, ALA607, CYS368, VAL369, VAL420 with bond distance 5.128, 4.839, 5.439, 4.947, 4.679, 4.952, 4.333 (Å) shown in Table 9.
Table 10 showed the interaction between the phytochemical constituents p-cymene, ɤ-terpinene, cis-sabinene, eucalyptol, α-campholenal, isoborneol, borneol, isopulegone, myrtenyl acetate, carvacrol, caryophyllene, ɤ-maaliene, valencene, caryophyllene oxide of essential oils obtained from O. grossii and co-crystallized ligand J6Q to antioxidant protein 6QME with docking score − 5.2, − 5.2, − 5.1, − 5.8, – 5.5, − 6.1, − 6.1, − 5.7, − 6.6, 5.9, − 7.5, − 7.4, − 7.9, − 7.2, − 8.3 respectively tabulated in Table 10. The cis-sabinene and eucalyptol, myrtenyl acetate, and valencene were bounded by hydrophobic bonds to ALA366 with bond distances 3.586, 5.327, 4.030, 3.831 (Å) respectively shown in Table 10.
The α-campholenal was bounded through hydrogen bond interaction to GLY464 and bounded hydrophobically to ALA366 with distances 2.564, and 4.372 (Å) respectively shown in Table 10. The Isoborneol represented two types of interactions, one was by hydrogen bond to residues ILE416, VAL463, GLY417 with bond distance 2.753, 2.592, 3.561 (Å) and interacted hydrophobically to residues ALA366 with bond distance 5.239 (Å) tabulated in Table 10.
The isopulegone showed the hydrophobic interaction to residues ALA556, and ARG415 with bond distances 3.987, 3.951 (Å); carvacrol was bounded through hydrogen bonds to residues VAL418, and VAL465 with bond distances 3.170, 2.389 (Å) and showed hydrophobic interaction to residues ALA366 with bond distance 3.759 (Å) shown in Table 10.
The first two highest top-ranked constituents of O. grossii with 6QME were already discussed above and represented in Figs. 6 and 8; while the third highest top-ranked compound ɤ-Maaliene with docking score − 7.4 (Kcal/mole) showed hydrophobic interaction to residues ALA366 with bond distance 4.556, 4.727 (Å) represented in Fig. 11 and Table 10.
The co-crystallized ligand J6Q showed two types of interactions, one was hydrogen bond to residues SER508, and SER602 with bond lengths 2.560, 2.152 (Å) while the other was hydrophobic interactions to residues ALA556, TYR525, and TYR525, TYR572, ALA556 with bond distance 3.779, 3.769, 4.388, 5.408, 4.137 with docking score − 8.3 (Kcal/mole) which was closely related to the highest 3 top ranked constituents of O. grossii and T. pallidus plants Fig. 12 and Table 10.
Discussion
The yields of both plants were higher compared to previous studies on similar species, which reported yields of 2% and 0.26% for O. grossii and T. pallidus, respectively9,12. The determination of the chemical constituents and flavonoid concentration of EOs holds considerable significance in the taxonomic classification and differentiation of various thyme species53. Notably, in T. pallidus, thymol emerged as the predominant compound, constituting a substantial proportion of 86% (Table 1). However, an alternative study reported a distinctive composition, with borneol representing the major constituent at 41.67%54. Furthermore, another study on the EOs reported the presence of 15 compounds, with γ-Terpinene emerging as the principal component, comprising 29.6% of the total composition55. For O. grossii, it is worth noting that although the primary component remained consistent, Figueredo56 observed structural variations in the chemical profile of O. grossii EO, suggesting inherent heterogeneity56. This heterogeneity in EO composition within the same species can be attributed to diverse factors, including environmental conditions, geographical location, timing of harvest, and variations in distillation techniques. Notably, climatic factors have been identified as particularly influential contributors57.
Antioxidant activity was evaluated through DPPH scavenging ability, Ion reducing ability and Total Antioxidant Activity. For DPPH activity, noteworthy, limited literature is available concerning O. grossii EO. Conversely, the EOs of T. pallidus exhibited a higher antioxidant activity compared to the findings reported by Laila El Bouzidi (IC50 = 345.11 ± 7.46 μg/mL)55, as well as Thymus vulgaris (IC50 = 0.259 ± 0.476 μg/mL) as reported in58.
As regards the ferric-reducing power capacity assay was employed to investigate the redox-modulating potential of EOs, as well as their ability to neutralize reactive species59. The underlying principle of this assay involves the reduction of hydroxyl radicals generated by the interaction of Fe2 + and H2O2, facilitated by the antioxidants present in the EOs, which subsequently chelate the resulting Fe2 + hydroxyl radicals60. Interestingly, the values of our test surpassed those reported for the EOs of other Origanum and Thymus species, including Origanum Vulgaris, T. satureioides, T. maroccanus, and T. broussonetii EOs, as documented by55,61. However, it is pertinent to note that the antioxidant activity of the tested EOs was notably lower than that of the pure reference antioxidant Quercetin (0.03 g/mL)62,63,64.
The total antioxidant capacity (TAC) of the investigated EOs, reference antioxidants, and BHT was assessed using the phosphomolybdenum method, as detailed by Pilar Prieto et al.25. Noteworthy, the methodology involves the conversion of Mo (VI) to Mo (V) in the presence of an antioxidant compound. The determination of TAC serves as a crucial parameter to evaluate the capacity of a substance to counteract unwanted oxidation processes. It holds biological significance by providing insights into the antioxidative strength exhibited by the studied substance. The antioxidant potential of phenolic compounds is attributed to the presence of hydroxyl groups, which confer their scavenging ability towards free radicals. Notably, a higher content of hydroxyl groups within these compounds corresponds to an enhanced capability to neutralize reactive species21,65.
The antioxidant property of phenolic compounds is closely linked to their structural features, which often include functional groups capable of binding and neutralizing free radicals66. Comparing the TPC and TFC results of T. pallidus in our study to those reported in the literature for Thymus species like T. vulgaris (19.2 ± 0.3 mg GAE/g)66, we observed that T. pallidus exhibited higher values. Moreover, T. daenensis subsp. and T. kotschyanus demonstrated TPC values of 295.93 ± 34.07 mg GAE/g and 337.00 ± 8.31 mg GAE/g, respectively67. Regarding TFC, T. capitatus displayed a flavonoid concentration of 10.62 ± 0.24 mg QE/g68.
Given that phenolic compounds often contain molecules that effectively bind and counteract free radicals, the antioxidative characteristic is inherently linked to its phenolic structure66. Consequently, to evaluate the antioxidant activity of O. grossii and T. pallidus essential oils, we examined the total flavonoid content (TFC) and total phenolic content (TPC) of each test sample. The results of this evaluation are presented in Table 4. TFC results were standardized against quercetin as a reference, and the outcomes were expressed in terms of mg Q E (milligrams of quercetin equivalents) per gram of essential oil, as shown in Table 4. Similarly, the findings of the comparison of TPC against the standard gallic acid were reported as mg GAE (milligrams of gallic acid equivalents) per gram of the sample. Notably, the essential oil from O. grossii exhibited the highest total flavonoid and phenolic content, measuring 0.207 ± 0.007 mg Q E/g EO and 0.136 ± 0.13 mg GAE/g EO, respectively69,70,71.
The analysis of Pearson’s Correlation revealed significant correlations between these variables. The total flavonoid content (TFC) exhibited strong positive correlations with the total antioxidant capacity (TAC) assay (r = 0.977), indicating that the flavonoids present in the samples contribute significantly to their antioxidative effects. Conversely, TFC showed negative linear correlations with the DPPH assay and the FRAP assay (r = − 0.997 and − 0.981, respectively), suggesting an inverse relationship between TFC and the scavenging of DPPH radicals and the reducing power of the samples. Furthermore, the DPPH assay demonstrated the highest correlation with the total phenolic content (TPC) among the antioxidant tests (r = − 0.852), indicating that polyphenols play a crucial role in the observed antioxidant activity measured by the DPPH assay. It is important to note that different phenolic compounds may exhibit varying responses to different antioxidant mechanisms, leading to variations in correlation strengths depending on the specific antioxidant assay employed. These findings highlight the significance of flavonoids and polyphenols in the antioxidant properties of the O. grossii and T. pallidus samples. The correlations observed between the total phenolic and flavonoid contents and the different antioxidant assays provide valuable insights into the specific mechanisms and compounds responsible for the observed antioxidant activity.
The antibacterial activity of O. grossii and T. pallidus EOs was evaluated against four bacterial strains: Salmonella sp, Streptococcus sp, S. aureus, and E. coli, which are known to be responsible for foodborne infections. These bacterial strains were obtained from the Center of the Hospital University of Fez, Morocco, and stored in the regional laboratory of epidemiological diagnosis and hygiene in Fez. The potent antibacterial activity of O. grossii and T. pallidus EOs can be attributed to their rich compositions of carvacrol and thymol, respectively. Previous studies investigating the antibacterial properties of Lippia sidoides EO and its main components, thymol, and carvacrol, have demonstrated their strong inhibitory effects against bacteria and fungi72,73. These findings highlight the potential of O. grossii and T. pallidus EOs as natural antibacterial agents due to their chemical compositions and strong inhibitory effects against both Gram-positive and Gram-negative bacteria74,75. Since Eos’ mechanism of action and its constituents (thymol and carvacrol) are hydrophobic, they may interact with the lipid bilayer of bacterial cytoplasmic membranes, causing a loss of integrity, increasing the fluidity and permeability of the membrane, and allowing cellular components like ions, ATP, and nucleic acids to leak out75.
Conclusions
Our study focused on the antioxidant capacity based on DPPH radical scavenging activity, CAT, FRAP, TPC, and TFC of that O. grossi and T. pallidus, and the antibacterial effect against bacteria species causing food poisoning. The results of this study suggest that the chemicals in O. grossii and T. pallidus plant EOs have antibacterial and antioxidant properties. These findings could lead to the development of new natural products with antibacterial and antioxidant activity. The chemicals in O. grossii that were found to have antibacterial activity were p-cymene, eucalyptol, and carvacrol. These chemicals are known to work by disrupting the cell membranes of bacteria, which can lead to cell death. The chemicals in T. pallidus that were found to have antibacterial activity were p-cymene, α-terpinene, and thymol. These chemicals are also known to work by disrupting the cell membranes of bacteria. The chemicals in both plants that were found to have antioxidant activity were p-cymene, eucalyptus, carvacrol, α-terpinene, and thymol. These chemicals are known to work by scavenging free radicals, which can damage cells. The findings of this study suggest that the chemicals in the studied EOs could be used to develop new natural products with antibacterial and antioxidant activity. These products could be used to treat a variety of conditions, including infections and inflammation.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Mancini, E. et al. Studies on chemical composition, antimicrobial and antioxidant activities of five Thymus vulgaris L. essential oils. Molecules 20, 12016–12028. https://doi.org/10.3390/molecules200712016 (2015).
Vale-Silva, L. et al. Correlation of the chemical composition of essential oils from Origanum vulgare subsp. virens with their in vitro activity against pathogenic yeasts and filamentous fungi. J. Med. Microbiol. 61, 252–260. https://doi.org/10.1099/jmm.0.036988-0 (2012).
Al-Asmari, A. K., Athar, M. T., Al-Faraidy, A. A. & Almuhaiza, M. S. Chemical composition of essential oil of Thymus vulgaris collected from Saudi Arabian market. Asian Pac. J. Trop. Biomed. 7, 147–150. https://doi.org/10.1016/j.apjtb.2016.11.023 (2017).
Javanmardi, J., Khalighi, A., Kashi, A., Bais, H. P. & Vivanco, J. M. Chemical characterization of basil (Ocimum basilicum L.) found in local accessions and used in traditional medicines in Iran. J. Agric. Food Chem. 50, 5878–5883. https://doi.org/10.1021/jf020487q (2002).
Laghmouchi, Y., Belmehdi, O., Senhaji, N. S. & Abrini, J. Chemical composition and antibacterial activity of Origanum compactum Benth. essential oils from different areas at northern Morocco. S. Afr. J. Bot. 115, 120–125. https://doi.org/10.1016/j.sajb.2018.02.002 (2018).
El Hafian, M., Benlandini, N., Elyacoubi, H., Zidane, L. & Rochdi, A. Étude floristique et ethnobotanique des plantes médicinales utilisées au niveau de la préfecture d’Agadir-Ida-Outanane (Maroc). J. Appl. Biosci. 81, 7198. https://doi.org/10.4314/jab.v81i1.8 (2014).
Mazars, G. Pharmacopée traditionnelle du Maroc: Jamal Bellakhdar, La Pharmacopée marocaine traditionnelle. Médecine arabe ancienne et savoirs populaires. Rev. Hist. Pharm. (Paris) 86, 465–466 (1998).
Bakha, M. et al. Genome size and chromosome number for six taxa of Origanum genus from Morocco. Bot. Lett. 164, 361–370. https://doi.org/10.1080/23818107.2017.1395766 (2017).
Bakha, M. et al. Chemical diversity of essential oil of the Moroccan endemic Origanum grosii in natural populations and after transplantation. S. Afr. J. Bot. 124, 151–159. https://doi.org/10.1016/J.SAJB.2019.05.014 (2019).
Belmalha, S., El Idrissi, M., Amechrouq, A. & Echchgadda, G. Chemical characterization of some species of Moroccan middle atlas thyme (Region of Midelt). Glob. J. Pure Appl. Chem. Res. 3, 43–52 (2015).
Benali, T. et al. GC-MS analysis, antioxidant and antimicrobial activities of achillea odorata subsp. pectinata and ruta Montana essential oils and their potential use as food preservatives. Foods https://doi.org/10.3390/foods9050668 (2020).
Ichrak, G. et al. Chemical composition, antibacterial and antioxidant activities of the essential oils from Thymus satureioides and Thymus pallidus. Nat. Prod. Commun. 6, 1507–1510. https://doi.org/10.1177/1934578x1100601025 (2011).
Abushaheen, M. A. et al. Antimicrobial resistance, mechanisms and its clinical significance. Dis. Mon. 66, 100971. https://doi.org/10.1016/j.disamonth.2020.100971 (2020).
Dadgostar, P. <p>Antimicrobial resistance: Implications and costs</p>. Infect. Drug Resist. 12, 3903–3910. https://doi.org/10.2147/IDR.S234610 (2019).
Habbadi, K. et al. Essential oils of Origanum compactum and Thymus vulgaris exert a protective effect against the phytopathogen Allorhizobium vitis. Environ. Sci. Pollut. Res. 25, 29943–29952. https://doi.org/10.1007/s11356-017-1008-9 (2018).
Hazzit, M., Baaliouamer, A., Faleiro, M. L. & Miguel, M. G. Composition of the essential oils of Thymus and Origanum species from Algeria and their antioxidant and antimicrobial activities. J. Agric. Food Chem. 54, 6314–6321. https://doi.org/10.1021/jf0606104 (2006).
European pharmacopoeia : Council of Europe : Free download, borrow, and streaming : Internet archive. https://archive.org/details/europeanpharmaco00euro (Accessed 28 May 2023).
Cavaleiro, C., Salgueiro, L. R., Miguel, M. G. & Proença Da Cunha, A. Analysis by gas chromatography-mass spectrometry of the volatile components of Teucrium lusitanicum and Teucrium algarbiensis. J. Chromatogr. A 1033, 187–190. https://doi.org/10.1016/j.chroma.2004.01.005 (2004).
van Tan, P. The determination of total alkaloid, polyphenol, flavonoid and saponin contents of Pogang gan (Curcuma sp.). Int. J. Biol. 10, 42. https://doi.org/10.5539/ijb.v10n4p42 (2018).
Budini, R., Tonelli, D. & Girotti, S. Analysis of total phenols using the Prussian blue method. J. Agric. Food Chem. 28, 1236–1238. https://doi.org/10.1021/JF60232A056/ASSET/JF60232A056.FP.PNG_V03 (1980).
Wu, W. M. et al. Free radical scavenging and antioxidative activities of caffeic acid phenethyl ester (CAPE) and its related compounds in solution and membranes: A structure-activity insight. Food Chem. 105, 107–115. https://doi.org/10.1016/j.foodchem.2007.03.049 (2007).
Blois, M. S. Antioxidant determinations by the use of a stable free radical. Nature 181, 1199–1200. https://doi.org/10.1038/1811199a0 (1958).
Blois, M. S. Antioxidant determinations by the use of a stable free radical [10]. Nature 181, 1199–1200. https://doi.org/10.1038/1811199A0 (1958).
Oyaizu, M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 44, 307–315. https://doi.org/10.5264/eiyogakuzashi.44.307 (1986).
Prieto, P., Pineda, M. & Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 269, 337–341. https://doi.org/10.1006/abio.1999.4019 (1999).
Dimitrijević, D. Antioxidant and antimicrobial activity of different extracts from leaves and roots of Jovibarba heuffelii (Schott.) A. Löve and D. Löve. J. Med. Plants Res. https://doi.org/10.5897/jmpr12.239 (2012).
Furtado, G. L. & Medeiros, A. A. Single-disk diffusion testing (Kirby-Bauer) of susceptibility of proteus mirabilis to chloramphenicol: Significance of the intermediate category. J. Clin. Microbiol. https://doi.org/10.1128/jcm.12.4.550-553.1980 (1980).
Counts, J. M., Astles, J. R., Tenover, F. C. & Hindler, J. Systems approach to improving antimicrobial susceptibility testing in clinical laboratories in the United States. J. Clin. Microbiol. 45, 2230–2234. https://doi.org/10.1128/JCM.00184-07 (2007).
Remmal, A., Bouchikhi, T., Rhayour, K., Ettayebi, M. & Tantaoui-Elaraki, A. Improved method for the determination of antimicrobial activity of essential oils in agar medium. J. Essent. Oil Res. 5, 179–184. https://doi.org/10.1080/10412905.1993.9698197 (1993).
da Silveira, S. M. et al. Chemical composition and antibacterial activity of Laurus nobilis essential oil towards foodborne pathogens and its application in fresh Tuscan sausage stored at 7 °C. LWT 59, 86–93. https://doi.org/10.1016/j.lwt.2014.05.032 (2014).
Qiu, X. et al. Crystal structure of Staphylococcus aureus tyrosyl-tRNA synthetase in complex with a class of potent and specific inhibitors. Protein Sci. 10, 2008–2016. https://doi.org/10.1110/PS.18001 (2001).
Declercq, J. P. et al. Crystal structure of human peroxiredoxin 5, a novel type of mammalian peroxiredoxin at 1.5 Å resolution. J. Mol. Biol. 311, 751–759. https://doi.org/10.1006/JMBI.2001.4853 (2001).
Qiu, X. et al. Crystal structure of Staphylococcus aureus tyrosyl-tRNA synthetase in complex with a class of potent and specific inhibitors. Protein Sci. 10(10), 2008–2016 (2001).
Gullapelli, K. et al. Synthesis, antibacterial and molecular docking studies of new benzimidazole derivatives. Egypt. J. Basic Appl. Sci. 4(4), 303–309 (2017).
Bouzian, Y. et al. Synthesis, spectroscopic characterization, crystal structure, DFT, molecular docking and in vitro antibacterial potential of novel quinoline derivatives. J. Mol. Struct. 1209, 127940 (2020).
Dincel, E. D. et al. Design, synthesis, biological evaluation, molecular docking, and dynamic simulation study of novel imidazo [2, 1-b] thiazole derivatives as potent antioxidant agents. J. Mol. Struct. 1258, 132673 (2022).
Alminderej, F. et al. Antioxidant activities of a new chemotype of Piper cubeba L. fruit essential oil (Methyleugenol/Eugenol): In Silico molecular docking and ADMET studies. Plants 9(11), 1534 (2020).
Aboul-Soud, M. A. et al. Antioxidant, anti-proliferative activity and chemical fingerprinting of centaurea calcitrapa against breast cancer cells and molecular docking of caspase-3. Antioxidants 11(8), 1514 (2022).
Sharma, D. et al. 4-(4-Bromophenyl)-thiazol-2-amine derivatives: Synthesis, biological activity and molecular docking study with ADME profile. BMC Chem. 13(1), 1–16 (2019).
Othman, I. M. et al. Toward a treatment of antibacterial and antifungal infections: Design, synthesis and in vitro activity of novel arylhydrazothiazolylsulfonamides analogues and their insight of DFT, docking and molecular dynamic simulations. J. Mol. Struct. 1243, 130862 (2021).
Abdipour, M., Younessi-Hmazekhanlu, M. & Ramazani, S. H. R. Artificial neural networks and multiple linear regression as potential methods for modeling seed yield of safflower (Carthamus tinctorius L.). Ind. Crops Prod. 127, 185–194 (2019).
Vadabingi, N. et al. Multiple molecular targets mediated antioxidant activity, molecular docking, ADMET, QSAR and bioactivity studies of halo substituted urea derivatives of α-Methyl-l-DOPA. Bioorg. Chem. 97, 103708 (2020).
Alam, A., Jawaid, T. & Alam, P. In vitro antioxidant and anti-inflammatory activities of green cardamom essential oil and in silico molecular docking of its major bioactives. J. Taibah Univ. Sci. 15(1), 757–768 (2021).
Egbujor, M. C., Okoro, U. C. & Okafor, S. Novel alanine-based antimicrobial and antioxidant agents: Synthesis and molecular docking. Indian J. Sci. Technol. 13(09), 1003–1014 (2020).
Morris, G. M. et al. Software news and updates AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 30, 2785–2791. https://doi.org/10.1002/JCC.21256 (2009).
Accelrys Software Inc. Discovery Studio Visualizer Vol. 2 (Accelrys Software Inc., 2005).
CambridgeSoft. ChemDraw Ultra 12.0 0 (Copyright) 1986 to 2009 (CambridgeSoft Corp., 2009).
CambridgeSoft. Chem 3D Pro 12.0 (Copyright) 1986 to 2009 (CambridgeSoft Corp., 2009).
Ferreira, L. G., Dos Santos, R. N., Oliva, G. & Andricopulo, A. D. Molecular docking and structure-based drug design strategies. Molecules 20, 13384–13421. https://doi.org/10.3390/MOLECULES200713384 (2015).
Zentgraf, M. et al. How reliable are current docking approaches for structure-based drug design? Lessons from Aldose Reductase. Angew. Chem. Int. Ed. 46, 3575–3578. https://doi.org/10.1002/anie.200603625 (2007).
Yusuf, D., Davis, A. M., Kleywegt, G. J. & Schmitt, S. An alternative method for the evaluation of docking performance: RSR vs RMSD. J. Chem. Inf. Model. 48, 1411–1422. https://doi.org/10.1021/ci800084x (2008).
Morris, G. M. et al. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 30, 2785–2791. https://doi.org/10.1002/jcc.21256 (2009).
Lalam, E. O. A., El-akhal, F., Ouedrhiri, W., Ouazzani Chahdi, F. & Greche, H. Composition chimique et activité antibactérienne des huiles essentielles de deux plantes aromatiques du centre nord marocain : Thymus vulagris et Thymus satureioïdis. Les Biotechnol. Lab. 8, 27–33 (2013).
Ramzi, H., Ismaili, M. R., Aberchane, M. & Zaanoun, S. Chemical characterization and acaricidal activity of Thymus satureioides C. & B. and Origanum elongatum E. & M. (Lamiaceae) essential oils against Varroa destructor Anderson & Trueman (Acari: Varroidae). Ind. Crops Prod. 108, 201–207. https://doi.org/10.1016/j.indcrop.2017.06.031 (2017).
El Bouzidi, L. et al. Chemical composition, antioxidant and antimicrobial activities of essential oils obtained from wild and cultivated Moroccan Thymus species. Ind. Crops Prod. 43, 450–456. https://doi.org/10.1016/j.indcrop.2012.07.063 (2013).
Figueredo, G. Etude chimique et statistique de la composition d’huiles essentielles d’origans (Lami-aceae) cultivés issus de graines d’origine méditerranéenne, Université Blaise Pascal - Clermont-Ferrand II (2007).
Amarti, F. et al. Composition chimique, activité antimicrobiennne et antioxydante de l’huile essentielle de Thymus zygis du Maroc. Phytotherapie 9, 149–157. https://doi.org/10.1007/s10298-011-0625-6 (2011).
Aazza, S., Lyoussi, B. & Miguel, M. G. Antioxidant and antiacetylcholinesterase activities of some commercial essential oils and their major compounds. Molecules https://doi.org/10.3390/molecules16097672 (2011).
Saraswati, P. E., Giriwono, D., Iskandriati, C. P. & Tan, N. Andarwulan, in-vitro anti-inflammatory activity, free radical (DPPH) scavenging, and ferric reducing ability (FRAP) of Sargassum cristaefolium lipid-soluble fraction and putative identification of bioactive compounds using UHPLC-ESI-ORBITRAP-MS/MS. Food Res. Int. 137, 109702. https://doi.org/10.1016/j.foodres.2020.109702 (2020).
Wu, N. et al. Antioxidant activities and xanthine oxidase inhibitory effects of extracts and main polyphenolic compounds obtained from geranium sibiricum L. J. Agric. Food Chem. 58, 4737–4743. https://doi.org/10.1021/jf904593n (2010).
Sarikurkcu, C. et al. Composition, antioxidant, antimicrobial and enzyme inhibition activities of two Origanum vulgare subspecies (subsp. vulgare and subsp. hirtum) essential oils. Ind. Crops Prod. 70, 178–184. https://doi.org/10.1016/j.indcrop.2015.03.030 (2015).
Hao, R., Roy, K., Pan, J., Shah, B. R. & Mraz, J. Critical review on the use of essential oils against spoilage in chilled stored fish: A quantitative meta-analyses. Trends Food Sci. Technol. 111, 175–190. https://doi.org/10.1016/J.TIFS.2021.02.054 (2021).
Radha Krishnan, K. et al. Antimicrobial and antioxidant effects of spice extracts on the shelf life extension of raw chicken meat. Int. J. Food Microbiol. 171, 32–40. https://doi.org/10.1016/j.ijfoodmicro.2013.11.011 (2014).
Vallverdú-Queralt, A. et al. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 154, 299–307. https://doi.org/10.1016/j.foodchem.2013.12.106 (2014).
C. Biology, Total antioxldant capacity Grzegorz Bartosz Department of Molecular Biophysics, University of L6di, L6di, Poland ; and Department of Biochemistry, 2423 (2003).
Yu, Y. M., Chao, T. Y., Chang, W. C., Chang, M. J. & Lee, M. F. Thymol reduces oxidative stress, aortic intimal thickening, and inflammation-related gene expression in hyperlipidemic rabbits. J. Food Drug Anal. 24, 556–563. https://doi.org/10.1016/j.jfda.2016.02.004 (2016).
Nickavar, B. & Esbati, N. Evaluation of the antioxidant capacity and phenolic content of three Thymus species. JAMS J. Acupunct. Meridian Stud. 5, 119–125. https://doi.org/10.1016/j.jams.2012.03.003 (2012).
Jabri-Karoui, I., Bettaieb, I., Msaada, K., Hammami, M. & Marzouk, B. Research on the phenolic compounds and antioxidant activities of Tunisian Thymus capitatus. J. Funct. Foods 4, 661–669. https://doi.org/10.1016/j.jff.2012.04.007 (2012).
Proestos, C., Chorianopoulos, N., Nychas, G. J. E. & Komaitis, M. RP-HPLC analysis of the phenolic compounds of plant extracts. Investigation of their antioxidant capacity and antimicrobial activity. J. Agric. Food Chem. 53, 1190–1195. https://doi.org/10.1021/jf040083t (2005).
Sebai, H. et al. Lavender (Lavandula stoechas L.) essential oils attenuate hyperglycemia and protect against oxidative stress in alloxan-induced diabetic rats. Lipids Health Dis. https://doi.org/10.1186/1476-511X-12-189 (2013).
Hyun, T. K., Kim, H. C. & Kim, J. S. Antioxidant and antidiabetic activity of Thymus quinquecostatus Celak. Ind. Crops Prod. 52, 611–616. https://doi.org/10.1016/j.indcrop.2013.11.039 (2014).
Botelho, M. A. et al. Antimicrobial activity of the essential oil from Lippia sidoides, carvacrol and thymol against oral pathogens. Braz. J. Med. Biol. Res. 40, 349–356. https://doi.org/10.1590/S0100-879X2007000300010 (2007).
De Guimarães, L. G. L., Da Silva, M. L. M., Reis, P. C. J., Costa, M. T. R. & Alves, L. L. General characteristics, phytochemistry and pharmacognosy of Lippia sidoides. Nat. Prod. Commun. 10, 1861–1867. https://doi.org/10.1177/1934578x1501001116 (2015).
Abass Bnyan, I., Tariq Abid, A. & Naji Obied, H. Antibacterial activity of carvacrol against different types of bacteria. J. Nat. Sci. Res. 4, 13–16 (2014).
Lambert, R. J. W., Skandamis, P. N., Coote, P. J. & Nychas, G. J. E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 91, 453–462. https://doi.org/10.1046/j.1365-2672.2001.01428.x (2001).
Funding
This work is financially supported by the Researchers Supporting Project number (RSP-2023R437), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Conceptualization, Z.H.; Methodology and software, Z.H., F.S.; validation, L.A., B.F.Z. and L.Y.; formal analysis, A.A.; writing—original draft preparation, Z.H.; writing—review and editing, Z.H. and L.Y.; supervision, T.M. and G.A.; project administration, A.A. Writing—original draft preparation, reviewing and editing, data validation, and data curation: F.S., M.B., A.M.S., H.-A.N. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zejli, H., Fitat, A., Lefrioui, Y. et al. Phytochemical analysis and biological activities of essential oils extracted from Origanum grossii and Thymus pallidus: in vitro and in silico analysis. Sci Rep 13, 20021 (2023). https://doi.org/10.1038/s41598-023-47215-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-47215-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.