Abstract
Longitudinal data sets for population abundance are essential for studies of imperiled organisms with long life spans or migratory movements, such as marine turtles. Population status trends are crucial for conservation managers to assess recovery effectiveness. A direct assessment of population growth is the enumeration of nesting numbers and quantifying nesting attempts (successful nests/unsuccessful attempts) and emergence success (number of hatchlings leaving the nest) because of the substantial annual variations due to nest placement, predation, and storm activity. We documented over 133,000 sea turtle crawls for 50.9 km of Florida Gulf of Mexico coastline from 1982 to 2021 for a large loggerhead turtle nesting aggregation and a recovering remnant population of green sea turtles. Over time both species have emerged to nest significantly earlier in the year and green sea turtle nesting seasons have extended. Nest counts and hatchling production for both species have significantly increased, but the rate of emergence success of hatchlings leaving nests has not changed for loggerheads and has declined for green sea turtles. Sea level rise and coastal developments undoubtedly influence coastal habitats in the long-term, impacting nest site selection and potential recruitment from the loss of emerged hatchlings. However, the present indications for steady Gulf of Mexico recovery of loggerhead and green sea turtles counter findings of the Florida Atlantic coasts. This study indicates that effective conservation practices can be detected within time scales of 1–2 turtle generations.
Similar content being viewed by others
Introduction
Long term data sets and analyses are essential to conservation plans of a species1,2,3. In ideal situations, observations of behavior can be combined with census counts of individuals4,5 thus allowing researchers to quantify how populations change over time. For many species, especially those that are threatened or endangered, these changes may be difficult to observe6,7 and proxies for direct observation must be used instead. This is especially true for animals that migrate from foraging grounds to breeding grounds8,9,10 and might not be easily accessible year-round, such as marine turtles.
Marine turtles are one of the few reptiles that migrate long-distances between foraging areas and breeding areas11,12. Observational data linked with mark-recapture histories indicate that migration cycles are resource driven and food availability can affect the time-frame between breeding seasons13,14,15. Remigration cycle shifts may influence recruitment rates, possibly impacting a population’s growth over time. Some demographic studies of marine turtles occur in-water through mark-recapture techniques16,17,18, but most estimates of population size are made during the breeding season from evidence of female tracks at nesting beaches. Females, their nests, and the resulting hatchlings are accessible on the beach during the nesting season and provide a baseline for population trends19,20,21. Marine turtles reach sexual maturity later than other reptiles and have a long lifespan22,23,24,25 reducing the likelihood that changes within a population are immediately apparent. Long term beach monitoring data can provide a reference point for the health of that breeding population26,27,28 and by coupling nest counts with reproductive success data researchers can predict future issues stemming from population fitness changes29.
The prevailing theory of marine turtle population structure suggests that data from one beach may not be enough to see the full picture of a breeding population30. Marine turtle genetic population structure is driven by female natal philopatry31 and gene flow occurs throughout the region as males travel along the coast toward their breeding areas32,33. More recent studies propose that population subdivision creates pockets of subpopulations30,34,35. It is therefore critical to document monitoring for more than a decade of contiguous nesting beaches to assess a region’s population trend36,37.
Marine turtles are threatened globally. In some regions they are hunted for their meat and shells, their eggs are poached, and turtles can be caught as bycatch38,39,40. Pollution in the ocean increases the likelihood of plastic ingestion or death and boat strikes are prevalent near populated areas41,42. Nesting beaches are at risk from armoring/sand nourishment activities, their nests are at risk of predation, and hatchling disorientation or misorientation from artificial lighting43,44,45. However, relative abundance research provides conservation managers with tools to better protect these animals. The loggerhead sea turtle (Caretta caretta) nesting aggregation on the Northwest Atlantic Ocean is the largest in the world46, and 90% of these clutches are laid in Florida47,48. Green sea turtle (Chelonia mydas) nesting populations have been rapidly increasing in Florida since 199849,50; these increases have resulted in the down-listing of this species from “endangered” to “threatened”51. Much of the research on these and other species of marine turtles occurs on the East coast of Florida, where nesting density is high52,53,54. Few studies have focused on Florida’s Gulf of Mexico coastline28,55,56, despite the growing nesting populations in this region. Programs that have monitored marine turtle nesting over time are invaluable to identifying trends of how populations have changed through time27,57,58.
The primary goal of this study was to determine how nesting counts and hatchling production have changed over time and to quantify how nesting turtles on these beaches are contributing to the greater Gulf of Mexico marine turtle populations. The secondary goal was to assess how the different beaches contribute to the population and to identify regions that are more or less productive to inform regional conservation managers of these trends.
Methods
Study sites
Since 1982, the Mote Marine Laboratory Sea Turtle Conservation and Research Program (MML STCRP) has standardized annual monitoring for 50.9 km of nesting habitat across six beaches in Sarasota County, Florida. With 40+ years of continuous data, STCRP is uniquely placed to identify trends in the growing population and to estimate how this population’s breeding behavior has changed over time. Sarasota County hosts the largest nesting aggregation of loggerheads within the Gulf of Mexico and is an excellent representation of the growth of nesting in the region59,60.
During the nesting season (April–October), MML STCRP staff and volunteers monitor sea turtle nesting during morning patrols on six beaches in Sarasota County. The majority of these barrier islands are considered to be critically eroded61, threatening suitable nesting habitat and residential property. Residents of these coastal communities have attempted to safeguard their beaches through beach nourishment and/or implementing coastal armoring techniques. These activities can negatively impact sea turtle nesting behavior62,63,64,65. Each beach varies in length, sand color and sand grain, armoring, nourishment, and profile (Table 1, Fig. 1).
Nesting beaches monitored by MML STCRP in Sarasota Florida. North to South they are Longboat Key, Lido Key, Siesta Key (North and South), Casey Key and Venice. Beaches vary in physical parameters (sand type, nourishment, obstructions, armoring) and these differences are highlighted in Table 1.
Coordinated citizen science nesting surveys
All beaches monitored by MML STCRP fall under the Statewide Nesting Beach Survey (SNBS) program under the Florida Fish and Wildlife Conservation Commission (FWC). Siesta Key is also part of a standardized data collection program called the Index Nesting Beach Survey (INBS). These two programs are designed to assess the distribution and relative abundance of sea turtle nesting across Florida to inform beach managers of coastal development issues and promote the recovery of sea turtle populations57,66. Survey data are collected through permit holders, but the core of the data could not be collected without the help of trained volunteers who patrol the beaches to identify and document sea turtle ocean emergences. One such volunteer group: Longboat Key Turtle Watch (LBKTW) started quantifying nesting activity in 1969 and was a pioneering example for other long-term projects in the region. Since 2005, volunteers log an average of 19,000+ hours every nesting season.
Annual daily beach monitoring was conducted from April 15 (May 1st prior to 2016) through October 31, or 3 days after the final hatchling emergence. Permitted personnel traveled the shoreline at dawn above the mean high-water line to document turtle nesting activity. Using Florida Fish and Wildlife’s marine turtle guidelines67, each activity was classified as a nest or a non-nesting emergence (also known as a false crawl) and the species was identified via crawl pattern. Nest counts were defined as the number of crawls that emerged from the ocean that resulted in the laying of a clutch of eggs within the sand before the turtle returned to the ocean. A non-nesting emergence was defined as an emergence that did not result in egg deposition. Nesting success was calculated as the proportion of emergences that resulted in a nest in relation to the total number of crawls and served as an indicator of a beach’s nesting suitability68,69,70,71,72:
Nesting density is an index of nest distribution and is used as a metric for conservation success when assessed over time73,74:
Most clutches were left in situ, but clutches laid during active nourishment projects were relocated by 9 a.m. the morning after deposition—eggs were transported to a location higher on the beach and placed into an artificially built nest chamber that closely resembled the original nest chamber in shape, size, and depth. Additionally, regulations reflecting the potential negative impacts of nest relocations were not set prior to 1987 and many nests were relocated to protective “hatcheries”.
Turtle crawl locations were documented in relation to the nearest Florida Department of Environmental Protection (FDEP) range monument to the north and, beginning in 2004, with global positioning system (GPS). Starting in 1997, personnel measured the distance from the nest or false crawl apex to the closest upland vegetation/barrier and from the nest or false crawl apex to the day’s mean high-water line. By adding these two values together, total beach width was estimated. These measurements were used to assess nest site selection in relation to the lower, middle, or upper thirds of the beach, regardless of beach width.
All nests prior to 2013 were monitored: eggs in the clutch were verified, nest sites were marked with stakes, and nest sites were checked daily for depredation, disorientation/misorientation, wash-overs/inundation, wash outs and hatchling emergences. By 2013, the nesting population had grown too large to monitor and stake every nest. Following communication with FWC, MML STCRP shifted to a new sampling protocol based on turtle species and beach nesting density. On moderate to high density non-nourished beaches all loggerhead clutches laid on Wednesdays were verified and thereafter monitored; all nests on low density beaches, all rare species [green, Kemp’s ridley (Lepidochelys kempii), leatherback (Dermochelys coriacea), and potential hybrid] nests, research nests, relocated nests, and all nests in nourishment areas were monitored.
Nest depredation occurred when a native or non-native organism preyed on incubating sea turtle eggs and hatchlings. In Sarasota County, depredation occurs from native predators (raccoons, armadillos, ghost crabs, coyotes) and from non-native predators (fire ants). Depredation events included predators digging into a nest and removing or damaging nest contents, subsequently impacting hatchling production. From 1982 to 2020, to mitigate depredation, self-releasing cages and/or screens were installed at all verified rare species nest sites and some loggerhead nest sites in high depredation areas.
After a monitored nest hatched and the hatchlings emerged, MML STCRP determined whether the hatchlings disoriented/misoriented en route to the Gulf. If a hatchling is disoriented, they will wander on the beach without direction (typically from skyglow), and if a hatchling is misoriented they typically travel to an artificial source of light and not the ocean75. Disorientation or misorientation events can be caused by natural and artificial lighting76 and can have an impact on hatchling survival77. MML STCRP documents every disorientation/misorientation event, but their impact on hatchling survival is not fully described here.
Incubation duration was assessed by subtracting the date hatchlings emerged from the nest by the date the nest was laid. MML STCRP staff excavated the nest three days following an observed emergence. If an emergence was not noted, excavations occurred after 70 days of incubation (80 days on North Siesta Key - where cooler sand temperature extends the incubation duration, and for all leatherback nests). During an excavation, nest contents were classified by counting hatched eggshells, hatchlings, and remaining unhatched eggs78. This method has not changed over the course of the study reducing error58. These counts were used to determine the emergence success of the nest. Emergence success quantified how many hatchlings exited the nest chamber and made it to the surface:
Emergence success was calculated for all nests from 1982 to 2012, but only for monitored nests from 2013 to 2021 (see above). To remove potential bias from relocations, only in situ nests were included in the emergence success calculations. Partial depredations and partial washouts were removed from overall success calculations because we could not ascertain how many eggs were lost. Full depredations and full washouts were included as zero (0) success because the entire clutch was removed from productivity. By compiling these data, the annual hatchling production was assessed using regression analysis by beach and year. Two values are provided here: (1) a minimum number of hatchlings confirmed from all excavations from 1982 to 2021 and (2) a higher estimated number of hatchlings if MML STCRP had monitored and excavated every nest post-2013 (a further explanation can be found in Supplementary Information A).
Data standardization and analysis
All data were assessed and analyzed between 1982 and 2021. All analyses were performed on loggerhead and green data sets. MML STCRP also identified nests and false crawls from Kemp’s ridleys, leatherbacks and loggerhead/hawksbill hybrids (confirmed by genetics, B. Shamblin pers comm.), but the total number of these rare sea turtles nesting in the region remains too low to make inferences about overall nesting behavior. Further, adjustments were made to the beach protocol over time (see above); thus, not all parameters could be compared and analyzed throughout the timeframe. Nest counts, nesting density, nesting success, and incubation duration were all compared for the full 40-year monitoring timeframe (emergence success for 35 years). Nest location placement was only compared for the period 1997–2021. A separate data set removing beaches by the year that they were nourished was analyzed and the results are presented in Supplementary Information B.
All analyses were run in Program R 4.0.379, all figures were made using package ggplot280, and all maps were made using QGIS 3.1681. All data were tested for normality (Shapiro–Wilk or Kolmogorov–Smirnov tests) and homoscedasticity (Levene’s test). Most data were non-normal and most tests were non-parametric. To identify if there were differences in nest counts, nesting density via beach or year, generalized linear models were assessed and post-hoc Dunn tests were performed (package dunn.test)82. Siegel nonparametric linear regression (package mblm)83 was used to determine if first and last nest dates had changed over the course of the study and for analysis of incubation duration and emergence success over time. Both nesting success and emergence success are proportions that range between values of 0 and 1. Modified beta regression models (package betareg)84 were run to explore whether nesting success or emergence success were affected by abiotic factors including Year, Beach Width, Date of Activity and Latitude (proxied by FDEP monument). Finally, Kruskal–Wallis tests were used to compare nest site selection in relation to the spatial placement on the beach.
Results
MML STCRP documented 133,957 marine turtle crawls from 1982 to 2021; 48.8% of these crawls resulted in nests (65,321). Of those, 99.0% (64,692) were laid by loggerheads. There were 608 green sea turtle nests, ten Kemp’s ridley nests, five leatherback nests and two known loggerhead/hawksbill hybrid nests.
Nesting counts for loggerheads and rare species have increased since monitoring began (Fig. 2). The nest count data for both loggerheads and green sea turtles were not normally distributed (Shapiro–Wilk: loggerheads: W = 0.71, p < 0.001; green sea turtles: W = 0.42, p < 0.001) and residuals were non-normal for both species. No other species data were analyzed due to small sample size. For loggerheads, there were significant differences in nest counts over time and by beach (χ2 = 84.59, df = 39, p < 0.001; χ2 = 142.83, df = 5, p < 0.001 respectively). Post-hoc tests revealed that Casey Key had significantly higher nest counts than the other beaches and Lido Key and North Siesta Key had significantly fewer nests than the other beaches. For green sea turtles, even though the nesting population was growing there was no significant difference in nest counts over time (χ2 = 28.03, df = 26, p = 0.36). However, green sea turtle nesting counts were significantly higher on Casey Key than on all of the other beaches (χ2 = 16.91, df = 5, p < 0.001).
Species phenology, density, and success
The earliest loggerhead clutch was laid on April 20 and the latest was laid on September 30, and for green sea turtles: May 21 and September 19, respectively. Emergences resulting in a nest have significantly shifted earlier in the year for both loggerheads (V = 37, df = 38, p < 0.001) and for green sea turtles (V = 76.5, df = 22, p = 0.037; Fig. 3). Nesting season has not significantly shifted longer for loggerheads (V = 437, df = 38, p = 0.104), but has shifted significantly later for green sea turtles (V = 298, df = 22, p < 0.001).
Nesting density for both loggerheads and green sea turtles were not normally distributed (W = 0.79, p < 0.001; W = 0.49, p < 0.001) and neither were the residuals. For loggerheads, nesting density (x̄: 30.7 nests/km) significantly increased over time and was significantly different between beaches (χ2 = 96.12, df = 39, p < 0.001; χ2 = 129.54, df = 5, p < 0.001; Fig. 4). Loggerhead nesting density was highest on Casey Key and lowest on North Siesta Key. For green sea turtles, nesting density (x̄: 0.822 nests/km) increased over time—but not significantly (χ2 = 30.43, df = 25, p = 0.21); however, there were significant differences by beach (χ2 = 16.36, df = 5, p = 0.01). Post-hoc tests determined that green sea turtles prefer to nest on Casey Key and South Siesta Key and have never nested on North Siesta Key.
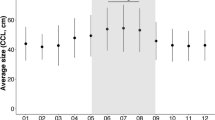
Loggerhead nesting density per km over time for the period 1982–2021. Nesting density significantly increased over time (the grey band represents the 95% confidence interval). Grey dots represent outliers from those years. Southern beaches had higher densities than northern beaches, resulting in high variation between the beaches from year to year.
Nesting success for both species was not normally distributed (loggerheads: W = 0.97, p < 0.001; green sea turtles: W = 0.91, p < 0.001) and neither were their residuals. Loggerhead nesting success declined over time, but not significantly (x̄: 0.49 ± 0.14; χ2 = 51.57, df = 39, p = 0.09). Loggerhead nesting success significantly varied between beaches (χ2 = 68.39, df = 5, p < 0.001). Nesting success was highest on South Siesta Key (0.57) and lowest on North Siesta Key (0.38). Nesting success for green sea turtles varied widely (x̄: 0.53 ± 0.33), but it did not significantly vary by year (χ2 = 30.967, df = 30, p = 0.42), nor by beach (χ2 = 4.577, df = 5, p = 0.47), despite the lack of nesting on North Siesta Key.
Species incubation and emergence success
The average incubation duration and emergence success for loggerheads across all beaches was 58.9 days and 52.1%, respectively (a full breakdown by beach is in Supplementary Information). Over the course of the study, neither incubation duration, nor emergence success were normally distributed (Kolmogorov–Smirnov: D = 1, p < 0.001; D = 0.5, p < 0.001 respectively), and neither were their residuals. Loggerhead incubation duration has significantly increased over time (V = 741, df = 37, p < 0.001) and was significantly different between beaches (χ2 = 113.39, df = 5, p < 0.001). The longest average incubation duration was on North Siesta Key (77.6 days in 2015) and the shortest average on Venice (50.9 days in 1994). Loggerhead emergence success decreased over time—but not significantly (V = 310, df = 38, p = 0.181), but emergence success did significantly vary between beaches (χ2 = 54.50, df = 5, p < 0.001). Casey Key had the highest average emergence success (61.1%) and North Siesta Key the lowest (28.3%). Over the course of the study, the minimum number of loggerhead hatchlings that emerged from these nests was 2,180,700. Using multiple regression models (outlined in Supplementary Information A), a higher value of 3,078,811 is estimated. Casey Key produced the most hatchlings annually (x̄: 24,000–33,500 per year, see Fig. 5).
Hatchlings emerged from 484 of the 608 green sea turtle nests (79.6%), resulting in 40,580 hatchlings. The average incubation duration was 58.4 days, and the average emergence success was 63.7%. Over the course of the study, neither incubation duration nor emergence success were normally distributed (W = 0.954, p < 0.001; W = 0.803, p < 0.001 respectively), nor were their residuals. Incubation duration has significantly increased over the course of the study (V = 226, df = 20, p = 0.001) and incubation duration significantly varied between beaches (χ2 = 45.87, df = 4, p < 0.001). Lido Key had a longer average incubation duration than both South Siesta Key and Venice. Green sea turtle emergence success has significantly decreased over time (V = 6, df = 21, p < 0.001). However, there was no significant difference in emergence success between beaches (χ2 = 6.69, df = 4, p = 0.15). There were 320 Kemp’s ridley hatchlings that emerged from nests (incubation x̄: 63.8 days, emergence success x̄: 27.3%) and 165 loggerhead/hawksbill hybrid hatchlings (incubation x̄: 54 days, emergence success x̄: 50%). None of the five leatherback nests hatched.
Effects of nest site selection on nest success
Only statistically significant values are explained below; full model breakdowns are in Supplementary Information.
For loggerheads, nesting success significantly increased from north to south (z = − 7.75, p < 0.001) and significantly decreased as the beach widened (z = − 12.84, p < 0.001). Nesting success was also significantly different when these terms interact (z = 7.28, p < 0.001). For green sea turtles: nesting success significantly decreased from north to south (z = − 2.41, p = 0.02) and significantly decreased as the beach widened (z = − 1.99, p = 0.046, Fig. 6). Emergence success for loggerheads significantly increased from north to south (z = 2.68, p = 0.007). Emergence success significantly decreased over the course of the nesting season (z = − 2.45, p = 0.014) and significantly decreased over the course of the study (z = − 2.69, p = 0.007). For green sea turtles, emergence success significantly increased over the course of the study (z = 2.45, p = 0.014); significantly increased from north to south (z = 2.05, p = 0.04), as the beach widened (z = 3.92, p < 0.001), and significantly decreased over the course of the season (z = − 2.71, p = 0.007).
For both species, nest site selection differed between the upper, middle, and lower portions of the beach (by width, Table 2). Loggerheads significantly preferred to nest in the upper portion of the beach—nearer to the dune (χ2 = 962.92, df = 2, p < 0.001), but they were significantly more successful at nesting in the middle (χ2 = 419.05, df = 2, p < 0.001). Loggerheads had a significantly higher emergence success in the upper portion of the beach (χ2 = 208.62, df = 2, p < 0.001), and a significantly lower incubation duration in the lower part of the beach (χ2 = 101.72, df = 2, p < 0.001). Green sea turtles significantly preferred to nest in the upper part of the beach near or in the dune (χ2 = 58.64, df = 2, p < 0.001), were significantly more successful at nesting in the upper portion (χ2 = 9.02, df = 2, p = 0.01), and had significantly lower emergence success in the lower portion (χ2 = 11.65, df = 2, p < 0.001). There was a significant difference in incubation duration in relation to location on the beach (χ2 = 6.06, df = 2, p = 0.048), nests in the upper portion of the beach had a higher average incubation duration.
During some years, storm activity and high surf effectively removed the lower and middle portions of the beach over the course of the season. To account for this spatial difference, separate models were run to determine if nest placement, nesting success, emergence success, and incubation duration were impacted by the continuous variable of distance to the upper barrier or dune. For loggerheads, nest placement was significantly more likely to be closer to the upland barrier (t = − 18.77, df = 9786, p < 0.001), nesting success was significantly greater closer to the upland barrier (z = − 28.33, df = 9787, p < 0.001); emergence success significantly declined further from the upland barrier (z = − 12.72, df = 5628, p < 0.001, Fig. 7), and incubation duration significantly increased further from the upland barrier (t = 5.29, df = 4935, p < 0.001). For green sea turtles, nest placement was significantly more likely closer to the upland barrier (t = − 7.03, df = 466, p < 0.001), but nesting success did not differ (z = − 1.28, df = 466, p = 0.20); emergence success significantly declined farther from the upland barrier (z = − 1.99, df = 235, p = 0.473), and incubation duration significantly declined farther from the upland barrier (t = − 2.75, df = 223, p = 0.006).
Discussion
Although loggerhead nesting counts have increased across Florida beaches59, evidence of a significant increase in the overall number of breeding females in the past 30 years is lacking66, hampering a strong recovery of the overall population. Few studies have looked at nesting trends on the Florida Gulf of Mexico coastline and their impact on the larger breeding population. Recent research in the Northern Gulf of Mexico suggests a reduction in the number of nests85. To the south on Keewaydin Island, although nest counts have increased, their hatchling production has declined28. Further, little information has been published about the slow recovery of green sea turtles in the Gulf of Mexico following the overharvesting of the species in the 1800’s86,87. However, the data presented in this paper shows that nest counts in Sarasota County have rapidly grown for both species, encouraging recovery. Beaches in Florida have partnered with FWC for their SNBS program since 1979 and the INBS program since 1989. For over 40 years, citizen scientists have provided data for the management of these beaches. Conservation efforts in this region appear to be effective and are needed.
One of the contributing factors of increased growth may be due to the implementation of turtle excluder devices (TEDs) on shrimp trawling vessels in the 1990s. Reducing bycatch invariably expands the number of sea turtles reaching sexual maturity—loggerheads reach sexual maturity between 27 and 38 years13,24 and green sea turtles reach sexual maturity between 19 and 44 years22,88,89. Thus increasing the number of potential nesters for future generations. There was an estimated 60% reduction in bycatch of all species of sea turtles in the Atlantic following the TED regulation (mortality: 94% reduction90). The usage of TEDs and the increased awareness in the region have provided 1–2 generations worth of potential nesters to aid in recruitment and growth of the population. Additionally, these increased recruitment rates could be due to ocean currents in the Gulf of Mexico.
Recruitment to sea turtle nesting aggregations is strongly influenced by proximity to the Gulf Stream System (GSS), which affects overall population structure91. Putman and colleagues modelled that the GSS accounted for 90% of the spatial variation in regional nesting density on the Atlantic and may explain why rookeries on the east coast of Florida will continue to dramatically increase in recruitment. In the Gulf of Mexico, the Loop Current passes near the western coast of Florida before joining the GSS92, and may explain increased nesting in the Sarasota County region in relation to the rest of the Florida rookeries on the Gulf of Mexico. From 2016 to 2021, loggerhead nesting counts in Sarasota County (which includes beaches monitored by MML STCRP and the Coastal Wildlife Club) accounted for 44.4% of all nesting on Florida beaches on the Gulf of Mexico and 50.1% for greens59,60. Genetically, the loggerhead population nesting in the region is distinct from management units to the north (St. Georges Island, Florida) and to the south (Keewaydin Island, Florida)93 and the central west region is one of the fastest growing nesting populations in Florida66. While biogeography may help explain the overall population shifts, individual assessments could explain the finer differences by beach.
Loggerheads and green sea turtles prefer to nest on beaches with less artificial light and on shorelines that are less developed94,95,96. Comparable to previous observations87,97, both species in the Sarasota region also prefer to nest closer to the upland barrier. Nesting success has significantly declined over the course of the study, likely due to increased coastal housing density and construction near the beach49,98. Increases in coastal development result in more obstructions on the beach deterring nesters71,99, more lighting issues that discourage nesters45,100, and more nourishment or beach armoring that can prevent nesters from approaching the beach43,65,101. Two of the beaches with the lowest nesting success and nesting density (Lido Key, and Longboat Key) are also the beaches that have been (re)nourished the most in the county. Casey Key is the least developed and it has the highest nesting density. Unfortunately, modeling of nesting beach inundation into the future suggests that sea level rise could cover 5–32% of suitable nesting beach widths by the year 2100102,103,104. A recent report from NOAA estimates that by 2050 sea levels will rise on average between 0.25 and 0.30 m, increasing the likelihood of coastal flooding, storm surge impact, and coastal erosion105. Nesting density has continued to increase in line with the nest counts, which could be an exacerbating factor if the suitable nesting areas decline due to sea level rise and coastal development.
Nesting females may increase their fitness by laying clutches earlier in the season, in varied locations over the course of the season, and by varying the number of eggs per clutch106. Loggerhead nests laid later in the season had a much lower emergence success rate—likely due to the increased level of tropical storms and predation over the course of the season. Regional temperature increases at foraging areas are influencing sea turtle nesting phenology107,108,109. Although longer-lived females consistently lay more clutches over the course of the season110, few estimates of true nest counts derived from satellite tagging data have been published15,111,112,113 and updates are needed. Further modeling of how clutch frequency and clutch size has changed over time in relation to rising temperature and sea levels should be prioritized for nesting beaches on the Gulf of Mexico.
Throughout Florida, air temperature rises over the course of the nesting season, but different abiotic factors influence how nest temperatures fluctuate. Increased temperature influences embryo mortality114, but nests in the Sarasota region typically get more rainfall than nests on the east coast of Florida at similar latitudes and thus tend to be cooler (Lolavar & Wyneken pers. comm). The six beaches have different sand particle size and reflectance (albedo) that affect incubation temperature and duration (e.g., nest incubation duration is shorter on the dark, coarse sand of Venice than on the white, silty sand of north Siesta). An assessment is needed for the region to determine how physical characteristics (sand type, slope, albedo, etc.) influence nest temperatures. Green turtles tend to dig deeper egg chambers, that are placed closer to the upper barrier resulting in few green turtle nests that fully washout (barring a hurricane). On the Gulf coast of Florida, green sea turtles are considered to be a rare species and most of their nests were caged to prevent depredation and increase potential recruitment. Previous caging protocols are a likely factor of the discrepancy in emergence success between the two species115. However, the green nesting population is recovering and since 2021, MML STCRP has stopped caging all green nests.
Following emergence, 6–7.6% of hatchlings do not make it to the water77,116, thus reducing potential recruitment. Sky glow and artificial lighting sources lead hatchlings towards the dune and increase the likelihood of depredation or exhaustion. From 2018 to 2021 misorientation/disorientation events in Sarasota County made up 9% of all recorded events in the state of Florida117. Models from Mazaris and colleagues identified that increasing hatchling recruitment even at a single site, would increase the overall hatchling production from entire nesting aggregations118. Future projects should assess lighting issues in relation to disorientation events and hatchling mortality in the Sarasota region.
The data presented here documented the expanding nesting population for a productive Gulf of Mexico rookery. Although total nesting counts in the Gulf of Mexico annually accounts for only 7% of all loggerhead nesting in Florida, these beaches have a significant hatchling production annually reinforcing recovery. Primary, adult, and breeding sex ratios should be assessed to quantify the population age structure and determine if there is any risk of a sex ratio skew that other rookeries are experiencing119,120. Projects should model how ocean level increases will decrease suitable nesting habitat and how coastal development exacerbates the issue103,105. When suitable nesting area decreases, will there be an effect on carrying capacity or will nesters migrate to less suitable beaches? Recruitment is critical to lasting recovery, and protection measures are needed to maintain population growth.
Data availability
The data sets generated during and/or analyzed during the current study will be available by request. Please contact the corresponding author at jlasala@mote.org for information.
References
Magurran, A. E. et al. Long-term datasets in biodiversity research and monitoring: Assessing change in ecological communities through time. Trends Ecol. Evol. 25(10), 574–582. https://doi.org/10.1016/j.tree.2010.06.016 (2010).
Ahumada, J. A., Hurtado, J. & Lizcano, D. Monitoring the status and trends of tropical forest terrestrial vertebrate communities from camera trap data: A tool for conservation. PLoS One https://doi.org/10.1371/journal.pone.0073707 (2013).
Leslie, S. C., Blackett, F. C., Stalio, M. & Black, S. A. Systems behavior charts for longitudinal data inform marine conservation management. J. Aquac. Mar. Biol. 6(5), 00171 (2017).
Trillmich, F. et al. On the challenge of interpreting census data: insights from a study of an endangered pinniped. PLoS One https://doi.org/10.1371/journal.pone.0154588 (2016).
Waterhouse, L. et al. Recovery of critically endangered Nassau grouper Eepinephelus striatus) in the Cayman Islands following targeted conservation actions. PNAS 117(3), 1587–1595. https://doi.org/10.5281/zenodo.3585864 (2020).
Gray, M. E., Dickson, B. G., Nussear, K. E., Esque, T. C. & Chang, T. A range-wide model of contemporary, omnidirectional connectivity for the threatened Mojave desert tortoise. Ecosphere https://doi.org/10.1002/ecs2.2847 (2019).
Beranek, C., Colyvas, K., Clulow, J. & Mahony, M. Novel use of statistics to determine precise relationship assignment to estimate breeding output of a threatened amphibian with tadpole genotypes. Authorea https://doi.org/10.22541/au.160611302.24625206/v1 (2020).
Acevedo, J. et al. Migratory preferences of humpback whales between feeding and breeding grounds in the eastern south pacific. Mar. Mamm. Sci. 33(4), 1035–1052. https://doi.org/10.1111/mms.12423 (2017).
Paxton, K. L. & Moore, F. R. Connecting the dots: Stopover strategies of an intercontinental migratory songbird in the context of the annual cycle. Ecol. Evol. 7(17), 6716–6728. https://doi.org/10.1002/ece3.3227 (2017).
Howard, C. et al. Disentangling the relative roles of climate and land cover change in driving the long-term population trends of European migratory birds. Divers. Distrib. 26(11), 1442–1455. https://doi.org/10.1111/ddi.13144 (2020).
Plotkin, P. T., Owens, D., Byles, R. A. & Patterson, R. Departure of male olive ridley turtles (Lepidochelys olivacea) from a nearshore breeding ground. Herpetologica 52, 1–7 (1996).
National Research Council (NRC). Assessment of Sea Turtle Status and Trends: Integrating Demography and Abundance (The National Academies Press, 2010).
Bjorndal, K. A., Bolten, A. B. & Chaloupka, M. Y. Green turtle somatic growth model: Evidence for density dependence. Ecol. Appl. 10, 269–282 (2000).
Chaloupka, M., Kamezaki, N. & Limpus, C. Is climate change affecting the population dynamics of the endangered pacific loggerhead sea turtle?. J. Exp. Mar. Biol. Ecol. 356, 136–143. https://doi.org/10.1016/j.jembe.2007.12.009 (2008).
Phillips, K. P., Mortimer, J. A., Jolliffe, K. G., Jorgensen, T. H. & Richardson, D. S. Molecular techniques reveal cryptic life history and demographic processes of a critically endangered marine turtle. J. Exp. Mar. Biol. Ecol. 455, 29–37. https://doi.org/10.1016/j.jembe.2014.02.012 (2014).
Casale, P., Mazaris, A. D., Freggi, D., Basso, R. & Argano, R. Survival probabilities of loggerhead sea turtles (Caretta caretta) estimated from capture-mark-recapture data in the Mediterranean sea. Sci. Mar. 71(2), 365–372 (2007).
Ehrhart, L. M., Redfoot, W. E. & Bagley, D. A. Marine turtles of the central region of the Indian River Lagoon system. Florida Sci. 70(4), 415–434 (2007).
Siegwalt, F. et al. High fidelity of sea turtles to their foraging grounds revealed by satellite tracking and capture-mark-recapture: New insights for the establishment of key marine conservation areas. Biol. Conserv. 20, 108742. https://doi.org/10.1016/j.biocon.2020.108742 (2020).
Addison, D. S. Mean annual nest frequency for renesting loggerhead turtles (Caretta caretta) on the southwest coast of Florida. Mar. Turtle Newsl. 75, 13–15 (1996).
Conant, T. A. et al. Loggerhead sea turtle (Caretta caretta) 2009 Status Review Under the U.S. Endangered Species Act. Loggerhead Biological Review Team (2009).
Lasala, J. A., Hughes, C. R. & Wyneken, J. Breeding sex ratio and population size of loggerhead turtles from southwestern Florida. PLoS One https://doi.org/10.1371/journal.pone.0191615 (2018).
Bjorndal, K., & Zug, G. Growth and age of sea turtles. In Biology and Conservation of Sea Turtles (1995).
Scott, R., Marsh, R. & Hays, G. C. Life in the really slow lane: Loggerhead sea turtles mature late relative to other reptiles. Funct. Ecol. 26, 227–235 (2011).
Chasco, B. E. et al. Integrated mixed-effect growth models for species with incomplete ageing histories: A case study for the loggerhead sea turtle Caretta caretta. Mar. Ecol. Prog. Ser. 636, 221–234. https://doi.org/10.3354/meps13222 (2020).
Girondot, M., Mourrain, B., Chevallier, D. & Godfrey, M. H. Maturity of a giant: Age and size reaction norm for sexual maturity for Atlantic leatherback turtles. Mar. Ecol. https://doi.org/10.1111/maec.12631 (2021).
Derville, S. et al. Long-term monitoring of green turtle nesting on Tromelin Island demonstrates stable reproduction and population parameters. Chelonian Conserv. Biol. 14, 11–20. https://doi.org/10.2744/ccab-14-01-11-20.1 (2015).
Garner, J. A., MacKenzie, D. S. & Gatlin, D. Reproductive biology of Atlantic leatherback sea turtles at Sandy Point, St. Croix: The first 30 years. Chelonian Conserv. Biol. 16, 29–43. https://doi.org/10.2744/CCB-1224.1 (2017).
Hoover, S. R. Long-Term Analyses (1986–2018) of the Loggerhead Sea Turtle (Caretta caretta) Nesting Data from Keewaydin Island, Florida (Florida Atlantic University, 2019).
Le Gouvello, D. Z. M., Girondot, M., Bachoo, S. & Nel, R. The good and bad news of long-term monitoring: An increase in abundance but decreased body size suggests reduced potential fitness in nesting sea turtles. Mar. Biol. https://doi.org/10.1007/s00227-020-03736-4 (2020).
Shamblin, B. M. et al. Genetic structure of the southeastern united states loggerhead turtle nesting aggregation: Evidence of additional structure within the peninsular Florida recovery unit. Mar. Biol. 158(3), 571–587. https://doi.org/10.1007/s00227-010-1582-6 (2011).
Bowen, B. W. et al. Natal homing in juvenile loggerhead turtles (Caretta caretta). Mol. Ecol. 13(12), 3797–3808. https://doi.org/10.1111/j.1365-294X.2004.02356.x (2004).
Arendt, M. D. et al. Migration, distribution, and diving behavior of adult male loggerhead sea turtles (Caretta caretta) following dispersal from a major breeding aggregation in the western North Atlantic. Mar. Biol. 159, 113–125. https://doi.org/10.1007/s00227-011-1826-0 (2012).
Wright, L. I. et al. Turtle mating patterns buffer against disruptive effects of climate change. Proc. R. Soc. B Biol. Sci. 279(1736), 2122–2127. https://doi.org/10.1098/rspb.2011.2285 (2012).
Shamblin, B. M. et al. Mitogenomic sequences better resolve stock structure of southern greater Caribbean green turtle rookeries. Mol. Ecol. 21(10), 2330–2340. https://doi.org/10.1111/j.1365-294X.2012.05530.x (2012).
Shamblin, B. M. et al. Green turtles nesting at their northern range limit in the United States represent a distinct subpopulation. Chelonian Conserv. Biol. 17(2), 314–319. https://doi.org/10.2744/CCB-1332.1 (2018).
Girondot, M. Estimating density of animals during migratory waves: A new model applied to marine turtles at nesting sites. Endanger. Species Res. 12, 95–105 (2010).
Whiting, A. U., Chaloupka, M., Pilcher, N., Basintal, P. & Limpus, C. J. Sampling nesting sea turtles: Optimizing survey design to minimize error. MEPS 674, 257–270 (2021).
Joseph, J. et al. Mass sea turtle slaughter at Pulau Tiga, Malaysia: Genetic studies indicate poaching locations and its potential effects. Glob. Ecol. Conserv. 17, e00586 (2019).
Mejías-Balsalobre, C. et al. Local community perceptions of sea turtle egg use in Tortuguero, Costa Rica. Ocean Coast. Manag. 201, 105423 (2021).
Putman, N. F. et al. Modeling juvenile sea turtle bycatch risk in commercial and recreational fisheries. iScience 26, 105977 (2023).
López-Martinez, S., Morales-Caselles, C., Kadar, J. & Rivas, M. L. Overview of global status of plastic presence in marine vertebrates. Glob. Change Biol. 27(4), 728–737 (2020).
Foley, A. M. et al. Characterizing watercraft-related mortality of sea turtles in Florida. J. Wildl. Manag. 83(5), 1057–1072 (2019).
Rumbold, D. G., Davis, P. W. & Perretta, C. Estimating the effect of beach nourishment on Caretta caretta (loggerhead sea turtle) nesting. Restor. Ecol. 9(3), 304–310. https://doi.org/10.1046/j.1526-100X.2001.009003304.x (2001).
Butler, Z. P. et al. Predation of loggerhead sea turtle eggs across Georgia’s barrier islands. Glob. Ecol. Conserv. 23, e01139 (2020).
Price, J. T., Drye, B., Domangue, R. J. & Paladino, F. V. Exploring the role of artificial lighting in loggerhead turtle (Caretta caretta) nest-site selection and hatchling disorientation. Herpetol. Conserv. Biol. 13(2), 415–422 (2018).
Casale, P., & Tucker, T. Caretta caretta. The IUCN Red List of Threatened Species (2017).
Encalada, S. E. et al. Population structure of loggerhead turtle (Caretta caretta) nesting colonies in the Atlantic and Mediterranean as inferred from mitochondrial DNA control region sequences. Mar. Biol. 130, 567–575 (1998).
Turtle Expert Working Group (TEWG). An assessment of the Kemp’s ridley (Lepidochelys kempii) and loggerhead (Caretta caretta) sea turtle populations in the western north Atlantic. NOAA Technical Memorandum NMFS-SEFSC-409 (1998).
Fuentes, M. M. P. B. et al. Conservation hotspots for marine turtle nesting in the united states based on coastal development. Ecol. Appl. 26(8), 2708–2719 (2016).
Sella, K. A. N. & Fuentes, M. M. P. B. Exposure of marine turtle nesting grounds to coastal modifications: Implications for management. Ocean Coast. Manag. 169(March), 182–190. https://doi.org/10.1016/j.ocecoaman.2018.12.011 (2019).
Seminoff, J. A. Marine turtle specialist group Chelonia mydas assessment. IUCN Marine Turtle Specialist Group, 34 (2004).
Lindborg, R. et al. An ethogram describing the nesting behavior of green sea turtles (Chelonia mydas). Herpetologica 75(2), 114–122. https://doi.org/10.1655/D-18-00015 (2019).
Page-Karjian, A. et al. Comprehensive health assessment of green turtles Chelonia mydas nesting in southeastern Florida, USA. Endanger. Species Res. 42, 21–35. https://doi.org/10.3354/ESR01036 (2020).
Reusche, M. R. Genetic Structure of Green Sea Turtle (Chelonia mydas) Foraging Aggregations on the East Coast of Florida (University of Central Florida, 2020). https://stars.library.ucf.edu/honorstheses/750.
Nielsen, J. T. Population Structure and the Mating System of Loggerhead Turtles (Caretta caretta) (University of Miami, 2010).
Lasala, J. A., Hughes, C. R. & Wyneken, J. Female loggerhead sea turtles (Caretta caretta L.) rarely remate during nesting season. Ecol. Evol. 10, 163–174. https://doi.org/10.1002/ece3.5869 (2020).
Witherington, B. E., Kubilis, P., Brost, B. & Meylan, A. Decreasing annual nest counts in a globally important loggerhead sea turtle population. Ecol. Appl. 19, 30–54 (2009).
Ceriani, S. A., Brost, B., Meylan, A. B., Meylan, P. A. & Casale, P. Bias in sea turtle productivity estimates: Error and factors involved. Mar. Biol. 168, 41 (2021).
FWC Florida Fish and Wildlife Research Institute (a). Statewide nesting beach survey program loggerhead nesting data 2016–2021. (2021). https://myfwc.com/media/23244/loggerheadnestingdata5years.pdf Accessed 23 Apr 2022.
FWC Florida Fish and Wildlife Research Institute (b). Statewide nesting beach survey program green nesting data 2016–2021. (2022). https://myfwc.com/media/23245/greenturtlenestingdata5years.pdf Accessed 23 Apr 2022.
Florida Department of Environmental Protection (FDEP). Critically eroded beaches in Florida. (2021). https://floridadep.gov/sites/default/files/FDEP-Critically-Eroded-Beaches-07-2021_0_1.pdf Accessed 24 Jan 2022.
Mosier, A. E. The impact of coastal armoring structures on sea turtle nesting behavior at three beaches on the east coast of Florida. St. Petersburg, FL, USA (1998).
Steinitz, M. J., Salmon, M. & Wyneken, J. Beach renourishment and loggerhead turtle reproduction: A seven-year study at Jupiter Island, Florida. J. Coast. Res. 14(3), 1000–1013 (1998).
Mosier, A. E., & Witherington, B. Documented effects of coastal armoring structures on sea turtle nesting behavior. In 20th Annual Sea Turtle Symposium, 304–305 (2000).
Rizkalla, C. E. & Savage, A. Impact of seawalls on loggerhead sea turtle (Caretta caretta) nesting and hatching success. J. Coast. Res. 27, 166–173 (2011).
Ceriani, S. A., Casale, P., Brost, M., Leone, E. H. & Witherington, B. Conservation implications of sea turtle nesting trends: Elusive recovery of a globally important loggerhead population. Ecosphere https://doi.org/10.1002/ecs2.2936 (2019).
Florida Fish Wildlife Conservation Commission. Marine Turtle Conservation Handbook. (2016). https://myfwc.com/media/3133/fwc-mtconservationhandbook.pdf 16 Feb 2023.
Godley, B. J., Broderick, A. C. & Hays, G. C. Nesting of green turtles (Chelonia mydas) at Ascension Island, South Atlantic. Biol. Conserv. 97, 151–158. https://doi.org/10.1016/S0006-3207(00)00107-5 (2001).
Mazaris, A. D. et al. Assessing the relative importance of conservation measures applied on sea turtles: Comparison of measures focusing on nesting success and hatching recruitment success. Amphibians Reptiles 30, 221–231 (2009).
Fujisaki, I. & Lamont, M. M. The effects of large beach debris on nesting sea turtles. J. Exp. Mar. Biol. Ecol. 482, 33–37 (2016).
Ware, M. & Fuentes, M. M. P. B. Leave no trace ordinances for coastal species management: Influences on sea turtle nesting success. Endanger. Species Res. 41, 197–207 (2020).
Hamann, M. et al. Patterns of nesting behavior and nesting success for green turtles at Raine Island, Australia. Endanger. Species Res. 47, 217–229 (2022).
Brothers, J. R. & Lohmann, K. J. Evidence for geomagnetic imprinting and magnetic navigation in the natal homing of sea turtles. Curr. Biol. 25, 392–396 (2015).
Fuentes, M. M. P. B. et al. Conservation hotspots for marine turtle nesting in the United States based on coastal development. Ecol. Appl. 26, 2708–2719 (2016).
Pankaew, K. & Milton, S. L. The effects of extended crawling on the physiology and swim performance of loggerhead and green sea turtle hatchlings. J. Exp. Biol. 221, jeb165225 (2018).
Dimitriadis, C., Fournari-Konstantinidou, I., Sourbès, L., Koutsoubas, D. & Mazaris, A. D. Reduction of sea turtle population recruitment caused by nightlight: Evidence from the mediterranean region. Ocean Coast. Manag. 153, 108–115. https://doi.org/10.1016/j.ocecoaman.2017.12.013 (2018).
Erb, V. & Wyneken, J. Nest-to-surf mortality of loggerhead sea turtle (Caretta caretta) hatchlings on Florida’s east coast. Front. Mar. Sci. https://doi.org/10.3389/fmars.2019.00271 (2019).
Miller, J. D. Determining clutch size and hatching success. In Research and Management Techniques for the Conservation of Sea Turtles. IUCN/SSC Marine Turtle Specialist Group Publication No. 4, (eds. Eckert, K. L., Bjorndal, K. A., Abreu-Grobois, F. A., Donnelly, M.) 124–129 (1999).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2020).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer, 2016).
QGIS.org. QGIS Geographic Information System. QGIS Association. (2022). http://www.qgis.org Accessed 24 Jan 2022.
Dinno, A. dunn.test: Dunn’s Test of Multiple Comparisons Using Rank Sums. R package version 1.3.5. (2017).
Komsta, L. mblm: Median-Based Linear Models. R package version 0.12.1. (2019).
Cribari-Neto, F. & Zeileis, A. Beta regression in R. J. Stat. Softw. 34(2), 1–24. https://doi.org/10.18637/jss.v034.i02 (2010).
Fujisaki, I., Lamont, M. & Carthy, R. Temporal shift of sea turtle nest sites in an eroding barrier island beach. Ocean Coast. Manag. 155, 24–29 (2018).
Shamblin, B. M. et al. Genetic structure of Florida green turtle rookeries as indicated by mitochondrial DNA control region sequences. Conserv. Genet. 16(3), 673–685. https://doi.org/10.1007/s10592-014-0692-y (2015).
Valverde, R. A., Holzwart, K. R. Sea turtles of the Gulf of Mexico. In Habitats and Biota of the Gulf of Mexico: Before the Deepwater Horizon Oil Spill (ed. Ward, C. H.) 1189–1352 (Rice University, 2017).
Goshe, L. R., Avens, L., Scharf, F. S. & Southwood, S. L. Estimation of age at maturation and growth of Atlantic green turtles (Chelonia mydas) using skeletochronology. Mar. Biol. 157(8), 1725–1740. https://doi.org/10.1007/s00227-010-1446-0 (2010).
Avens, L. et al. Population characteristics, age structure, and growth dynamics of neritic juvenile green turtles in the northeastern Gulf of Mexico. Mar. Ecol. Prog. Ser. 458(July), 213–229. https://doi.org/10.3354/meps09720 (2012).
Finkbeiner, E. M. et al. Cumulative estimates of sea turtle bycatch and mortality in USA fisheries between 1990 and 2007. Biol. Conserv. 144(11), 2719–2727. https://doi.org/10.1016/j.biocon.2011.07.033 (2011).
Putman, N. F., Bane, J. M. & Lohmann, K. J. Sea turtle nesting distributions and oceanographic constraints on hatchling migration. Proc. R. Soc. B Biol. Sci. 277, 3631–3637. https://doi.org/10.1098/rspb.2010.1088 (2010).
Shanmugam, G. Slides, slumps, debris flows, turbidity currents, and bottom currents: Implications ☆. In Encyclopedia of Ocean Sciences (Third Edition), vol. 4, 228–257 (Elsevier, 2019) https://doi.org/10.1016/b978-0-12-409548-9.04380-3.
Shamblin, B. M. et al. Geographic patterns of genetic variation in a broadly distributed marine vertebrate: New insights into loggerhead turtle stock structure from expanded mitochondrial DNA sequences. PLoS One 9, e85956. https://doi.org/10.1371/journal.pone.0085956 (2014).
Kikukawa, A., Kamezaki, N. & Otal, H. Factors affecting nesting beach selection by loggerhead turtles (Caretta caretta); a multiple regression approach. J. Zool. 249(4), 447–454 (1999).
Weishampel, J. F., Bagley, D. A., Ehrhart, L. M. & Rodenbeck, B. L. Spatiotemporal patterns of annual sea turtle nesting behaviors along an east central Florida beach. Biol. Conserv. 110(2), 295–303 (2003).
Weishampel, Z. A., Cheng, W. H. & Weishampel, J. F. Sea turtle nesting patterns in Florida vis-à-vis satellite-derived measures of artificial lighting. Remote Sens. Ecol. Conserv. 2(1), 59–72. https://doi.org/10.1002/rse2.12 (2016).
Garmestani, A. S., Percival, H. F., Portier, K. M. & Rice, K. G. Nest-site selection by the loggerhead sea turtle in Florida’s ten thousand islands. J. Herpetol. 34(4), 504–510 (2000).
Kaska, Y. et al. Natural and anthropogenic factors affecting the nest-site selection of loggerhead turtles, Caretta caretta, on Dalaman-Sarıgerme beach in south-west Turkey”. Zool. Middle East 50, 47–58. https://doi.org/10.1080/09397140.2010.10638411 (2010).
Oliver de la Esperanza, A., Martínez, A. A., Tuz, M. T. & Pérez-Collazos, E. Are anthropogenic factors affecting nesting habitat of sea turtles? The case of Kanzul Beach, Riviera Maya-Tulum (Mexico). J. Coast. Conserv. 21, 85–93. https://doi.org/10.1007/s11852-016-0473-5 (2017).
Witherington, B. E. Behavioral responses of nesting sea turtles to artificial lighting. Herpetologica 48, 31–39 (1992).
Hirsch, S. E., Kedzuf, S. & Perrault, J. R. Impacts of a geotextile container dune core on marine turtle nesting in Juno Beach, Florida, United States. Restor. Ecol. 27(2), 431–439. https://doi.org/10.1111/rec.12878 (2019).
Fish, M. R. et al. Construction setback regulations and sea-level rise: Mitigating sea turtle nesting beach loss. Ocean Coast. Manag. 51(4), 330–341. https://doi.org/10.1016/j.ocecoaman.2007.09.002 (2008).
Fuentes, M. M. P. B., Limpus, C. J., Hamann, M. & Dawson, J. Potential impacts of projected sea-level rise on sea turtle rookeries. Aquat. Conserv. Mar. Freshw. Ecosyst. 20(2), 132–139. https://doi.org/10.1002/aqc.1088 (2010).
Lyons, M. P., von Holle, B., Caffrey, M. A. & Weishampel, J. F. Quantifying the impacts of future sea level rise on nesting sea turtles in the southeastern United States. Ecol. Appl. 30(5), e02100. https://doi.org/10.1002/eap.2100 (2020).
Sweet, W. V. et al. Global and regional sea level rise scenarios for the United States: Updated mean projections and extreme water level probabilities along U.S. coastlines. NOAA Technical Report NOS 01. National Oceanic and Atmospheric Administration, National Ocean Service, Silver Spring, MD (2022). https://oceanservice.noaa.gov/hazards/sealevelrise/noaa-nostechrpt01-global-regional-SLR-scenarios-US.pdf Accessed 2 Mar 2022.
Cassill, D. L. Multiple maternal risk-management adaptations in the loggerhead sea turtle (Caretta caretta) mitigate clutch failure caused by catastrophic storms and predators. Sci. Rep. 11, 2491 (2021).
Mazaris, A. D., Kallimanis, A. S., Tzanopoulos, J., Sgardelis, S. P. & Pantis, J. D. Sea surface temperature variations in core foraging grounds drive nesting trends and phenology of loggerhead turtles in the Mediterranean Sea. J. Exp. Mar. Biol. Ecol. 379, 23–27 (2009).
Neeman, N., Robinson, N. J., Paladino, F. V., Spotila, J. R. & O’Connor, M. P. Phenology shifts in leatherback turtles (Dermochelys coriacea) due to changes in sea surface temperature. J. Exp. Mar. Biol. Ecol. 462, 113–120 (2015).
Monsingjon, J., Lopez-Mendilaharsu, M., Lara, P., Santos, A. & dei Marcovaldi, M.A.G., Girondot, M., Fuentes, M.M.P.B.,. Effects of temperature and demography on the phenology of loggerhead sea turtles in Brazil. Mar. Ecol. Prog. Ser. 623, 209–219 (2019).
Frazer, N. B. A model for assessing mean age-specific fecundity in sea turtle populations. Herpetologica 40(3), 281–291 (1984).
Kendall, W. L. & Bjorkland, R. Using open robust design models to estimate temporary emigration from capture-recapture data. Biometrics 57, 1113–1122 (2001).
Pfaller, J. B. et al. One beach amongst many: How weak fidelity to a focal nesting site can bias demographic rates in marine turtles. Mar. Biol. https://doi.org/10.1007/s00227-021-03991-z (2021).
Tucker, A. D. Nest site fidelity and clutch frequency of loggerhead turtles are better elucidated by satellite telemetry than by nocturnal tagging efforts: Implications for stock estimation. J. Exp. Mar. Biol. Ecol. 383, 48–55 (2010).
Bladow, R. A. & Milton, S. L. Embryonic mortality in green (Chelonia mydas) and loggerhead (Caretta caretta) sea turtle nests increases with cumulative exposure to elevated temperatures. J. Exp. Mar. Biol. Ecol. 518(September), 151180. https://doi.org/10.1016/j.jembe.2019.151180 (2019).
Brost, B. et al. Sea turtle hatchling production from Florida (USA) beaches, 2002–2012, with recommendations for analyzing hatching success. Endanger. Species Res. 27, 53–68. https://doi.org/10.3354/esr00653 (2015).
Villegas Vindiola, B. Measuring Nest-to-Surf Mortality of Florida’s East and West Coast Loggerhead Sea Turtle (Caretta caretta) Hatchlings (Florida Atlantic University, 2019).
FWC Florida Fish and Wildlife Research Institute (c). FWC Sea Turtle Disorientation Survey 2018–2021 (2021). https://myfwc.maps.arcgis.com/apps/dashboards/53699c9690024c079ed60aaa74a763c8 Accessed 23 Apr 2022.
Mazaris, A. D., Schofield, G., Gkazinou, C., Almpanidou, V. & Hays, G. Global sea turtle conservation successes. Sci. Adv. 3(9), e1600730 (2017).
Jensen, M. P. et al. Environmental warming and feminization of one of the largest sea turtle populations in the world. Curr. Biol. 28, 154-159.e4. https://doi.org/10.1016/j.cub.2017.11.057 (2018).
Tuttle, J. & Rostal, D. Effects of nest relocation on nest temperature and embryonic development of loggerhead sea turtles (Caretta caretta). Chelonian Conserv. Biol. 9, 1–7 (2010).
Acknowledgements
Data were collected under the guidance and permitting of the Florida Fish and Wildlife Conservation Commission’s (FWC) Marine Turtle Permits: 027, 028, 048, 054, 070, and RP #915. This paper would not have been possible without the help from hundreds of interns, volunteers and MML staff. Thanks in particular to E. Argo, J. Beggs, B. Bendel, P. Block, E. Briner, C. Brinton-Perz, J. Budzynkiewicz, K. Carter, P. Clark, K. Cochran, S. Condran, J. Cormany, J. Coulter, M. Crocker, J. Drenth, R. Edman, V. Erb, A. Ferrera, H. Fitzgerald, J. Flint, S. Gaillard, S. Gofman, J. Grimes, A. Hallbach, M. Hansen, A. Harper, A. Hays, M. Herron, S. Hirsch, C. Homer, S. Hoover, L. Kabat, K. Klaustermeier, K. Klingensmith, D. Lytwynec, C. Marshall, B. McIntyre, S. Morello, M. Mota, T. Muller, J. Neill, K. Noiseux, C. Olsen, N. Parks, M. Robinson, R. Romanowski, K. Schanzle S. Shaw, J. Sobin, P. Solomon, R. Warren, R. Welsh, S. Whitebread, and V. Wiese.
Author information
Authors and Affiliations
Contributions
J.A.L., M.C.M., and K.T.M. conceived the idea of the project and compiled the data; J.A.L. drafted the original manuscript; all other authors contributed to collecting the data, writing and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lasala, J.A., Macksey, M.C., Mazzarella, K.T. et al. Forty years of monitoring increasing sea turtle relative abundance in the Gulf of Mexico. Sci Rep 13, 17213 (2023). https://doi.org/10.1038/s41598-023-43651-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-43651-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.