Abstract
Erosive esophagitis (EE) is the part of gastroesophageal reflux disease (GERD) spectrum and may progress to esophageal adenocarcinoma. Due to its progressivity and unclear prevalence, we aim to identify the factors contributing in EE to decide the need for further examination. We performed a PRISMA 2020-based systematic search through PubMed and other resources up to June 2, 2022. Study quality was assessed using the Newcastle–Ottawa Scale (NOS). The odds ratio (OR) of each factor and worldwide prevalence of EE were measured. There are 114 observational studies included with a total of 759,100 participants. Out of 29 factors, the significant risk factors are age ≥ 60 y.o. (OR 2.03 [1.81–2.28]), White/Caucasian (OR 1.67 [1.40–1.99]), unmarried (OR 1.08 [1.03–1.14]), having GERD ≥ 5 years (OR 1.27 [1.14–1.42]), general obesity (OR 1.78 [1.61–1.98]), central obesity (OR 1.29 [1.18–1.42]), diabetes mellitus (DM) (OR 1.24 [1.17–1.32]), hypertension (OR 1.16 [1.09–1.23]), dyslipidemia (OR 1.15 [1.06–1.24]), hypertriglyceridemia (OR 1.42 [1.29–1.57]), hiatal hernia (HH) (OR 4.07 [3.21–5.17]), and non-alcoholic fatty liver disease (NAFLD) (OR 1.26 [1.18–1.34]). However, H. pylori infection (OR 0.56 [0.48–0.66]) and atrophic gastritis (OR 0.51 [0.31–0.86]) are protective towards EE. This study demonstrates that age, ethnicity, unmarried, long-term GERD, metabolic diseases, HH, and NAFLD act as risk factors for EE, whereas H. pylori infection and atrophic gastritis act as protective factors. These findings may enable a better understanding of EE and increase greater awareness to address its growing burden.
Similar content being viewed by others
Introduction
Gastroesophageal reflux disease (GERD) is a condition that develops when there is a retrograde flow of stomach contents back into the esophagus1,2,3. Long-term exposure to gastric contents may irritate the esophageal epithelium, leading to a spectrum of disease in three different phenotypes—non-erosive reflux disease (NERD), erosive esophagitis (EE), and Barrett’s esophagus (BE)—when inspected through endoscopy and/or histopathology4,5,6. Typical clinical presentations of GERD are heartburns and regurgitation, with atypical clinical presentations, such as epigastric pain, odynophagia, dysphagia, nausea, chronic cough, dental erosion, laryngitis, and asthma7,8.
Approximately 30% of GERD cases may progress to EE, and 1–13% of EE cases may also continue to develop BE6. However, reports of EE cases around the globe remain unclear, yet experts estimate the number hits approximately 1% of the population7. Aside from the burden on quality of life9, prolonged esophagitis may further induce esophageal epithelium metaplasia and progression of adenocarcinoma10. Due to its long-term morbidity, it is crucial to identify clear-cut risk factors that contribute to the development of EE to decide the need for endoscopy and/or histopathology analysis, to detect an early mucosal erosion, and to prevent its progression to BE and esophageal adenocarcinoma.
Given the burden on health-related quality of life, it is important for physicians to provide proper management and care from well-established knowledge of EE risk factors. Therefore, this meta-analysis aims to outline the detailed risk factors contributing to the development of EE as the primary outcome from the perspective of demography, comorbidities, and medication history. Furthermore, a secondary outcome of the global, regional, and local prevalence will also be depicted in this study since the exact number of cases reported is still unclear.
Results
Overview of literature search and included studies
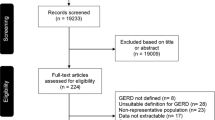
The initial search yields a total of 3145 studies, out of which, 1636 studies are removed due to duplication of studies. We obtain 306 studies with eligible titles and abstracts and review 253 studies, as the full-texts of 53 studies are irretrievable. Finally, only 114 eligible studies with a total of 759,100 participants are included in this study. The overall process is illustrated in Fig. 1. The summary of qualitative synthesis of the included studies is provided in Table 1.
Approximately 25.53% of participants are diagnosed as EE through upper gastrointestinal (UGI) endoscopy. The mean age is 47.56 years; two studies did not report the mean age of their study population11,12. To avoid proportional bias, we cannot report the gender proportion because 28 studies are missing this information. Among the 114 included studies, 36 are case–control, 11 are prospective cohort, 6 are retrospective cohort, and 61 are cross-sectional studies. In terms of regions, 84 studies are in Asia, 15 studies in America, 11 studies in Europe, and 4 studies in Africa.
Demographical factors
The demographical factors chosen for this analysis are as follows: sex, age, race, employment status, marital status, educational status, educational duration, and disease duration (Table 2). The forest and funnel plots are provided in Supplementary Fig. S1–S8 online. Evidence of high heterogeneity is detected in sex (I2 = 77%), age (I2 = 96%), race (I2 = 71%), employment status (I2 = 91%), and educational status (I2 = 85%). All heterogeneity tests are performed using REM. Four factors are found as risk factors: (1) Age ≥ 60 y.o. with OR 2.03 (95% CI = 1.81–2.28, n = 92 studies); (2) White/Caucasian race with OR 1.67 (95% CI = 1.40–1.99, n = 10 studies); (3) Being single with OR 1.08 (95% CI = 1.03–1.14, n = 7 studies); and (4) Having GERD ≥ 5 years with OR 1.27 (95% CI = 1.14–1.42, n = 2 studies). We define ‘having GERD ≥ 5 years’ as having symptomatic GERD that is not diagnosed by endoscopy for 5 years or more. The rest – being male, employed workers, being students of college or higher educational degree, and study duration ≥ 12 years—are not risk nor protective factors.
The sensitivity analysis on employment status shows that the pooled effect of EE in employed patients is changed from nonsignificant to significant after removing one study by Kulig et al.13. However, the pooled effects of two other factors in the leave-one-out sensitivity analyses are changed from significant to nonsignificant after removing one study, either Chung et al.14, Kulig et al.13, Mun et al.15, or Sadiku et al.16, for marital status, and Kulig et al.13 for disease duration. The sensitivity analyses of the remaining demographical factors suggest that the pooled effects are not influenced by any single study.
Comorbidities
Fifteen comorbidities are included in this analysis consisting general obesity, central obesity, diabetes mellitus (DM) or hyperglycemia, hypertension or elevated blood pressure (BP), dyslipidemia, hypertriglyceridemia, hypercholesterolemia, high low density lipoprotein cholesterol (LDL-C), low high density lipoprotein cholesterol (HDL-C), hiatal hernia (HH), H. pylori infection, gastric ulcer, duodenal ulcer, atrophic gastritis, and non-alcoholic fatty liver disease (NAFLD) (Table 2). The forest and funnel plots are provided in Supplementary Fig. S9–S23 online. We detect moderate to high heterogeneity in 11 out of 15 comorbidities, including general obesity (I2 = 85%), central obesity (I2 = 69%), hypertriglyceridemia (I2 = 70%), hypercholesterolemia (I2 = 67%), high LDL-C (I2 = 69%), low HDL-C (I2 = 56%), HH (I2 = 95%), H. pylori infection (I2 = 91%), gastric ulcer (I2 = 77%), duodenal ulcer (I2 = 61%), and atrophic gastritis (I2 = 84%). All heterogeneity tests are performed using REM. Based on the ORs, eight comorbidities that can be considered risk factors are as follows: (1) general obesity with OR 1.78 (95% CI = 1.61–1.98, n = 50 studies); (2) central obesity with OR 1.29 (95% CI = 1.18–1.42, n = 25 studies); (3) DM or hyperglycemia with OR 1.24 (95% CI = 1.17–1.32, n = 38 studies); (4) hypertension or elevated BP with OR 1.16 (95% CI = 1.09–1.23, n = 36 studies); (5) dyslipidemia with OR 1.15 (95% CI = 1.06–1.24, n = 10 studies); (6) hypertriglyceridemia with OR 1.42 (95% CI = 1.29–1.57, n = 22 studies); (7) HH with OR 4.07 (95% CI = 3.21–5.17, n = 57 studies); and (8) NAFLD with OR 1.26 (95% CI = 1.18–1.34, n = 8 studies). On the contrary, H. pylori infection (OR 0.56 [0.48–0.66]; n = 39 studies) and atrophic gastritis (OR 0.51 [0.31–0.86]; n = 8 studies) act as protective factors. Other factors – hypercholesterolemia, high LDL-C, low HDL-C, gastric ulcer, and duodenal ulcer – are not risk nor protective factors.
After removing a study by Cho et al.17 in the sensitivity analysis of duodenal ulcer, the pooled effect is shifted from nonsignificant to significant. On the contrary, the pooled OR of atrophic gastritis is shifted from significant to nonsignificant following the removal of a study by Ko et al.18. The leave-one-out sensitivity analyses of the remaining comorbidities suggest that the provided overall effects are robust and not affected by any single study.
Medication history
We include five pharmacological medications: Non-steroidal anti-inflammatory drug (NSAID) only, aspirin only, NSAID and/or aspirin, proton pump inhibitor (PPI), H2 receptor antagonist (H2RA), and antacids (Table 2). The forest and funnel plots are provided in Supplementary Fig. S24–S29 online. NSAID and/or aspirin (I2 = 58%) and H2RA (I2 = 53%) have moderate heterogeneity, while PPI (I2 = 93%) and antacids (I2 = 84%) have high heterogeneity. All heterogeneity tests are performed using REM. There is no medication history considered as risk nor protective factors in the current analysis: NSAID only (OR 1.02 [0.94–1.10]), aspirin only (OR 1.09 [0.96–1.24]), NSAID and/or aspirin (OR 1.21 [0.79–1.86]), PPI (OR 0.65 [0.30–1.39]), H2RA (OR 1.23 [0.63–2.39]), and antacids (OR 1.97 [0.98–3.93]).
The sensitivity analysis of the antacids use reveals that the overall effect is changed from nonsignificant to significant following the removal of one study by Kang et al.19. On the other hand, no study has a notable influence in the leave-one-out sensitivity analyses of the remaining medication histories, proving the robustness of the pooled results.
EE prevalence
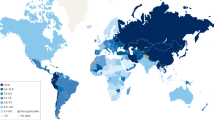
We perform meta-analysis of EE prevalence based on the geographic regions (Table 3) along with the substantial variations of the EE worldwide prevalence (Fig. 2 and Supplementary Fig. S30 online). There are 193,819 participants who are diagnosed with EE giving an overall pooled prevalence of 28% (95% CI = 24%–31%). The two highest pooled prevalence of EE are Africa (47% [95% CI = 27%–68%]) and the Middle East (43% [95% CI = 28%–60%]), while the lowest is Asia (24% [95% CI = 22%–27%]). Interestingly, the prevalence of EE in America (36% [95% CI = 30%–42%]) and Europe (34% [95% CI = 25%–44%]) are both higher than that in Asia. The top five countries in terms of prevalence are as follows: Indonesia (55% [95% CI = 42%–68%]), India (52% [95% CI = 44%–59%]), Nigeria (50% [95% CI = 42%–58%]), Peru (50% [95% CI = 44%–56%]), and Albania (48% [95% CI = 43%–52%]). The country with lowest pooled prevalence is Sweden (17% [95% CI = 15%–19%]).
The distribution map of worldwide erosive esophagitis (EE) prevalence (created with https://www.mapchart.net/).
Publication bias and quality assessment
The funnel plots of central obesity (Supplementary Fig. S10B online), high LDL-C (Supplementary Fig. S16B online), and low HDL-C (Supplementary Fig. S17B online) show an asymmetrical distribution of studies, revealing the potential of publication bias. These findings are further confirmed by significant Egger’s test result in each factor (Z = 2.03 and p = 0.04 for central obesity, Z = 2.16 and p = 0.03 for high LDL-C, Z = -2.23 and p = 0.03 for low HDL-C). On the contrary, no potential of publication bias is found in the rest of the factors since their funnel plots show a rather symmetrical distribution of studies, further supported by their insignificant Egger’s test results (Table 2).
The quality of each study is shown in Table S1–S3. The overall quality of the included case–control studies (Supplementary Table S1 online) is good in 27 studies, while the rest (n = 9) is moderate. Of the 17 cohort studies, thirteen and four studies have good- and moderate-quality, respectively (Supplementary Table S2 online). The qualities of 61 cross-sectional studies (Supplementary Table S3 online) are as follows: (1) very good for 37 studies; (2) good for 19 studies; and (3) satisfactory for 5 studies. There are no poor-quality and unsatisfactory studies in the current meta-analysis.
Discussion
To the best of our understanding, this meta-analysis is the first to thoroughly analyze the risk factors and prevalence of EE across the world from 1997 to 2021. Our results indicate that several demographical factors—age ≥ 60 y.o., White/Caucasian, single or unmarried, and having GERD ≥ 5 years—increase the risk of having EE. Interestingly, we find both risk and protective factors towards EE in the comorbidities. Obesity, DM, hypertension, dyslipidemia, hypertriglyceridemia, HH, and NAFLD are found to increase the risk of EE, while H. pylori infection and atrophic gastritis are found to be protective towards EE. Our results also indicate that medication history is not significantly increasing the risk nor protective of EE. The prevalence of EE in each of America, Africa, and Europe is higher than that in Asia and the highest prevalence is found to be in Africa and the Middle East.
Our study indicates that the risk of EE in males is twice than that in females. Previous studies have suggested that the combination of behavioral, immunologic, and metabolic aspects, especially in men, can increase the risk of EE and affect its prevalence. For example, Erol and Karpyak20 and Matsuzaki et al.21 suggest that cigarette smoking and alcohol consumption are more common in men and may increase the risk of having EE in men, approximately two to three times more than women. A longitudinal study by Adachi et al.22 also indicates that the prevalence of EE in men during 10-year period is increasing mainly due to aging, high BMI, and large diaphragmatic hiatus. This change, however, is not found in women. Furthermore, previous studies by Yoon Kim et al.23 and Sun Kim et al.24 suggest the protective effects of estrogen, although the studies use animal models.
Our study shows that the risk of EE in the Western (White/Caucasian) population is approximately two-fold higher than that in the non-White/Caucasian population. Previous studies have suggested that lifestyle factors, anatomical, and genetic variance can also explain the high risk of EE in the Western population. In terms of lifestyle factors, Wirth et al.25, Abraham et al.26, and Ko et al.18 indicate the differences in the risk can be attributed to the differences in eating habits or cultures (e.g. high fat diet and alcohol drinking in the Western population), distribution of visceral fat tissues, and body composition between the Western and Eastern populations25,26. In terms of anatomical differences, previous studies also suggest that the mass of gastric parietal cells of Western population is greater than that in the Asian population, which explains the higher gastric acid production in the Western population18,25. Moreover, in terms of genetic variance, some previous studies indicate that the difference in the ABH-secretor and Lewis histo-blood group may explain the difference of risk in the Western population. In particular, Wirth et al.25 and Suzuki et al.27 indicate that individuals with group A and non-secretors (common in the Western population) are prone to have EE.
This study finds that HH increases the risk of EE and this may be explained by anatomical and physiological factors. HH may diminish the augmenting effect of diaphragmatic crus to prevent gastric reflux28. Previous study mentions that the size of the HH is the most important risk factor of EE in individuals with GERD29. Some etiologies, such as pregnancies, surgical history, being elderly, and overweight, may increase the probability of HH30,31.
Obese individuals tend to experience more frequent and intense reflux symptoms compared to non-obese individuals32. Anatomically, obesity may promote esophagitis by increasing intra-abdominal pressure (IAP) and inducing lower esophageal sphincter (LES) relaxation33. Another evidence also reveals that obesity increases the transvesically-measured IAP34. Another mechanism thought to be involved in EE is related to adipose tissue. It may act as an endocrine tissue releasing inflammatory cytokines and leptin, which may further exacerbate the esophageal inflammatory process35.
In terms of metabolic diseases other than obesity, DM may cause esophageal dysfunction, which results in the amplitude reduction of esophageal contractions, less peristaltic waves, decreased LESP (lower esophageal sphincter pressure), and abnormal gastroesophageal reflux36,37. This is consistent with our finding that the risk of EE is increased in diabetic patients. Interestingly, the esophageal dysfunction in diabetic patients is also associated with autonomic neuropathy involving the vagal nerve, especially when the patient is in hyperglycemic state or has diabetes for 5–10 years after onset38,39. Gastric emptying can be disrupted due to this process, which triggers EE39. This process is further worsened by the fact that reflux symptoms may be more frequent in diabetic patients with three major complications (retinopathy, neuropathy, nephropathy) and longer duration of DM40,41.
In this study, we find that hypertension increases the risk of EE. This finding is first confirmed by Gudlaugsdottir et al.42, which finds a significantly higher systolic blood pressure (SBP) in EE compared to the controls, although the underlying pathophysiology is still unclear. The relationship between hypertension and esophageal reflux is further confirmed by Hu et al.43, which observes a significant improvement in the hypertension control after laparoscopic fundoplication during a 3.5 year follow-up period.
Our overall analysis finds dyslipidemia to be a risk factor for EE. However, most studies included in the analysis do not find dyslipidemia to be a risk factor. To evaluate this finding, we also separately analyzed several components of dyslipidemia, such as hypercholesterolemia, hypertriglyceridemia, high LDL-C, and low HDL-C. Our results suggest that hypertriglyceridemia is a risk factor of EE, but not dyslipidemia and its other components. Several studies have suggested triglyceride (TG) as an independent risk factor for EE related to humoral components that altered LESP and the frequency of transient relaxation44,45. TG has also been correlated with high fat intake, causing delayed gastric emptying time46,47,48. Moreover, hypertriglyceridemia is a significant predictive factor of EE severity, possibly related to fatty liver and insulin resistance49. The chronic inflammation in EE due to gastric acid injury may cause abnormal lipid metabolism, increasing TG47. Yet, several studies do not find TG to be an independent risk factor of EE50,51.
NAFLD also reaches statistical significance as a risk factor for EE. A study reports that only NAFLD is associated with EE, but not obesity45. NAFLD also increases the systemic oxidative stress and decreases the antioxidant capacity, which disrupts the gastric mucus layer and further causing esophageal mucosal damage and increasing the risk of EE45.
Interestingly, both gastric atrophy and H. pylori infection show to be protective factors for EE. The gastric atrophy can be classified into closed-type (C-type) and open type (O-type) according to the endoscopic atrophic border. According to Kim et al.52, the ambulatory pH monitoring study indicates that the O-type is associated with a lesser number of reflux symptoms and EE than the C-type. The O-type is characterized by an increasing number of impaired acid secreting parietal gastric cells will hinder more the gastric acid production, which will lead to hypochlorhydria, lessen the esophageal acidity, and further contribute to the pathogenesis of EE52,53. In a similar manner, the H. pylori infection may present protective mechanism since H. pylori chronic inflammation can cause gastric atrophy and further decreases the acid secretory capacity of the gastric lining54,55. It is only observable in O-type cases, while missing in the C-type, which produces higher gastrin and acid secretion56. However, this finding should be interpreted carefully since uneradicated H. pylori still carries a high risk of gastric cancer through several complex mechanisms57. Therefore, even though H. pylori is protective towards EE in our study, its eradication should still be well-considered to prevent the incidence of gastric cancer in later life.
To the best of our understanding, there has been no study that focuses on the meta-analysis of EE prevalence. We find that the prevalence of EE in America and Europe is higher than that in Asia. Recent meta-analyses on the prevalence of GERD58 and BE59 show similar results. A study by Qumseya et al.60 also finds a higher pooled prevalence of BE in low-risk Western populations compared to non-Western populations. One explanation for this distribution may be the difference in lifestyles. The typical Western diet is known to be high in fat, sodium, calories, and sugar, while it is low in fiber, fruits and vegetables. Concurrently, we have identified that White/Caucasian and individuals with obesity, type 2 diabetes mellitus, hypertension, dyslipidemia, and associated disease, such as NAFLD, are more significantly at risk of suffering from EE. Additionally, our meta-analysis shows a higher pooled prevalence of EE in Africa and the Middle East compared to those in other regions. This finding is in contrast to a previous BE meta-analysis by Eusebi et al.59, which finds the prevalence of BE in African and Middle Eastern countries to be lower than that in American countries.
We acknowledge several limitations in our study. First, we find some considerable high heterogeneities in most of the analyzed factors, mainly between the studies, such as population characteristics, various EE diagnostic criteria, differences in UGI study indications, and comorbidities along with various diagnostic criteria and cut-off values for their diagnosis. Second, although the EE diagnosis in the included studies is based on endoscopic result and the associated diagnostic criteria, endoscopy is still relatively an operator dependent-investigation, which may affect the EE prevalence in each country. Third, the number of included studies in several factors is still less than 10 studies; hence, the results should be carefully interpreted. Fourth, the included studies are mostly conducted in Asia (84 studies) and America (15 studies). This may affect the prevalence and risk factors of EE, and their interpretations in our study. Accordingly, we encourage more researchers from regions other than Asia to conduct more studies regarding the prevalence and risk factors of EE. However, regardless of the limitations, our study carries some strengths. The numbers of our included studies and their participants are relatively sufficient to cover a wide range of geographical areas; therefore, we can analyze the worldwide EE prevalence.
As the conclusion, we find several risk and protective factors of EE classified in three groups of factors, including demographical factors, comorbidities, and medication history. In the demographical factors, the risk of EE is increased due to age ≥ 60 y.o., being White/Caucasian, being single or unmarried, and having GERD ≥ 5 years. Interestingly, both risk and protective factors of EE are found in the comorbidities. Obesity, DM, hypertension, dyslipidemia, hypertriglyceridemia, HH, and NAFLD act as risk factors, while H. pylori infection and atrophic gastritis act as protective factors. The EE prevalence in each of America, Africa, and Europe are higher than that in Asia. Given these findings, an integrated care pathways of EE—including the decision regarding the timing of endoscopy based on the risk factors—is expected to be constructed, which then may help medical professionals to give proper and comprehensive managements for patients who are at a high risk of EE.
Methods
This systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) latest statement61. The protocol of this study has been previously registered to the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42023418716).
Search strategy
A systematic computerized data searching of relevant studies was conducted in four electronic medical databases, including PubMed, Scopus, Cumulative Index to Nursing and Allied Health Literature (CINAHL) Plus database via EBSCOhost, and Web of Science, by two authors (A.P.W. and B.S.W.) from inception to June 2, 2022. The construction of keywords was performed based on Medical Subject Headings (MeSH) terms combined with their variance and other additional terms as following: “risk”, “predict”, “erosive esophagitis”, “gastroesophageal reflux disease”, and the variations of those terms. Boolean operators’ combinations were also applied in order to broaden and narrow the search results. The search was restricted to human participants only with no language and publication date restrictions.
Eligibility criteria
The relevant studies were included if they met several following inclusion criteria: (1) study design of observational study; (2) study participants consisted of adult patients aged 18 years or older who had undergone upper gastrointestinal (UGI) endoscopy, either to screen or to diagnose EE; and (3) the measured outcomes were odds ratios (ORs) of any possible risk factors related to EE and number of EE events. The exclusion criteria were as follows: (1) duplicate studies; (2) irrelevant titles and/or abstracts; (3) irretrievable full-texts; and (4) incorrect study design (review articles, clinical trials, systematic reviews, meta-analyses, case reports or series, letter to editors, conference abstracts).
Data extraction and quality assessment
All relevant studies were independently screened by seven of the co-authors. Any disagreements were resolved in a consensus involving all authors. The extracted data from the included studies were the author, year of publication, study location (country and region), study design, diagnostic guideline for EE, age, specific population characteristic, sample size, number of EE events, EE-related risk factors expressed in ORs, and the adjustment factors. We assessed the quality of the included studies using the Newcastle–Ottawa Scale (NOS) tool. For cohort and case–control studies, their quality was considered as good, moderate, or poor if their score was 7–9, 4–6, and 0–3, respectively. For cross-sectional studies, a score of 9–10 was considered as very good, 7–8 as good, 5–6 as satisfactory, and 0–4 as unsatisfactory. The quality assessment was conducted collaboratively through a group discussion by all authors, and the final decision was also taken based on the agreement of all authors.
Statistical analysis
Meta-analyses were performed for the outcome of pooled ORs in each EE-related risk factor using RevMan ver. 5.4 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark). We also performed meta-analysis of pooled EE prevalence in each study using STATA ver. 16.0 (Stata Corporation, College Station, TX, USA) as the secondary outcome. The heterogeneity among studies was assessed using chi-square test (Cochran’s Q statistic). Then, we quantified the level of heterogeneity with the Higgins’ I2 statistic as follows: 0% was considered negligible heterogeneity, < 25% as low heterogeneity, 25–75% as moderate heterogeneity, and > 75% as high heterogeneity62. Since there was a considerable variability and diversity among studies and the characteristics of the study participants, we primarily applied the random-effect model (REM) for risk factors and prevalence analyses. P-value < 0.05 was considered statistically significant. The publication bias was visually assessed using funnel plot and quantitatively assessed using Egger’s test. Sensitivity analysis was carried out using the leave-one-out method.
Data availability
All data generated or analyzed during this study are included in this article and its supplementary material files. Further enquiries can be directed to the corresponding author.
References
Antunes, C., Aleem, A. & Curtis, S. A. Gastroesophageal reflux disease (2023).
Azami, M., Salamati, M., Ranjbar, R. & Sahebkar, A. The association between metabolic syndrome and erosive esophagitis: A systematic review and meta-analysis. EXCLI J. 20, 1532–1543 (2021).
Cheng, J. & Ouwehand, A. C. Gastroesophageal reflux disease and probiotics: A systematic review. Nutrients 12 (2020).
Fass, R. & Ofman, J. J. Gastroesophageal reflux disease—Should we adopt a new conceptual framework?. Am. J. Gastroenterol. 97, 1901–1909 (2002).
Ierardi, E. et al. Metabolic syndrome and gastro-esophageal reflux: A link towards a growing interest in developed countries. World J. Gastrointest. Pathophysiol. 1, 91–96 (2010).
Savarino, E. et al. Epidemiology and natural history of gastroesophageal reflux disease. Minerva Gastroenterol. Dietol. 63, 175–183 (2017).
Antunes, C. & Sharma, A. Esophagitis (2023).
Sandhu, D. S. & Fass, R. Current trends in the management of gastroesophageal reflux disease. Gut Liver 12, 7–16 (2018).
Wiklund, I. Review of the quality of life and burden of illness in gastroesophageal reflux disease. Dig. Dis. 22, 108–114 (2004).
Wang, R.-H. From reflux esophagitis to Barrett’s esophagus and esophageal adenocarcinoma. World J. Gastroenterol. 21, 5210–5219 (2015).
Chue, K. M. et al. The Hill’s classification is useful to predict the development of postoperative gastroesophageal reflux disease and erosive esophagitis after laparoscopic sleeve gastrectomy. J. Gastrointest Surg. Off. J. Soc. Surg. Aliment. Tract 26, 1162–1170 (2022).
Nurleili, R. A. et al. Visceral fat thickness of erosive and non-erosive reflux disease subjects in Indonesia’s tertiary referral hospital. Diabetes Metab. Syndr. 13, 1929–1933 (2019).
Kulig, M. et al. Risk factors of gastroesophageal reflux disease: Methodology and first epidemiological results of the ProGERD study. J. Clin. Epidemiol. 57, 580–589 (2004).
Chung, T.-H., Lee, J., Jeong, I.-D. & Lee, K.-C. Effect of weight changes on the development of erosive esophagitis. Korean J. Fam. Med. 41, 14–19 (2020).
Mun, E., Kim, D., Lee, Y., Lee, W. & Park, S. Association between Shift Work and Reflux Esophagitis: The Kangbuk Samsung Health Study. Int. J. Environ. Res. Public Health 18 (2021).
Sadiku, E. et al. Extra-esophageal symptoms in individuals with and without erosive esophagitis: A case-control study in Albania. BMC Gastroenterol. 21, 76 (2021).
Cho, J. H. et al. Old age and male sex are associated with increased risk of asymptomatic erosive esophagitis: Analysis of data from local health examinations by the Korean National Health Insurance Corporation. J. Gastroenterol. Hepatol. 26, 1034–1038 (2011).
Ko, S.-H., Baeg, M. K., Jung, H. S., Kim, P. & Choi, M.-G. Russian Caucasians have a higher risk of erosive reflux disease compared with East Asians: A direct endoscopic comparison. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 29 (2017).
Kang, M. S. et al. Abdominal obesity is an independent risk factor for erosive esophagitis in a Korean population. J. Gastroenterol. Hepatol. 22, 1656–1661 (2007).
Erol, A. & Karpyak, V. M. Sex and gender-related differences in alcohol use and its consequences: Contemporary knowledge and future research considerations. Drug Alcohol Depend. 156, 1–13 (2015).
Matsuzaki, J. et al. Association of visceral fat area, smoking, and alcohol consumption with reflux esophagitis and Barrett’s Esophagus in Japan. PLoS ONE 10, e0133865 (2015).
Adachi, K., Mishiro, T., Tanaka, S., Hanada, K. & Kinoshita, Y. Gender differences in the time-course changes of reflux esophagitis in Japanese patients. Intern. Med. 54, 869–873 (2015).
Kim, S. Y. et al. Gender specific differences in prevalence and risk factors for gastro-esophageal reflux disease. J. Korean Med. Sci. 34, e158 (2019).
Kim, Y. S., Kim, N. & Kim, G. H. Sex and gender differences in gastroesophageal reflux disease. J. Neurogastroenterol. Motil. 22, 575–588 (2016).
Wirth, H.-P. & Yang, M. Different pathophysiology of gastritis in east and west? A western perspective. Inflamm. Intest. Dis. 1, 113–122 (2016).
Abraham, A. et al. Erosive esophagitis in the obese: the effect of ethnicity and gender on its association. Gastroenterol. Res. Pract. 2016, 7897390 (2016).
Suzuki, H. & Mori, H. Different pathophysiology of gastritis between east and west?. An Asian Perspective. Inflamm. Intest. Dis. 1, 123–128 (2016).
Torresan, F. et al. A new mechanism of gastroesophageal reflux in hiatal hernia documented by high-resolution impedance manometry: a case report. Ann. Gastroenterol. 29, 548–550 (2016).
Jones, M. P. et al. Hiatal hernia size is the dominant determinant of esophagitis presence and severity in gastroesophageal reflux disease. Am. J. Gastroenterol. 96, 1711–1717 (2001).
Sfara, A. & Dumitrascu, D. L. The management of hiatal hernia: an update on diagnosis and treatment. Med. Pharm. reports 92, 321–325 (2019).
Che, F., Nguyen, B., Cohen, A. & Nguyen, N. T. Prevalence of hiatal hernia in the morbidly obese. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 9, 920–924 (2013).
Rogers, B. D., Patel, A., Wang, D., Sayuk, G. S. & Gyawali, C. P. Higher esophageal symptom burden in obese subjects results from increased esophageal acid exposure and not from dysmotility. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 18, 1719–1726 (2020).
Baeg, M. K., Ko, S.-H., Ko, S. Y., Jung, H. S. & Choi, M.-G. Obesity increases the risk of erosive esophagitis but metabolic unhealthiness alone does not: A large-scale cross-sectional study. BMC Gastroenterol. 18, 82 (2018).
Smit, M. et al. How central obesity influences intra-abdominal pressure: A prospective, observational study in cardiothoracic surgical patients. Ann. Intensive Care 6, 99 (2016).
Nam, S. Y. et al. The effect of abdominal visceral fat, circulating inflammatory cytokines, and leptin levels on reflux esophagitis. J. Neurogastroenterol. Motil. 21, 247–254 (2015).
Kinekawa, F. et al. Relationship between esophageal dysfunction and neuropathy in diabetic patients. Am. J. Gastroenterol. 96, 2026–2032 (2001).
Annese, V. et al. Gastrointestinal motor dysfunction, symptoms, and neuropathy in noninsulin-dependent (type 2) diabetes mellitus. J. Clin. Gastroenterol. 29, 171–177 (1999).
Moki, F. et al. Association between reflux oesophagitis and features of the metabolic syndrome in Japan. Aliment. Pharmacol. Ther. 26, 1069–1075 (2007).
Loke, S.-S., Yang, K. D., Chen, K.-D. & Chen, J.-F. Erosive esophagitis associated with metabolic syndrome, impaired liver function, and dyslipidemia. World J. Gastroenterol. 19, 5883–5888 (2013).
Kinekawa, F. et al. Esophageal function worsens with long duration of diabetes. J. Gastroenterol. 43, 338–344 (2008).
Nishida, T. et al. Gastroesophageal reflux disease related to diabetes: Analysis of 241 cases with type 2 diabetes mellitus. J. Gastroenterol. Hepatol. 19, 258–265 (2004).
Gudlaugsdottir, S., Verschuren, W., Dees, J., Stijnen, T. & Wilson, J. Hypertension is frequently present in patients with reflux esophagitis or Barrett’s esophagus but not in those with non-ulcer dyspepsia. Eur. J. Intern. Med. 13, 369 (2002).
Hu, Z. et al. Improved control of hypertension following laparoscopic fundoplication for gastroesophageal reflux disease. Front. Med. 11, 68–73 (2017).
Chung, S. J. et al. Metabolic syndrome and visceral obesity as risk factors for reflux oesophagitis: A cross-sectional case-control study of 7078 Koreans undergoing health check-ups. Gut 57, 1360–1365 (2008).
Hung, W.-C. et al. Nonalcoholic fatty liver disease vs. obesity on the risk of erosive oesophagitis. Eur. J. Clin. Invest. 44, 1143–1149 (2014).
Hsieh, Y.-H. et al. What is the impact of metabolic syndrome and its components on reflux esophagitis? A cross-sectional study. BMC Gastroenterol. 19, 33 (2019).
Chua, C.-S. et al. Metabolic risk factors associated with erosive esophagitis. J. Gastroenterol. Hepatol. 24, 1375–1379 (2009).
Chih, P.-C. et al. Overweight associated with increased risk of erosive esophagitis in a non-obese Taiwanese population. PLoS ONE 8, e77932 (2013).
Park, J.-H. et al. Metabolic syndrome is associated with erosive esophagitis. World J. Gastroenterol. 14, 5442–5447 (2008).
Ha, N. R. et al. Differences in clinical characteristics between patients with non-erosive reflux disease and erosive esophagitis in Korea. J. Korean Med. Sci. 25, 1318–1322 (2010).
Ou, J.-L. et al. Prevalence and risk factors of erosive esophagitis in Taiwan. J. Chin. Med. Assoc. 75, 60–64 (2012).
Kim, D.-H. et al. Endoscopic grading of atrophic gastritis is inversely associated with gastroesophageal reflux and gastropharyngeal reflux. Korean J. Intern. Med. 22, 231–236 (2007).
Ghoshal, U. C. & Chourasia, D. Gastroesophageal reflux disease and: what may be the relationship?. J. Neurogastroenterol. Motil. 16, 243–250 (2010).
Han, Y. M. et al. Inverse correlation between gastroesophageal reflux disease and atrophic gastritis assessed by endoscopy and serology. World J. Gastroenterol. 28, 853–867 (2022).
Koike, T. et al. Helicobacter pylori infection prevents erosive reflux oesophagitis by decreasing gastric acid secretion. Gut 49, 330–334 (2001).
Amieva, M. R. & El-Omar, E. M. Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology 134, 306–323 (2008).
Díaz, P., Valenzuela Valderrama, M., Bravo, J. & Quest, A. F. G. Helicobacter pylori and gastric cancer: adaptive cellular mechanisms involved in disease progression. Front. Microbiol. 9, 5 (2018).
Nirwan, J. S., Hasan, S. S., Babar, Z.-U.-D., Conway, B. R. & Ghori, M. U. Global prevalence and risk factors of gastro-oesophageal reflux disease (GORD): Systematic review with meta-analysis. Sci. Rep. 10, 5814 (2020).
Eusebi, L. H., Cirota, G. G., Zagari, R. M. & Ford, A. C. Global prevalence of Barrett’s oesophagus and oesophageal cancer in individuals with gastro-oesophageal reflux: A systematic review and meta-analysis. Gut 70, 456–463 (2021).
Qumseya, B. J. et al. Systematic review and meta-analysis of prevalence and risk factors for Barrett’s esophagus. Gastrointest. Endosc. 90, 707-717.e1 (2019).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, n71 (2021).
Higgins, J. P. T., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003).
Adekanle, O., Olowookere, S. A., Ijarotimi, O., Ndububa, D. A. & Komolafe, A. O. Relationship between body mass index, waist circumference, waist hip ratio and erosive gastroesophageal reflux disease in a tertiary centre in Nigeria: A case control study. Alexandria J. Med. 54, 605–609 (2018).
Al Shammaa, A. S. M., Abdulmuhsen, F. K. & Hatem, R. M. Fatty liver disease and the risk of erosive esophagitis in a sample of Iraqi patients: A cross sectional study. Rev. Latinoam. Hipertens. 15, 8–14 (2021).
Avidan, B., Sonnenberg, A., Schnell, T. G. & Sontag, S. J. Risk factors for erosive reflux esophagitis: A case-control study. Am. J. Gastroenterol. 96, 41–46 (2001).
Avidan, B., Sonnenberg, A., Schnell, T. G., Budiman-Mak, E. & Sontag, S. J. Risk factors of oesophagitis in arthritic patients. Eur. J. Gastroenterol. Hepatol. 13, 1095–1099 (2001).
Avidan, B., Sonnenberg, A., Schnell, T. G. & Sontag, S. J. Acid reflux is a poor predictor for severity of erosive reflux esophagitis. Dig. Dis. Sci. 47, 2565–2573 (2002).
Barreda Costa, C. et al. Lower prevalence of Helicobacter pylori infection observed in patients with erosive esophagitis. Rev. Gastroenterol. del Peru organo Of. la Soc Gastroenterol. del Peru 34, 33–37 (2014).
Chang, C.-H. et al. Alcohol and tea consumption are associated with asymptomatic erosive esophagitis in Taiwanese men. PLoS ONE 12, e0173230 (2017).
Chen, C.-N., Wu, M.-S., Lien, G.-S. & Suk, F.-M. Influence of replacing percutaneous endoscopic gastrostomy for nasogastric tube feeding on gastroesophageal reflux disease with erosive esophagitis. Adv. Dig. Med. 3, 49–55 (2016).
Cheng, H.-H., Chang, C.-S., Wang, H.-J. & Wang, W.-C. Interleukin-1beta and -10 polymorphisms influence erosive reflux esophagitis and gastritis in Taiwanese patients. J. Gastroenterol. Hepatol. 25, 1443–1451 (2010).
Cheng, H. et al. Supplementation of Los Angeles classification with esophageal mucosa index of hemoglobin can predict the treatment response of erosive reflux esophagitis. Surg. Endosc. 25, 2478–2486 (2011).
Chiba, H. et al. A cross-sectional study on the risk factors for erosive esophagitis in young adults. Intern. Med. 51, 1293–1299 (2012).
Choi, J. S. et al. Fatty liver disease and the risk of erosive oesophagitis in the Korean population: A cross-sectional study. BMJ Open 9, e023585 (2019).
Chung, H. et al. Noninvasive prediction of erosive esophagitis using a controlled attenuation parameter (CAP)-based risk estimation model. Dig. Dis. Sci. 61, 507–516 (2016).
Chung, T. H., Lee, J. & Kim, M. C. Impact of night-shift work on the prevalence of erosive esophagitis in shipyard male workers. Int. Arch. Occup. Environ. Health 89, 961–966 (2016).
Deppe, H. et al. Erosive esophageal reflux vs. non erosive esophageal reflux: Oral findings in 71 patients. BMC Oral Health 15, 84 (2015).
El-Serag, H. B. & Sonnenberg, A. Association of esophagitis and esophageal strictures with diseases treated with nonsteroidal anti-inflammatory drugs. Am. J. Gastroenterol. 92, 52–56 (1997).
El-Serag, H. B., Satia, J. A. & Rabeneck, L. Dietary intake and the risk of gastro-oesophageal reflux disease: a cross sectional study in volunteers. Gut 54, 11–17 (2005).
El-Serag, H. B., Graham, D. Y., Satia, J. A. & Rabeneck, L. Obesity is an independent risk factor for GERD symptoms and erosive esophagitis. Am. J. Gastroenterol. 100, 1243–1250 (2005).
Filiberti, R. A. et al. Alcohol consumption pattern and risk of Barrett’s oesophagus and erosive oesophagitis: An Italian case-control study. Br. J. Nutr. 117, 1151–1161 (2017).
Fujiwara, Y. et al. Differences in clinical characteristics between patients with endoscopy-negative reflux disease and erosive esophagitis in Japan. Am. J. Gastroenterol. 100, 754–758 (2005).
Gaddam, S. et al. The impact of pre-endoscopy proton pump inhibitor use on the classification of non-erosive reflux disease and erosive oesophagitis. Aliment. Pharmacol. Ther. 32, 1266–1274 (2010).
Gado, A., Ebeid, B., Abdelmohsen, A. & Axon, A. Prevalence of reflux esophagitis among patients undergoing endoscopy in a secondary referral hospital in Giza, Egypt. Alexandria J. Med. 51, 89–94 (2015).
Gatopoulou, A. et al. Impact of Helicobacter pylori infection on histological changes in non-erosive reflux disease. World J. Gastroenterol. 10, 1180–1182 (2004).
Gunji, T. et al. Risk factors for erosive esophagitis: A cross-sectional study of a large number of Japanese males. J. Gastroenterol. 46, 448–455 (2011).
Ham, H. et al. Esophagogastric junction contractile integral and morphology: Two high-resolution manometry metrics of the anti-reflux barrier. J. Gastroenterol. Hepatol. 32, 1443–1449 (2017).
Heo, C. M. et al. Impact of sarcopenia on the risk of erosive esophagitis. Korean J. Gastroenterol. 75, 132–140 (2020).
Hsu, C.-S. et al. Increasing insulin resistance is associated with increased severity and prevalence of gastro-oesophageal reflux disease. Aliment. Pharmacol. Ther. 34, 994–1004 (2011).
Hung, H.-H. et al. Establishing a risk scoring system for predicting erosive esophagitis. Adv. Dig. Med. 3, 95–100 (2016).
Hung, W.-C. et al. Gender differences in the association of non-alcoholic fatty liver disease and metabolic syndrome with erosive oesophagitis: A cross-sectional study in a Taiwanese population. BMJ Open 6, e013106 (2016).
Isshi, K. et al. Clinical features and therapeutic responses to proton pump inhibitor in patients with severe reflux esophagitis: A multicenter prospective observational study. JGH Open Open Access J. Gastroenterol. Hepatol. 5, 99–106 (2021).
Jo, S. Y. et al. Comparison of gastroesophageal reflux disease symptoms and proton pump inhibitor response using gastroesophageal reflux disease impact scale questionnaire. J. Neurogastroenterol. Motil. 19, 61–69 (2013).
Jonaitis, L. V., Kiudelis, G. & Kupcinskas, L. Characteristics of patients with erosive and nonerosive GERD in high-Helicobacter-pylori prevalence region. Dis. Esophagus Off J. Int. Soc. Dis. Esophagus 17, 223–227 (2004).
Jonaitis, L., Kupčinskas, J., Kiudelis, G. & Kupčinskas, L. D. novo erosive esophagitis in duodenal ulcer patients related to pre-existing reflux symptoms, smoking, and patient age, but not to Helicobacter pylori eradication: A one-year follow-up study. Medicina (Kaunas). 46, 454–459 (2010).
Jones, M. P., Sloan, S. S., Jovanovic, B. & Kahrilas, P. J. Impaired egress rather than increased access: An important independent predictor of erosive oesophagitis. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 14, 625–631 (2002).
Jung, J. G. et al. Vegetarianism as a protective factor for reflux esophagitis: A retrospective, cross-sectional study between Buddhist priests and general population. Dig. Dis. Sci. 58, 2244–2252 (2013).
Kainuma, M. et al. The association between objective tongue color and endoscopic findings: Results from the Kyushu and Okinawa population study (KOPS). BMC Complement. Altern. Med. 15, 372 (2015).
Kavitt, R. T. et al. Esophageal mucosal impedance pattern is distinct in patients with extraesophageal reflux symptoms and pathologic acid reflux. J. Voice 31, 347–351 (2017).
Kawai, T. et al. Helicobacter pylori infection and reflux esophagitis in young and middle-aged Japanese subjects. J. Gastroenterol. Hepatol. 25(Suppl 1), S80–S85 (2010).
Kim, H. Y. Association between erosive esophagitis and the anthropometric index in the general Korean population. Balkan Med. J. 36, 169–173 (2019).
Kim, J. G. et al. Halimeter ppb levels as the predictor of erosive gastroesophageal reflux disease. Gut Liver 4, 320–325 (2010).
Kim, J. Y. et al. Association of sleep dysfunction and emotional status with gastroesophageal reflux disease in Korea. J. Neurogastroenterol. Motil. 19, 344–354 (2013).
Koo, J. S. et al. Abdominal obesity as a risk factor for the development of erosive esophagitis in subjects with a normal esophago-gastric junction. Gut Liver 3, 276–284 (2009).
Lee, D., Lee, K. J., Kim, K. M. & Lim, S. K. Prevalence of asymptomatic erosive esophagitis and factors associated with symptom presentation of erosive esophagitis. Scand. J. Gastroenterol. 48, 906–912 (2013).
Lee, Y.-C. et al. Comparative analysis between psychological and endoscopic profiles in patients with gastroesophageal reflux disease: A prospective study based on screening endoscopy. J. Gastroenterol. Hepatol. 21, 798–804 (2006).
Lee, H. L. et al. Association between GERD-related erosive esophagitis and obesity. J. Clin. Gastroenterol. 42, 672–675 (2008).
Lee, H. L. et al. Association between erosive esophagitis and visceral fat accumulation quantified by abdominal CT scan. J. Clin. Gastroenterol. 43, 240–243 (2009).
Lee, E. S. et al. Comparison of risk factors and clinical responses to proton pump inhibitors in patients with erosive oesophagitis and non-erosive reflux disease. Aliment. Pharmacol. Ther. 30, 154–164 (2009).
Lee, S. D., Keum, B., Chun, H. J. & Bak, Y.-T. Gastroesophageal reflux disease in type ii diabetes mellitus with or without peripheral neuropathy. J. Neurogastroenterol. Motil. 17, 274–278 (2011).
Lee, S.-W. et al. Impact of body mass index and gender on quality of life in patients with gastroesophageal reflux disease. World J. Gastroenterol. 18, 5090–5095 (2012).
Lee, S.-W. et al. Characteristics of symptom presentation and risk factors in patients with erosive esophagitis and nonerosive reflux disease. Med. Princ. Pract. Int. J. Kuwait Univ. Heal. Sci. Cent. 23, 460–464 (2014).
Lee, S.-W. et al. Association of metabolic syndrome with erosive esophagitis and Barrett’s esophagus in a Chinese population. J. Chin. Med. Assoc. 80, 15–18 (2017).
Lee, S.-W. et al. Impact of obesity on a Chinese population with Erosive Esophagitis and Barrett’s Esophagus. Gut Liver 11, 377–382 (2017).
Lee, H. et al. Relationship between obesity and development of erosive reflux disease: A mediation analysis of the role of cardiometabolic risk factors. Sci. Rep. 7, 6375 (2017).
Li, C.-H. et al. Different risk factors between reflux symptoms and mucosal injury in gastroesophageal reflux disease. Kaohsiung J. Med. Sci. 31, 320–327 (2015).
Lien, H.-C. et al. Increasing prevalence of erosive esophagitis among Taiwanese aged 40 years and above: a comparison between two time periods. J. Clin. Gastroenterol. 43, 926–932 (2009).
Lippmann, Q. K., Crockett, S. D., Dellon, E. S. & Shaheen, N. J. Quality of life in GERD and Barrett’s esophagus is related to gender and manifestation of disease. Am. J. Gastroenterol. 104, 2695–2703 (2009).
Lord, R. V. N. et al. Hiatal hernia, lower esophageal sphincter incompetence, and effectiveness of Nissen fundoplication in the spectrum of gastroesophageal reflux disease. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 13, 602–610 (2009).
Mahdi, B. M., Hasan, R. M. & Salih, W. H. Human leukocyte antigen HLADRB1 determinants susceptibility to gastroesophageal reflux disease. Arq. Gastroenterol. 54, 41–45 (2017).
Matsuda, R. et al. Gastroesophageal reflux disease-related disorders of systemic sclerosis based on the analysis of 66 patients. Digestion 98, 201–208 (2018).
Matsuki, N. et al. Lifestyle factors associated with gastroesophageal reflux disease in the Japanese population. J. Gastroenterol. 48, 340–349 (2013).
Matsumura, T. et al. Endoscopic-guided measurement of mucosal admittance can discriminate gastroesophageal reflux disease from functional heartburn. Clin. Transl. Gastroenterol. 8, e94 (2017).
Meira, A. T. D. S., Tanajura, D. & Viana, I. D. S. Clinical and endoscopic evaluation in patients with gastroesophageal symptoms. Arq. Gastroenterol. 56, 51–54 (2019).
Migaczewski, M. et al. The prevalence of, and risk factors for, Barrett’s oesophagus after sleeve gastrectomy. Wideochirurgia i inne Tech. maloinwazyjne Videosurgery other miniinvasive Tech. 16, 710–714 (2021).
Minatsuki, C. et al. Background factors of reflux esophagitis and non-erosive reflux disease: A cross-sectional study of 10,837 subjects in Japan. PLoS ONE 8, e69891 (2013).
Nam, S. Y. et al. Different effects of dietary factors on reflux esophagitis and non-erosive reflux disease in 11,690 Korean subjects. J. Gastroenterol. 52, 818–829 (2017).
Noh, Y. W., Jung, H.-K., Kim, S.-E. & Jung, S.-A. Overlap of erosive and non-erosive reflux diseases with functional gastrointestinal disorders according to Rome III criteria. J. Neurogastroenterol. Motil. 16, 148–156 (2010).
Ohashi, S. et al. Visceral fat obesity is the key risk factor for the development of reflux erosive esophagitis in 40–69-years subjects. Esophagus 18, 889–899 (2021).
Oikawa, T. et al. Gene polymorphisms of NOD1 and interleukin-8 influence the susceptibility to erosive esophagitis in Helicobacter pylori infected Japanese population. Hum. Immunol. 73, 1184–1189 (2012).
Park, C. H. et al. Differences in the risk factors of reflux esophagitis according to age in Korea. Dis. Esophagus 27, 116–121 (2012).
Rafat, M. N. et al. Adiponectin level changes among Egyptians with gastroesophageal reflux disease. JGH Open Open Access J. Gastroenterol. Hepatol. 2, 21–27 (2018).
Ronkainen, J. et al. High prevalence of gastroesophageal reflux symptoms and esophagitis with or without symptoms in the general adult Swedish population: A Kalixanda study report. Scand. J. Gastroenterol. 40, 275–285 (2005).
Savarino, E. et al. Characteristics of reflux episodes and symptom association in patients with erosive esophagitis and nonerosive reflux disease: Study using combined impedance-pH off therapy. Am. J. Gastroenterol. 105, 1053–1061 (2010).
Savarino, E. et al. Overweight is a risk factor for both erosive and non-erosive reflux disease. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 43, 940–945 (2011).
Shaker, A. & Magdy, M. Frequency of obstructive sleep apnea (OSA) in patients with gastroesophageal reflux disease (GERD) and the effect of nasal continuous positive airway pressure. Egypt. J. Chest Dis. Tuberc. 65, 797–803 (2016).
Shimamoto, T. et al. No association of coffee consumption with gastric ulcer, duodenal ulcer, reflux esophagitis, and non-erosive reflux disease: A cross-sectional study of 8,013 healthy subjects in Japan. PLoS ONE 8, e65996 (2013).
Shimatani, T. et al. Gastric acid normosecretion is not essential in the pathogenesis of mild erosive gastroesophageal reflux disease in relation to Helicobacter pylori status. Dig. Dis. Sci. 49, 787–794 (2004).
Sogabe, M., Okahisa, T., Kimura, Y., Hibino, S. & Yamanoi, A. Visceral fat predominance is associated with erosive esophagitis in Japanese men with metabolic syndrome. Eur. J. Gastroenterol. Hepatol. 24, 910–916 (2012).
Sogabe, M. et al. Differences in several factors in the development of erosive esophagitis among patients at various stages of metabolic syndrome: A cross-sectional study. Diabetes. Metab. Syndr. Obes. 14, 1589–1600 (2021).
Tai, C.-M. et al. The relationship between visceral adiposity and the risk of erosive esophagitis in severely obese Chinese patients. Obesity (Silver Spring) 18, 2165–2169 (2010).
Tai, C.-M. et al. Increase in gastroesophageal reflux disease symptoms and erosive esophagitis 1 year after laparoscopic sleeve gastrectomy among obese adults. Surg. Endosc. 27, 1260–1266 (2013).
Vaishnav, B., Bamanikar, A., Maske, P., Reddy, A. & Dasgupta, S. Gastroesophageal reflux disease and its association with body mass index: Clinical and endoscopic study. J. Clin. Diagn. Res. 11, OC01–OC04 (2017).
Wang, F.-W. et al. Erosive esophagitis in asymptomatic subjects: Risk factors. Dig. Dis. Sci. 55, 1320–1324 (2010).
Wang, P.-C. et al. Male sex, hiatus hernia, and Helicobacter pylori infection associated with asymptomatic erosive esophagitis. J. Gastroenterol. Hepatol. 27, 586–591 (2012).
Wang, K. et al. A population-based survey of gastroesophageal reflux disease in a region with high prevalence of esophageal cancer in China. Chin. Med. J. (Engl.) 132, 1516–1523 (2019).
Wei, T.-Y., Hsueh, P.-H., Wen, S.-H., Chen, C.-L. & Wang, C.-C. The role of tea and coffee in the development of gastroesophageal reflux disease. Ci ji yi xue za zhi Tzu-chi Med. J. 31, 169–176 (2019).
Wu, P. et al. The association of metabolic syndrome with reflux esophagitis: A case-control study. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 23, 989–994 (2011).
Wu, P. et al. Dietary intake and risk for reflux esophagitis: a case-control study. Gastroenterol. Res. Pract. 2013, 691026 (2013).
Yamamoto, T. et al. Prevalence of erosive esophagitis among Japanese patients taking low-dose aspirin. J. Gastroenterol. Hepatol. 25, 792–794 (2010).
Yang, Y.-J., Sheu, B.-S., Chang, W.-L., Cheng, H.-C. & Yang, H.-B. Increased body mass index after H. pylori eradication for duodenal ulcer predisposes to erosive reflux esophagitis. J. Clin. Gastroenterol. 43, 705–710 (2009).
Yasuhara, H. et al. Large waist circumference is a risk factor for reflux esophagitis in Japanese males. Digestion 81, 181–187 (2010).
Ye, B.-X. et al. Association between body mass index, hiatal hernia and gastroesophageal reflux in patients with gastroesophageal reflux disease. Shijie Huaren Xiaohua Zazhi 20, 3375–3379 (2012).
Ze, E. Y., Kim, B. J., Kang, H. & Kim, J. G. Abdominal visceral to subcutaneous adipose tissue ratio is associated with increased risk of erosive esophagitis. Dig. Dis. Sci. 62, 1265–1271 (2017).
Author information
Authors and Affiliations
Contributions
A.P.W. and B.S.W. conceptualized and designed the study. A.P.W., B.S.W., S.L.P., L.C.R., N.A.P.W., T.K., and A.J.E.P. collected the data and writing original draft preparation. A.P.W., B.S.W., and C.D.K.W. performed statistical analyses. S.L.P., L.C.R., N.A.P.W., T.K., and A.J.E.P. assessed the quality of the studies. C.D.K.W., A.Z.M., and A.G. writing review and editing. All author gave a significant contribution to data interpretation, reviewed the manuscript, and approved it as submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Witarto, A.P., Witarto, B.S., Pramudito, S.L. et al. Risk factors and 26-years worldwide prevalence of endoscopic erosive esophagitis from 1997 to 2022: a meta-analysis. Sci Rep 13, 15249 (2023). https://doi.org/10.1038/s41598-023-42636-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-42636-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.