Abstract
Chemokines are chemotactic cytokines that can cause directed migration of leukocytes. The aim of this study was to examine differences in single nucleotide polymorphisms (SNP) of chemokine in AITD patients compared to normal controls. A total of 86 Korean pediatric patients were included in the patient group and 183 adults were included in the normal control group. To compare influences of several chemokine gene polymorphisms, 25 SNPs in 16 chemokine genes were analyzed. Genotype frequencies of CCL11(rs3744508)AA(OR = 6.9) and CCR2(rs1799864)AA(OR = 3.8) were higher in the AITD patients than in the controls, whereas CCL17(rs223828)CC was lower in the AITD patients than in the controls(OR = 0.4). In comparison between Graves' disease (GD) patients and controls, genotype frequency of CCL17(rs223828)CC(OR = 0.4) was lower in the GD group, whereas those of CCR2(rs1799864)AA(OR = 4.8) were higher in the GD group. The genotype frequency of CCL11(rs3744508)AA(OR = 11.3) was higher in Hashimoto's thyroiditis (HT) patients, whereas that of CXCL8(rs2227306)CC(OR = 0.4) was lower in HT patients. Polymorphisms of CCL11(rs3744508), CCL17(rs223828), and CCR2(rs1799864) might be associated with AITD, with CCL17(rs223828), CCR2(rs1799864) and CXCR2(rs2230054, rs1126579) affecting GD and CCL11(rs3744508) and CXCL8(rs2227306) affecting HT in Korean children.
Similar content being viewed by others
Introduction
Chemokines are small, chemotactic cytokines that play a crucial role in regulating cell trafficking of various types of leukocytes in development, homeostasis, and inflammation by binding to specific receptors1,2. In addition to guiding immune cells to infected or inflamed sites, chemokines coordinate interactions between immune cells, making them a crucial part of various diseases such as infections, cancer, healing recovery, angiogenesis, and autoimmunity2,3. Chemokines are divided into four subfamilies (CC, CXC, CX3C, and XC) based on their cysteine configurations, while chemokine receptors are transmembrane receptors that can induce cell migration, adhesion, and other biological responses1,3,4. There are also chemokine antagonists being used or studied for treatment5.
Autoimmune thyroid disease (AITD) encompasses a range of conditions where the immune system targets the thyroid gland. Classic AITD includes Graves’ disease (GD) and Hashimoto's thyroiditis (HT), both characterized by clinical manifestations of hyperthyroidism in GD and hypothyroidism in HT, along with thyroid infiltration, and the production of thyroid autoantibodies by T and B cells that react with thyroid antigens6. AITD may develop when genetically susceptible individuals are exposed to environmental triggers such as infection, iodine, and stress7,8. Thyroid associated ophthalmopathy (TAO) is an autoimmune disease that affects ocular and peripheral tissues due to shared autoantigen9. Although the recruitment and maintenance of lymphocytes in the thyroid gland is not yet fully understood, considerable clinical and experimental evidence supports the role of lymphocytes in triggering and maintaining AITD10. Early onset AITD is likely more strongly influenced by genetic susceptibility than late onset11.
AITD stands out as the most prevalent among autoimmune disorders, impacting 15 out of every 1000 individuals in the United States12. In a meta-analysis of thyroid dysfunction incidence and prevalence in Europe, it was revealed that the yearly incidence rate for hyperthyroidism stood at 0.51 cases per 1000 individuals within the population13. Based on a nationwide population-based cohort study in Korea that focused on the annual incidence and prevalence of thyroid disease, it was identified that the prevalence of individuals undergoing treatment for hyperthyroidism was 2.76 per 1000 people and the annual incidence of new diagnoses for hyperthyroidism, with corresponding ongoing treatment, was found to be 0.55 cases per 1000 individuals in the year 201514.
A number of studies have investigated the relationship between chemokines and AITD, given the important role of chemokines in autoimmune processes15,16,17. Our study aims to determine the relationship between chemokine and AITD by analyzing 25 Single nucleotide polymorphisms (SNPs) of 16 chemokine genes in Korean pediatric AITD patients, and comprehensively examining the associations of these SNPs with different groups of AITD.
Materials and methods
Subjects
The present study was conducted on 86 patients diagnosed with AITD [36 with HT and 50 with GD (intractable GD, n = 30) (TAO, n = 24; non-TAO, n = 26)] who were treated at the Pediatric Endocrine Clinics of Seoul St. Mary’s Hospital between March 2009 and August 2021. The age of patients in the study was 13.2 ± 3.3 years at enrollment, and 11.3 ± 3.2 years at diagnosis of AITD (Table 1).
For the control group, a total of 183 healthy Korean adults (52.5% females and 47.5% males; average age: 29.9 ± 3.7 years) without a history of AITD comprising staff and students from the College of Medicine at the Catholic University of Korea were included. All participants provided an informed consent for a genetic study. This study was approved by the Institutional Review Board (IRB) of the Catholic University of Korea (IRB Number: KC09FISI0042, MC13SISI0126), Seoul, Korea, and the study was conducted in accordance with the Declaration of Helsinki.
GD was diagnosed based on clinical symptoms and biochemical confirmation of hyperthyroidism, including diagnosis of goiter, elevated radioactive iodine uptake, antibodies against the thyroid-stimulating hormone (TSH) receptor, and elevated thyroid hormone levels. Patients with other forms of autoimmune diseases, hematologic diseases, or endocrine diseases were excluded. TAO was diagnosed based on the presence of typical clinical features. It was classified according to the system recommended by the American Thyroid Association (ATA) Committee18. Patients with no symptoms or the lid lag sign only were included in the non-TAO group, whereas those with soft tissue changes, proptosis, extraocular muscle dysfunction, or the latter two were considered to have an eye disease19. In GD, a remission was defined as consistent with improvement of clinical features and restoration of euthyroidism or induction of hypothyroidism after antithyroid drug (ATD) therapy. We defined intractable GD as hyperthyroidism persistent over two years of ATD therapy, relapse after ATD withdrawal, or treatment with ATD for at least 5 years20,21,22. HT was diagnosed when at least three of the following criteria established by Fisher et al.23 were met: (1) goiter, diffuse goiter, and decreased radionuclide uptake during thyroid scan, (2) circulating thyroglobulin or microsomal autoantibodies, and (3) hormonal evidence of hypothyroidism.
DNA extraction
Genomic DNA was extracted from 4 ml of peripheral blood mixed with ethylenediaminetetra acetic acid (EDTA) using an AccuPrep DNA extraction Kit (Bioneer, Daejeon, Republic of Korea): After 20 μl of proteinase K and 200 μl of lysis buffer (200 mM Tris–HCl, 25 mM EDTA, 300 mM NaCl, 1.2% sodium dodecyl sulfate) were added to peripheral blood leukocytes, the mixture was incubated at 60 °C for 10 min. The lysate was extracted with an equal volume of isopropanol. After washing with 500 μl of washing buffer and ethanol, the pellet was dried and suspended in 200 μl of sterile distilled water. DNA extracts were stored at − 20 °C.
Target gene primer design and polymerase chain reaction (PCR)
Genomic DNA was obtained from multiple samples and the pediatric patient and control groups with AITD were suitable for analysis using a PCR template of 50 ng or lower. The primers used in this study are presented in Table 2. PCR amplifications were performed in 50 µl of reaction mixtures in 96-well thin walled trays (Nippon Genetics, Tokyo, Japan). The reaction mixtures consisted of 10 μmol/l target-specific primers, 150–300 ng genomic DNA, 1 × buffer (60 mmol/l Tris–Cl, 15 mmol/l ammonium sulfate, and 100 mmol/l MgCl2), 250 μmol/l dNTPs (dATP/dGTP/dCTP/dTTP, 250 μmol/l), and 1 U Taq DNA polymerase (Bioprince, Enzynomics, Daejeon, Korea)24. In PCR, the extracted genomic DNA was amplified in a ProFlex 96-Well PCR System (Thermo Fisher Scientific, Waltham, MA, USA) using the following PCR conditions: a preliminary step of 1 cycle at 95 °C for 15 min followed by 40 cycles of denaturation at 94 °C for 30 s, annealing at 63 °C for 90 s, and extension at 72 °C for 30 s. The final extension was performed at 60 °C for 30 min25.
Sequencing
Sanger sequencing was performed using a Big Dye Terminator v3.1 (Amersham Pharmacia) and reactions were analyzed with ABI PRISM 3730XL analyzer (PE Applied Biosystems, Foster City, CA, USA). Sequencing data was analyzed using the FinchTV 1.4 software (Geospiza, Inc., Seattle, Washington, USA).
Statistical analysis
The allele frequencies were calculated using Microsoft Office Excel. The statistical significance was assessed using the χ2 test and Fisher’s exact test. The p values were adjusted by the Bonferroni method and OR was calculated using Haldane’s modification of Woolf’s method26,27. A two-tailed p value < 0.05 was considered statistically significant. The Hardy–Weinberg equilibrium (HWE) of each SNPs were evaluated using the Haploview software version 4.228 and all SNPs fit HWE. The power of our study was calculated based on an available sample size of 50 GD cases and 186 controls. The power for rs223828 minor allele frequency (MAF) (MAF: 25.6%) was 0.75 when OR was 0.4. Sample size and power were calculated using Quanto software version 1.2.4 (University of Southern California; Los Angeles, CA, USA).
Results
Comparison of genotype and allele frequencies of 25 SNPs of 16 chemokine genes in AITD patients and controls
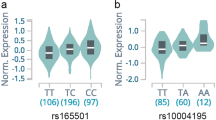
An analysis of the genotype of the SNP in the entire patient group, including those with GD and HT is shown in Table 3. Genotype frequencies of CCL11(rs3744508) AA (7.1% vs. 1.1%, OR = 6.9, Pc = 0.023) and CCR2(rs1799864) AA (16.3% vs. 4.9%, OR = 3.8, Pc = 0.006) were higher in the total AITD patent group than in the control group, whereas that of CCL17(rs223828) CC (21.4% vs. 39.3%, OR = 0.4, Pc = 0.012) was lower in the total AITD patient group than in the control group. Allele frequency of CCL17(rs223828) T (51.6% vs. 42.2%, OR = 1.5, Pc = 0.041) was higher in the total AITD patient group than in the control group. Other SNPs were not statistically significant between AITD patients and controls (Table 4).
Comparison of genotype and allele frequencies of four chemokine genes in GD patients and controls (Table 5)
In comparison between GD patients and controls, the genotype frequency of CCL17(rs223828) CC (20.4% vs. 39.3%, OR = 0.4, Pc = 0.041) was lower in GD patients, whereas genotype frequencies of while CCR2(rs1799864) AA (20.0% vs. 4.9%, OR = 4.8, Pc = 0.002), CXCR2(rs2230054) CC (61.2% vs. 42.6%, OR = 2.1, Pc = 0.02), and CXCR2(rs1126579) TT (58.0% vs. 40.4%, OR = 2.0, Pc = 0.027) were higher in GD patients. In the intractable GD group, genotype frequencies of CCL17(rs223828) TT (33.3% vs. 16.9%, OR = 2.5, Pc = 0.035), T (57.4% vs. 44.4%, OR = 1.9, Pc = 0.049) and CCR2(rs1799864) AA (16.7% vs. 4.9%, OR = 3.9, Pc = 0.048) were higher, whereas that of CCL17(rs223828) CC (10% vs. 39.3%, OR = 0.2, Pc = 0.005) was lower than in the control. In the TAO group, CCL17(rs223828) CC (16.7% vs. 39.4%, OR = 0.3, Pc = 0.030) had lower frequency, whereas CCR2(rs1799864) AA (16.7% vs. 4.9%, OR = 3.9, Pc = 0.026) had higher frequency than the control group. In the non-TAO group CCR2(rs1799864) AA (23.1% vs. 4.9%, OR = 5.8, Pc = 0.002), CXCR2(rs2230054) CC (68.0% vs. 42.6%, OR = 3.9. Pc = 0.026) and CXCR2(rs1126579) TT (61.5% vs. 40.4%, OR = 2.4, Pc = 0.042) had higher frequencies than the control group.
Comparison of genotype and allele frequencies of four chemokine genes in HT patients and controls (Table 6)
In comparison between control and HT patients, two SNPs showed statistical differences: CCL11(rs3744508) AA (11.1% vs. 1.1%, OR = 11.3, Pc = 0.002) had a higher frequency in HT patients, whereas CXCL8(rs2227306) CC (27.8% vs. 49.2%, OR = 0.4, Pc = 0.018) had lower frequency in HT patients.
Discussion
In this study, we observed significant differences in the frequencies of SNPs CCL11(rs3744508), CCL17(rs223828), and CCR2(rs1799864) were observed between the control group and AITD in Korean children. The AITD group had a higher T allele frequency in CCL17(rs223828), higher genotype frequencies in CCL11(rs3744508)AA and CCR2(rs1799864)AA, and a lower frequency in CCL17(rs223828)CC compared to controls. Upon dividing AITD into GD and HT, we found significant differences in CCL17(rs223828), CCR2(rs1799864), and CXCR2(rs2230054, rs1126579) between the control group and the GD group. Among GD patients, frequencies of CCR2(rs1799864)AA, CXCR2(rs2230054)CC, and CXCR2(rs1126579)TT were higher, and CCL17(rs223828)CC was lower compared to controls. In HT patients, CCL11(rs3744508)AA was higher, and CXCL8(rs2227306)CC was lower than controls.
Associations between chemokine SNPs and immune-related diseases, including AITD, have been previously documented. The T allele in CCL17(rs223828) was associated with increased serum CCL17 levels as well as increased coronary artery disease risk in a Chinese Han Population29. In relation to CCL17, Aso et al.30 confirmed with reports of higher levels of CCL17 and CXCL10 in serum in GD patients accompanied by type 1 diabetes. SNPs such as CCL11(rs1129844) have also been connected with elevated plasma chemokine levels in conditions like Fibromyalgia31. In the population of India, a significant association was found between SNPs CCR2 (rs1799864A) and Japanese encephalitis32, an observation supported by the overexpression of CCL2 (a ligand that can bind to CCR2) in GD33. Even though no direct connections have been reported between cxcr2 polymorphism and AITD, research has uncovered associations with other medical conditions. For instance, in China, the CXCR2 (rs2230054) CC genotype occurred more frequently in patients with thoracic aortic aneurysm34. Additionally, individuals carrying the CXCR2 rs1126579 TT genotype showed a significant increase in the likelihood of HCV spontaneous clearance35. Early studies focusing on chemokines of HT have revealed the role of CXCL836. Weetman et al.37 demonstrated that thyroid cells express CXCL8 upon stimulation with inflammatory cytokines such as IFN-r, TNF-α, and IL-1. Interestingly, the CC genotype of CXCL8(rs2227306) was found to be associated with susceptibility to sepsis in males38, indicating a broader impact of these polymorphisms on immune function and disease susceptibility.
In addition to the chemokine SNPs significantly identified in our study, various other chemokine SNPs have been associated with GD or HT, underlining the complexity of the relationship between these genetic variations and AITD. Several chemokines, including CCL3, CCL4, CCL21, CXCL9, CXCL10, and CXCL11, have been reported to link specifically to GD and HT37,39,40,41,42,43,44,45. In the early stage of GD, relationships are seen between chemokines and immune cells. CXCL10, made by follicular epithelial cells in the thyroid, attracts specific immune cells called CXCR3-expressing Th1 cells, leading to inflammation41. Research has shown that people with GD have more CXCL10 in their blood than people of the same age and gender without GD42. This same pattern has been found with other similar proteins, CXCL9 and CXCL11, where higher levels were found in the blood of people with GD compared to those without the condition43. The functional dynamics of chemokines in AITD have also been explored. Other research has examined the interaction of chemokines and other cytokines in primary thyrocyte cultures from GD patients, discovering that PPAR-α ligands can inhibit the secretion of CXCL9, CXCL10, and CXCL11 in a dose-dependent manner44,45. This finding provides potential insights into therapeutic pathways. Furthermore, Garcia-Lopez et al. identified increased expression levels of CXCL9 and CXCL10 in the thyroid of HT patients, thus revealing a specific association of CXCL9 and CXCL10 with HT46. These observations collectively illuminate the multifaceted role of chemokines in the pathogenesis of AITD, and underscore the need for continued investigation into these complex interactions.
This study uncovered statistically significant differences in the frequencies of SNPs in CCL17, CCR2, and CXCR2 between the GD group and the control group. Additionally, we identified significant differences in the frequencies of SNPs of CCL11 and CXCL8 between the HT group and the control group. These findings not only confirm the association between the CXCL8 gene polymorphism and HT but also suggest that CCL11 might be a potential contributing factor to HT. In practical terms, these insights could lead to novel therapeutic avenues. Targeting chemokines through antagonists, such as CCR4, CCR5, and CXCR4, might present a new strategy for treating AITD and other related conditions. This emphasizes the importance of understanding these genetic variations, as they could provide key information for the development of future treatments.
This study has some limitations. First, the power of statistics is inevitably low because of small number of patients. Second, we did not investigate how the specific genetic variations, or SNPs, we identified influence the levels of chemokines in the patient’s blood. Understanding this relationship could offer useful insights for predicting the disease. Future research should focus on exploring how these SNPs affect serum levels, as this could provide a more comprehensive understanding of the underlying mechanisms in AITD. In conclusion, this study identified six SNPs in five genes (CCL11, CCL17, CCR2, CXCR2, and CXCR8) that may be linked to an increased risk of AITD, GD, and HT in Korean pediatric patients. These findings point to a potential genetic relationship between these specific SNPs and the development of AITD, offering a new perspective on the underlying mechanisms of the disease. This requires larger sample sizes and possibly the inclusion of samples from diverse regions, to build a more comprehensive and definitive understanding of these genetic influences on AITD.
Data availability
The datasets generated and/or analysed during the current study are available in the Harvard dataverse repository, https://doi.org/10.7910/DVN/IAWYPJ.
References
Zlotnik, A. & Yoshie, O. Chemokines: A new classification system and their role in immunity. Immunity 12, 121–127 (2000).
Liu, C., Papewalis, C., Domberg, J., Scherbaum, W. A. & Schott, M. Chemokines and autoimmune thyroid diseases. Horm. Metab. Res. 40, 361–368 (2008).
Sokol, C. L. & Luster, A. D. The chemokine system in innate immunity. Cold Spring Harb. Perspect. Biol. 7, a016303 (2015).
Nomiyama, H., Osada, N. & Yoshie, O. Systematic classification of vertebrate chemokines based on conserved synteny and evolutionary history. Genes Cells 18, 1–16 (2013).
Miao, M., De Clercq, E. & Li, G. Clinical significance of chemokine receptor antagonists. Expert. Opin. Drug Metab. Toxicol. 16, 11–30 (2020).
Braverman, L. E. & Cooper, D. Werner & Ingbar's The Thyroid: A Fundamental and Clinical Text (Lippincott Williams & Wilkins, 2012).
Tomer, Y. & Davies, T. F. Searching for the autoimmune thyroid disease susceptibility genes: From gene mapping to gene function. Endocr. Rev. 24, 694–717 (2003).
Rho, J. G. et al. Long-term outcomes of Graves’ disease in children and adolescents receiving antithyroid drugs. Ann. Pediatr. Endocrinol. Metab. 26, 266–271 (2021).
Seiff, S. R. & Wagner, L. H. Management of graves myopathy: Thyroid-associated orbitopathy: When should we operate?. J. AAPOS 22, 251–252 (2018).
Weetman, A. P. Cellular immune responses in autoimmune thyroid disease. Clin. Endocrinol. (Oxf.) 61, 405–413 (2004).
Shin, D.-H. et al. HLA alleles, especially amino-acid signatures of HLA-DPB1, might contribute to the molecular pathogenesis of early-onset autoimmune thyroid disease. PLoS ONE 14, e0216941 (2019).
Jacobson, D. L., Gange, S. J., Rose, N. R. & Graham, N. M. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin. Immunol. Immunopathol. 84, 223–243 (1997).
Garmendia-Madariaga, A., Santos-Palacios, S., Guillén-Grima, F. & Galofré, J. C. The incidence and prevalence of thyroid dysfunction in Europe: A meta-analysis. J. Clin. Endocrinol. Metab. 99, 923–931 (2014).
Kwon, H. et al. Prevalence and annual incidence of thyroid disease in Korea from 2006 to 2015: A nationwide population-based cohort study. Endocrinol. Metab. (Seoul) 33, 260–267 (2018).
Kimura, H. & Caturegli, P. Chemokine orchestration of autoimmune thyroiditis. Thyroid 17, 1005–1011 (2007).
Rotondi, M., Chiovato, L., Romagnani, S., Serio, M. & Romagnani, P. Role of chemokines in endocrine autoimmune diseases. Endocr. Rev. 28, 492–520 (2007).
Crescioli, C. et al. Methimazole inhibits CXC chemokine ligand 10 secretion in human thyrocytes. J. Endocrinol. 195, 145–155 (2007).
Wartofsky, L. Classification of eye changes of Graves’ disease. Thyroid 2, 235–236 (1992).
Frecker, M. et al. Genetic factors in Graves’ ophthalmopathy. Clin. Endocrinol. 25, 479–485 (1986).
Gastaldi, R. et al. Graves disease in children: Thyroid-stimulating hormone receptor antibodies as remission markers. J. Pediatr. 164, 1189–1194 (2014).
Inoue, N. et al. Association of functional polymorphisms in promoter regions of IL5, IL6 and IL13 genes with development and prognosis of autoimmune thyroid diseases. Clin. Exp. Immunol. 163, 318–323 (2011).
Graffelman, J. & Weir, B. S. Testing for Hardy-Weinberg equilibrium at biallelic genetic markers on the X chromosome. Heredity (Edinb.) 116, 558–568 (2016).
Fisher, D. A., Oddie, T. H., Johnson, D. E. & Nelson, J. C. The diagnosis of Hashimoto’s thyroiditis. J. Clin. Endocrinol. Metab. 40, 795–801 (1975).
Baek, I. C. et al. Distributions of HLA-A, -B, and -DRB1 alleles typed by amplicon-based next generation sequencing in Korean volunteer donors for unrelated hematopoietic stem cell transplantation. Hla 97, 112–126 (2021).
Cho, W. K. et al. GPR174 and ITM2A gene polymorphisms rs3827440 and rs5912838 on the X chromosome in Korean children with autoimmune thyroid disease. Genes (Basel) 11, 858 (2020).
Baek, I. C. et al. Association of HLA class I and II genes with Middle East respiratory syndrome coronavirus infection in Koreans. Immun. Inflamm. Dis. 10, 111–116 (2022).
Menyhart, O., Weltz, B. & Gyorffy, B. MultipleTesting.com: A tool for life science researchers for multiple hypothesis testing correction. PLoS ONE 16, e0245824 (2021).
Barrett, J. C., Fry, B., Maller, J. & Daly, M. J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 21, 263–265 (2005).
Ye, Y. et al. Association between a CCL17 genetic variant and risk of coronary artery disease in a Chinese Han population. Circ. J. 82, 224–231 (2017).
Aso, Y. et al. Profound reduction in T-helper (Th) 1 lymphocytes in peripheral blood from patients with concurrent type 1 diabetes and Graves’ disease. Endocr. J. 53, 377–385 (2006).
Zhang, Z. et al. SNPs in inflammatory genes CCL11, CCL4 and MEFV in a fibromyalgia family study. PLoS ONE 13, e0198625 (2018).
Chowdhury, P. & Khan, S. A. Significance of CCL2, CCL5 and CCR2 polymorphisms for adverse prognosis of Japanese encephalitis from an endemic population of India. Sci. Rep. 7, 13716 (2017).
Yin, X. et al. mRNA-Seq reveals novel molecular mechanisms and a robust fingerprint in Graves’ disease. J. Clin. Endocrinol. Metab. 99, E2076-2083 (2014).
Zhao, H., Xu, Y. & Cui, J. CXCR2 (rs3890158 and rs2230054) and CXCL4 (rs352008 and rs1801572) gene polymorphisms in patients with thoracic aortic aneurysm. Biotechnol. Genet. Eng. Rev. 2023, 1–13 (2023).
Zang, F. et al. Association of CXCR2 genotype variations with HCV clearance in a Chinese population. Arch. Virol. 163, 2711–2718 (2018).
Hirooka, Y., Mitsuma, T., Nogimori, T. & Ishizuki, Y. Deregulated production of interleukin-8 (IL-8) in autoimmune thyroid disease studied by newly developed IL-8 radioimmunoassay. Endocr. Regul. 27, 11–15 (1993).
Weetman, A. P., Bennett, G. L. & Wong, W. L. Thyroid follicular cells produce interleukin-8. J. Clin. Endocrinol. Metab. 75, 328–330 (1992).
Hu, D. et al. Investigation of association between IL-8 serum levels and IL8 polymorphisms in Chinese patients with sepsis. Gene 594, 165–170 (2016).
Ashhab, Y. et al. A one-tube polymerase chain reaction protocol demonstrates CC chemokine overexpression in Graves’ disease glands. J. Clin. Endocrinol. Metab. 84, 2873–2882 (1999).
Qi, Y. et al. Increased chemokine (C-C motif) ligand 21 expression and its correlation with osteopontin in Graves’ disease. Endocrine 50, 123–129 (2015).
Romagnani, P. et al. Expression of IP-10/CXCL10 and MIG/CXCL9 in the thyroid and increased levels of IP-10/CXCL10 in the serum of patients with recent-onset Graves’ disease. Am. J. Pathol. 161, 195–206 (2002).
Antonelli, A. et al. Interferon-gamma-inducible alpha-chemokine CXCL10 involvement in Graves’ ophthalmopathy: Modulation by peroxisome proliferator-activated receptor-gamma agonists. J. Clin. Endocrinol. Metab. 91, 614–620 (2006).
Antonelli, A., Ferrari, S. M., Corrado, A., Ferrannini, E. & Fallahi, P. Increase of interferon-gamma inducible CXCL9 and CXCL11 serum levels in patients with active Graves’ disease and modulation by methimazole therapy. Thyroid 23, 1461–1469 (2013).
Antonelli, A. et al. CXCL9 and CXCL11 chemokines modulation by peroxisome proliferator-activated receptor-alpha agonists secretion in Graves’ and normal thyrocytes. J. Clin. Endocrinol. Metab. 95, E413-420 (2010).
Antonelli, A. et al. Peroxisome proliferator-activated receptor alpha agonists modulate Th1 and Th2 chemokine secretion in normal thyrocytes and Graves’ disease. Exp. Cell Res. 317, 1527–1533 (2011).
Garcia-Lopez, M. A., Sancho, D., Sanchez-Madrid, F. & Marazuela, M. Thyrocytes from autoimmune thyroid disorders produce the chemokines IP-10 and Mig and attract CXCR3+ lymphocytes. J. Clin. Endocrinol. Metab. 86, 5008–5016 (2001).
Acknowledgements
This study was supported by a grant of the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2022R1F1A1062761) and the Korean Society of Pediatric Endocrinology Grant (2016 -001).
Author information
Authors and Affiliations
Contributions
Conceptualization: T.G.K., B.K.S., W.K.C. Methodology and statistical analysis: I.C.B., W.K.C. Writing—original draft: C.S., I.C.B., W.K.C. Writing—review & editing: T.G.K., B.K.S., I.C.B., W.K.C.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shin, C., Baek, IC., Cho, W. et al. Comprehensive analysis of chemokine gene polymorphisms in Korean children with autoimmune thyroid disease. Sci Rep 13, 15642 (2023). https://doi.org/10.1038/s41598-023-42021-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-42021-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.