Abstract
Maintaining cancer patients’ exercise capacity and therefore patients’ ability to live a self-determined life is of huge importance, but little is known about major determinants. We sought to identify determinants of exercise capacity in patients with a broad spectrum of cancer types, who were already receiving cancer treatment or about to commence such therapy. Exercise capacity was assessed in 253 consecutive patients mostly suffering from advanced cancer using the 6-min walk test (6-MWT). All patients underwent echocardiography, physical examination, resting electrocardiogram, hand grip strength (HGS) measurement, and laboratory assessments. Patients were divided into two groups according to the median distance in the 6-MWT (459 m). Patients with lower exercise capacity were older, had significantly lower HGS and haemoglobin and higher values of high sensitive (hs) Troponin T and NT-proBNP (all p < 0.05). Whilst the co-morbidity burden was significantly higher in this group, no differences were detected for sex, body mass index, tumor type, or cachexia (all p > 0.2). Using multivariable logistic regression, we found that the presence of anaemia (odds ratio (OR) 6.172, 95% confidence interval (CI) 1.401–27.201, p = 0.016) as well as an increase in hs Troponin T (OR 3.077, 95% CI 1.202–5.301, p = 0.019) remained independent predictors of impaired exercise capacity. Increasing HGS was associated with a reduced risk of a lower exercise capacity (OR 0.896, 95% CI 0.813–0.987, p = 0.026). Screening patients for elevated hs troponin levels as well as reduced HGS may help to identify patients at risk of lower exercise capacity during cancer treatment.
Similar content being viewed by others
Introduction
In 2018, the World Health Organization (WHO) reported 18.1 million new cases of cancer worldwide, with lung, breast, and prostate cancer being the most frequent entities1. Cardiotoxicity of anti-cancer treatments has become an increasingly important aspect in the care of such patients, leading to the involvement of cardiologists and the deployment of cardio-oncology services in comprehensive cancer centers in recent years2.
Cardio-oncology, however, involves more aspects than the treatment of cardiotoxic adverse events, it also covers cancer development in patients with manifest cardiac diseases like heart failure or other cardiovascular diseases in patients diagnosed with cancer3,4. Close interaction between the two fields is important, because patients are not only threatened by high mortality rates, but also by reduced exercise capacity and consequently impaired quality of life (QoL)5,6. Current evidence also suggests an interplay between cardiovascular and metabolic parameters and QoL in patients with cancer7.
Identifying patients at risk of diminishing exercise capacity and the identification of early interventional approaches may be a pivotal aspect in the care of affected patients8. Since similar problems are observed in patients with chronic heart failure, we aimed to assess cancer patients’ exercise capacity from a cardiology perspective. Even though this point is receiving increasing attention in recent years, advanced cardiovascular investigations do still not always belong to the routine clinical care among patients with cancer in general or in those scheduled to undergo systemic therapy.
Clinical trials in oncology are mainly driven by endpoints that target patients’ survival. Whilst this is clearly important, many patients in the clinical setting ask questions about maintaining mobility and QoL. Cardio-oncology is devoted to understanding the pathophysiological changes in the cardiovascular system in patients with cancer, offering an intriguing approach to improving cardiovascular function and possibly survival.
Parameters of interest have so far included left ventricular ejection fraction (LVEF), heart rate, blood pressure, heart rate variability, cardiac biomarkers, and strain imaging. Particularly LVEF has been in the focus of attention when cardiotoxic reactions are being assessed. Limited information is available on exercise capacity as measured using typical tests usually performed in clinical trials in cardiology. These include tests like spiroergometry (cardiopulmonary exercise testing) or the frequently performed 6-min walk test (6-MWT). Whilst spiroergometry allows detection of maximal and submaximal exercise capacity, the 6-MWT allows reliable assessment of everyday exercise performance.
Data from patients with colorectal cancer indicate that exercise capacity is impaired already in chemotherapy-naïve patients, but the start of respective therapies worsens this development9. Recent analyses using available treatment data from patients with heart failure have highlighted the immense difficulties to improve exercise capacity with the most robust data supporting therapeutic decrease of high heart rate values and the treatment of iron deficiency10. We sought to identify risk factors for the development of impaired exercise capacity in a mixed cohort of patients with cancer from different etiologies in order to counteract the deterioration in physical performance.
Methods
We prospectively enrolled 253 cancer patients between November 2017 and July 2019 at the University Medical Center Göttingen and the Charité Medical School, Campus Benjamin Franklin, Berlin, both in Germany. Only patients with histologically confirmed cancer that were ≥ 18 years of age were included in this study.
The following criteria were defined as reasons for exclusion: (1) clinical signs of an acute infection or antibiotic treatment due to an infection, (2) relevant cardiovascular disease (e.g. coronary artery disease, prior myocardial infarction, or left ventricular ejection fraction (LVEF) < 50%, atrial fibrillation), (3) severe chronic obstructive pulmonary disease, defined as Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage > II (except in lung cancer patients all GOLD stages were allowed), (4) and any other cancer diagnosis in the 5 years preceding enrolment11.
The inclusion and exclusion criteria result in a heterogenous cancer patient population and as a result cancer specific therapy varies as well. However, this approach allows an overview of general principles yielding reduced exercise performance measured by the 6-MWT, which are not restricted to a specific cancer type or a specific cancer therapy. Due to the all-comer status of the patients included in this analysis, most participants were already receiving cancer treatment or were about to commence such therapy.
All participants underwent a battery of tests, which included the acquisition of the patient’s medical history, a physical examination, a resting electrocardiogram (ECG), transthoracic echocardiography, blood collection as well as a 6-MWT and hand grip strength (HGS) assessment.
Blood collection
Full blood count and clinical chemistry parameters were analyzed from a venous blood draw by the local laboratory. High sensitive (hs) Troponin T and N-terminal pro-B-type natriuretic peptide (NT-proBNP) were assessed using assays provided by Roche Germany Holding GmbH (Grenzach-Wyhlen, Germany).
Cardiovascular assessment
Following the recording of the medical history, a detailed physical examination including measurement of body weight was performed. The blood pressure of all patients was assessed in a sitting position in a quiet environment after a rest of at least 5 min according to current guidelines12. Three measurements were performed 2 min apart using a Boso Medicus electronical sphygmomanometer (Bosch + Sohn GmbH und Co.KG, Juningen, Germany). Results were recorded as an average of the last two blood pressure readings. A 12 lead-electrocardiogram was recorded in a supine position using a MAC™ 3500 Resting ECG System (GE Healthcare, Chicago, Illinois, United States). Additionally, 24-h holter ECG data were acquired using a DMS300-4L recorder (DMS-Service, Los Angeles, California, United States). Echocardiography was performed with Vivid E9 ultrasound device (GE Healthcare, Chicago, Illinois, United States) to exclude a reduced ejection fraction as well as severe valve diseases.
Exercise capacity was evaluated by the 6-MWT, which was performed according to standard protocol13. Furthermore, physical strength was evaluated by measuring HGS. HGS measurements were performed in a sitting position using a Jamar® Plus + Digital Hand Dynamometer (Performance Health Holding, Inc., Warrenville, Illinois USA). While the elbow was flexed at 90°, shoulder and wrist were in a neutral position (0 degrees). HGS was evaluated in both hands, and the average of 3 tests of the stronger hand noted. Cachexia was defined as a weight loss of at least 5% of body weight within the last 12 month or a combination of 2% weight loss and a body mass index (BMI) < 20 kg/m2.
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 26 for Windows (International Business Machines Corporation, Armonk, New York, USA). Normal distribution was tested using the Shapiro–Wilk test. Parametric data are expressed as mean ± standard deviation. Non-parametric data were compared using Mann–Whitney U and Kruskal–Wallis tests as appropriate and expressed as median an interquartile range. For between-group comparisons in parametric data t- or analysis of variance (ANOVA) testing was performed as appropriate. For binary variables the intergroup comparison was performed using the chi2 test. Simple regression was used to analyze first line associations between continuous variables. Univariable and multivariable logistic regression models were used to identify clinical determinants of exercise capacity in patients with advanced cancer and expressed as odds ratio and 95% confidence interval (CI). A p-value < 0.05 was considered statistically significant. All significant univariable parameters as well as creatinine and sex were included in the multivariable logistic regression model.
Institutional Review Board statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the local ethic committee of the University Medical Centre Göttingen (approval code 22/8/17; approval date 13.09.2017).
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Results
Study population characteristics
A total 253 patients were included. Patients’ characteristics are displayed in Table 1: 57.7% of the study cohort were male. Mean age was 60.4 ± 12.5 years with age ranging from 20 to 83 years. Whilst most patients were diagnosed with a solid tumor (77.1%), only a minority had haematological malignancies (22.9%) (Fig. 1). Most of the patients were diagnosed with advanced disease stages: 89.4% patients were classified as UICC stadium ≥ III and 56.4% as Ann-Arbor-Stadium ≥ III. Follow-up was censored in February 2020 and the mean follow-up period was 12.1 ± 6.2 months. Until this point, a total of 84 (33.2%) patients had died. Mean body-mass-index (BMI) was 25.4 ± 4.6 kg/m2 and ranged from 14.9 to 41.6 kg/m2. The most common co-morbidities were anaemia (61.7%)—defined as a haemoglobin value < 13 g/dl in male and < 12 g/dl in female—followed by cachexia (46.6%) and hypertension (43.5%). Dyspnoea of any cause was reported in 30.4%, current smoking status in 15.0% and diabetes mellitus in 11.5% of patients.
Exercise capacity
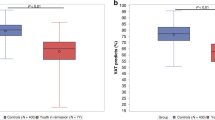
Patients were divided into two groups based on the median distance in the 6-MWT (459 m). Patients with lower exercise capacity were older and had higher values of serum hs Troponin T and NT-proBNP (all p < 0.05). In this group, the co-morbidity burden was significantly higher with regards to anaemia (p < 0.001), hypertension (p < 0.001), and diabetes mellitus (p = 0.035). Patients with lower exercise capacity were significantly more likely to report dyspnoea on exertion (p = 0.003). No significant differences between the groups with lower vs. higher exercise capacity were found for sex (p = 0.23), BMI (p = 0.25), tumor type (p = 0.53), survival (p = 0.1), presence of cachexia (p = 0.66) and current smoking status (p = 0.55) (Table 1). Haemoglobin, transferrin, albumin, and sodium values were all significantly lower in patients with reduced exercise capacity (6-MWT below median, all p < 0.05). All laboratory findings are summarized in Table 2. Whilst there were no significant differences in LVEF between the two groups, heart rate, and diastolic blood pressure (all p > 0.05), systolic blood pressure (p = 0.007) was slightly lower and the HGS higher in patients with higher exercise capacity (p < 0.0001) (Table 3 and Fig. 2).
Predictors of lower exercise capacity
Using univariable logistic regression, we found that hs Troponin T, NT-proBNP, age, history of hypertension, anaemia, diabetes mellitus, and dyspnoea all predicted lower exercise capacity (all p < 0.04). The same was true for haemoglobin and HGS in which decreasing values were associated with lower exercise capacity (Table 4, Fig. 3) (all p < 0.001). In addition, the presence of a hypalbuminaemia was associated with a lower exercise capacity in the univariable logistic regression model (p = 0.009). After multivariable adjustment, hs troponin T, anaemia, history of hypertension, and HGS all remained independent predictors of impaired exercise capacity (Table 4). NT-proBNP lost its predictive power after multivariable adjustment.
Discussion
Our data show that several factors are associated with reduced exercise capacity in patients with cancer. After multivariable adjustment, the presence of anaemia, increasing levels of hs troponin T and decreasing values in HGS remained significant predictors of impaired exercise capacity.
In clinical practice, reduced exercise capacity is reported by many patients with cancer, and this finding negatively impacts activity of daily living in cancer survivors14. In turn, reduced physical activity negatively impacts patients’ QoL and the so-called hard endpoints like cancer mortality and cancer recurrence15. In contrast, it is well known that being physically active is beneficial with regards to better physical and social functioning and also to coping with cancer and treatment-related side effects16.
Unfortunately, the number of studies trying to identify risk factors for reduced exercise capacity in patients with cancer remains limited. Miller et al. identified age, higher body fat content, methotrexate exposure and extremes of LV mass as predictors of lower exercise capacity in long-term paediatric cancer survivors measured as peak oxygen consumption (peak VO2). In line with the findings of our study, NT-proBNP failed as a useful predictor17. In contrast to the aforementioned study, our study found hs Troponin T to be a predictor of reduced exercise capacity in oncological patients even after multivariable adjustment. This is in line with data published by deFilippi et al. who found an association between troponin levels, exercise capacity and the risk of developing heart failure18. Interestingly, increasing troponin levels were also described as an independent predictor of mortality in the elderly population19. The same was found for respiratory diseases including COVID-19, renal disease and in patients suffering from coronary heart disease as well as from heart failure20,21,22,23,24. In the oncological setting, cardiac biomarkers, especially B-type natriuretic peptide as well as troponin, are usually used to detect cardiac side effects of cancer treatment or even the cancer itself25.
In recent years, the interest in cardiac biomarkers has increased in oncological patients. Indeed, Cardinale et al. were able to show that troponin values at baseline and post chemotherapy allow risk stratification regarding impairments in LV function26. Because of its prognostic value, it is not surprising that cardiac biomarkers, including troponin, were entered into most of the recently published baseline cardiovascular risk stratification models27. In contrast to troponin, it is more obvious that the presence of anaemia predicts impaired exercise capacity due to reduced oxygen supply to skeletal muscles in anaemic patients. Anaemia is an extraordinarily common comorbidity in patients with cancer and its presence is described in 39% at the time of patients’ enrolment and in up to 67% during chemotherapy in the European Cancer Anaemia Survey28. Among our patients, anaemia was present in 61.7%, whereby its prevalence was significantly higher in patients with reduced exercise capacity, and multivariable analysis revealed anaemia to be an independent predictor of impaired exercise capacity. The mechanisms of developing anaemia in cancer patients are multiple and are spanning from decreased erythropoiesis to directly destroying blood cells or blood loss itself. In the clinical setting, cancer patients often complain about fatigue, which is a typical sign of anaemia and also often occurs in the context of physical exhaustion29. The relation between anaemia and reduced exercise capacity or physical activity is not only described by our group but also is in line with many publications considering especially patients with heart failure30,31,32,33. With regards to cancer patients, the evidence of an influence of anaemia on exercise capacity has been much less investigated. However, there are no reasons to assume a different relationship in oncological patients. On the other hand, exercise directly affects the number of red blood cells. Drouin et al. have shown physical activity to have the potential to stabilise red cell counts and therefore to prevent anaemia in a breast cancer population34. This finding is confirmed in a large meta-analysis by Hu and Lin35. In patients with chronic heart failure, the presence of anaemia was also found to be an independent predictor of increased mortality36. It remains unclear if this result can be translated to cancer patients. Nevertheless, clinicians should consider anaemia to improve patients’ exercise tolerance. If anaemia is the result of iron deficiency, the supply of intravenous iron may help in the correction of anaemia. Additionally, iron deficiency was shown to be a predictor of reduced exercise capacity in patients with chronic heart failure37,38. The fact that iron deficiency was not predicting lower exercise capacity in our study could be the result of equal distribution of iron deficiency between patients with lower and better exercise capacity. If iron deficiency is not present and therefore unlikely to be a chief reason for anaemia development, the attending clinician has to choose between blood transfusion treatment and the use of erythropoietin containing drugs. Using the latter imposes an increased risk of thrombosis. To maintain the achieved level of erythrocytes, exercise training should be prescribed as well.
HGS assessment can help to identify patients at high risk of being affected in their exercise capacity. In our analysis, HGS was positively associated with exercise capacity, which is not astonishing because HGS represents the total muscle mass, and a reduced muscle mass may lead to reduced exercise capacity. In agreement with our findings, the relationship between HGS and exercise capacity has already been described for other diseases like COPD and coronary artery disease39,40. Furthermore, various studies have documented the correlation between HGS and QoL41,42,43. Cancer patients usually wish to be directly involved in their cancer treatment. Emphasising the meaning of physical activity may give patients therefore the feeling to directly influence the underlying condition. This may be especially important since recent studies were able to illustrate a direct correlation of HGS and cancer mortality44,45,46.
In summary, increasing troponin values are an important predictor of reduced exercise capacity. Due to the ease of their assessment, troponin values should be measured during cancer treatment in order to identify patients at high risk of reductions in exercise capacity. Furthermore, correction of anaemia and assessing HGS may be helpful in maintaining patients’ higher exercise capacity level.
Limitations
Some limitations need to be addressed. First, the current study included all-comers of a regional cancer centres specialised in the treatment of specific cancers. The all-comer status resulted in different cancer therapeutic strategies, which may have affected the results. Second, the all-comer status implies that our results cannot be directly transferred to a specific cancer type or treatment option. Third, we did not perform analyses of echocardiography parameters and exercise capacity, because patients with relevant cardiovascular diseases (coronary artery disease, prior myocardial infarction, or left ventricular ejection fraction (LVEF) < 50%, atrial fibrillation) were excluded as per study design. Fourth, due to the small sample size some effects may not have reached statistical significance even if there may be a correlation (e.g. present of cachexia and a lower 6MWT). Finally, we decided to define low exercise capacity as a 6-MWT distance below the median of the study cohort, which may have affected the results. However, due to the lack of a universally valid threshold to define low exercise capacity using the median deemed to be most reasonable.
Data availability
Data are not publicly available due to ethical and legal issues. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Mattiuzzi, C. & Lippi, G. Current cancer epidemiology. J. Epidemiol. Glob. Health 9, 217–222. https://doi.org/10.2991/jegh.k.191008.001 (2019).
Ewer, M. S. & Ewer, S. M. Cardiotoxicity of anticancer treatments: What the cardiologist needs to know. Nat. Rev. Cardiol. 7, 564–575. https://doi.org/10.1038/nrcardio.2010.121 (2010).
Anker, M. S., von Haehling, S., Landmesser, U., Coats, A. J. S. & Anker, S. D. Cancer and heart failure-more than meets the eye: Common risk factors and co-morbidities. Eur. J. Heart Fail. 20, 1382–1384. https://doi.org/10.1002/ejhf.1252 (2018).
Ameri, P. et al. Cancer diagnosis in patients with heart failure: Epidemiology, clinical implications and gaps in knowledge. Eur. J. Heart Fail. 20, 879–887. https://doi.org/10.1002/ejhf.1165 (2018).
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. https://doi.org/10.3322/caac.21660 (2021).
Anker, M. S., Hulsmann, M. & Cleland, J. G. What do patients with heart failure die from? A single assassin or a conspiracy?. Eur. J. Heart Fail. 22, 26–28. https://doi.org/10.1002/ejhf.1689 (2020).
Evertz, R. et al. Cardiovascular and metabolic determinants of quality of life in patients with cancer. ESC Heart Fail. https://doi.org/10.1002/ehf2.14175 (2022).
Buffart, L. M. et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: An individual patient data meta-analysis of 34 RCTs. Cancer Treat. Rev. 52, 91–104. https://doi.org/10.1016/j.ctrv.2016.11.010 (2017).
Cramer, L. et al. Cardiovascular function and predictors of exercise capacity in patients with colorectal cancer. J. Am. Coll. Cardiol. 64, 1310–1319. https://doi.org/10.1016/j.jacc.2014.07.948 (2014).
von Haehling, S. et al. Improving exercise capacity and quality of life using non-invasive heart failure treatments: Evidence from clinical trials. Eur. J. Heart Fail. 23, 92–113. https://doi.org/10.1002/ejhf.1838 (2021).
Marcoa, R. et al. Classification of chronic obstructive pulmonary disease (COPD) according to the new global initiative for chronic obstructive lung disease (GOLD) 2017: Comparison with GOLD 2011. COPD 15, 21–26. https://doi.org/10.1080/15412555.2017.1394285 (2018).
Williams, B. et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Ital. Cardiol. (Rome) 19, 3S-73S. https://doi.org/10.1714/3026.30245 (2018).
ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 166, 111–117. https://doi.org/10.1164/ajrccm.166.1.at1102 (2002).
Ness, K. K. et al. Exercise intolerance, mortality, and organ system impairment in adult survivors of childhood cancer. J. Clin. Oncol. 38, 29–42. https://doi.org/10.1200/JCO.19.01661 (2020).
Cormie, P., Zopf, E. M., Zhang, X. & Schmitz, K. H. The impact of exercise on cancer mortality, recurrence, and treatment-related adverse effects. Epidemiol. Rev. 39, 71–92. https://doi.org/10.1093/epirev/mxx007 (2017).
Banzer, W. et al. Changes in exercise capacity, quality of life and fatigue in cancer patients during an intervention. Eur. J. Cancer Care (Engl.) 23, 624–629. https://doi.org/10.1111/ecc.12201 (2014).
Miller, A. M. et al. Exercise capacity in long-term survivors of pediatric cancer: An analysis from the Cardiac Risk Factors in Childhood Cancer Survivors Study. Pediatr. Blood Cancer 60, 663–668. https://doi.org/10.1002/pbc.24410 (2013).
deFilippi, C. R. et al. Physical activity, change in biomarkers of myocardial stress and injury, and subsequent heart failure risk in older adults. J. Am. Coll. Cardiol. 60, 2539–2547. https://doi.org/10.1016/j.jacc.2012.08.1006 (2012).
Eggers, K. M., Venge, P., Lindahl, B. & Lind, L. Cardiac troponin I levels measured with a high-sensitive assay increase over time and are strong predictors of mortality in an elderly population. J. Am. Coll. Cardiol. 61, 1906–1913. https://doi.org/10.1016/j.jacc.2012.12.048 (2013).
Waschki, B. et al. High-sensitivity troponin I and all-cause mortality in patients with stable COPD: An analysis of the COSYCONET study. Eur. Respir. J. https://doi.org/10.1183/13993003.01314-2019 (2020).
Lorente-Ros, A. et al. Myocardial injury determination improves risk stratification and predicts mortality in COVID-19 patients. Cardiol. J. 27, 489–496. https://doi.org/10.5603/CJ.a2020.0089 (2020).
Dierkes, J. et al. Cardiac troponin T predicts mortality in patients with end-stage renal disease. Circulation 102, 1964–1969. https://doi.org/10.1161/01.cir.102.16.1964 (2000).
Hudson, M. P. et al. Implications of elevated cardiac troponin T in ambulatory patients with heart failure: A prospective analysis. Am. Heart J. 147, 546–552. https://doi.org/10.1016/j.ahj.2003.10.014 (2004).
Lippi, G., Cervellin, G. & Sanchis-Gomar, F. Predicting mortality with cardiac troponins: Recent insights from meta-analyses. Diagnosis (Berl) 8, 37–49. https://doi.org/10.1515/dx-2019-0061 (2021).
Lyon, A. R. et al. ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. https://doi.org/10.1093/eurheartj/ehac244 (2022).
Cardinale, D. et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation 109, 2749–2754. https://doi.org/10.1161/01.CIR.0000130926.51766.CC (2004).
Lyon, A. R. et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: A position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur. J. Heart Fail. 22, 1945–1960. https://doi.org/10.1002/ejhf.1920 (2020).
Ludwig, H. et al. The European Cancer Anaemia Survey (ECAS): A large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur. J. Cancer 40, 2293–2306. https://doi.org/10.1016/j.ejca.2004.06.019 (2004).
Ament, W. & Verkerke, G. J. Exercise and fatigue. Sports Med. 39, 389–422. https://doi.org/10.2165/00007256-200939050-00005 (2009).
Mayer, G., Thum, J. & Graf, H. Anaemia and reduced exercise capacity in patients on chronic haemodialysis. Clin. Sci. (Lond.) 76, 265–268. https://doi.org/10.1042/cs0760265 (1989).
Ebner, N. et al. The impact of iron deficiency and anaemia on exercise capacity and outcomes in patients with chronic heart failure. Results from the studies investigating co-morbidities aggravating heart failure. Int. J. Cardiol. 205, 6–12. https://doi.org/10.1016/j.ijcard.2015.11.178 (2016).
Piepoli, M. F., Corra, U. & Agostoni, P. Cardiopulmonary exercise testing in patients with heart failure with specific comorbidities. Ann. Am. Thorac. Soc. 14, S110–S115. https://doi.org/10.1513/AnnalsATS.201610-803FR (2017).
Kalra, P. R. et al. Effect of anemia on exercise tolerance in chronic heart failure in men. Am. J. Cardiol. 91, 888–891. https://doi.org/10.1016/s0002-9149(03)00030-4 (2003).
Drouin, J. S. et al. Random control clinical trial on the effects of aerobic exercise training on erythrocyte levels during radiation treatment for breast cancer. Cancer 107, 2490–2495. https://doi.org/10.1002/cncr.22267 (2006).
Hu, M. & Lin, W. Effects of exercise training on red blood cell production: Implications for anemia. Acta Haematol. 127, 156–164. https://doi.org/10.1159/000335620 (2012).
Szachniewicz, J. et al. Anaemia is an independent predictor of poor outcome in patients with chronic heart failure. Int. J. Cardiol. 90, 303–308. https://doi.org/10.1016/s0167-5273(02)00574-0 (2003).
Anker, S. D. et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N. Engl. J. Med. 361, 2436–2448. https://doi.org/10.1056/NEJMoa0908355 (2009).
Ponikowski, P. et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiencydagger. Eur. Heart J. 36, 657–668. https://doi.org/10.1093/eurheartj/ehu385 (2015).
Kyomoto, Y. et al. Handgrip strength measurement in patients with chronic obstructive pulmonary disease: Possible predictor of exercise capacity. Respir. Investig. 57, 499–505. https://doi.org/10.1016/j.resinv.2019.03.014 (2019).
Kim, W. et al. Handgrip strength as a predictor of exercise capacity in coronary heart disease. J. Cardiopulm Rehabil. Prev. 40, E10–E13. https://doi.org/10.1097/HCR.0000000000000458 (2020).
Jakobsen, L. H., Rask, I. K. & Kondrup, J. Validation of handgrip strength and endurance as a measure of physical function and quality of life in healthy subjects and patients. Nutrition 26, 542–550. https://doi.org/10.1016/j.nut.2009.06.015 (2010).
Kim, H., Yoo, S., Kim, H., Park, S. G. & Son, M. Cancer survivors with low hand grip strength have decreased quality of life compared with healthy controls: The Korea National Health and Nutrition Examination Survey 2014–2017. Korean J. Fam. Med. 42, 204–211. https://doi.org/10.4082/kjfm.20.0060 (2021).
Paek, J. & Choi, Y. J. Association between hand grip strength and impaired health-related quality of life in Korean cancer survivors: A cross-sectional study. BMJ Open 9, e030938. https://doi.org/10.1136/bmjopen-2019-030938 (2019).
Zhuang, C. L. et al. Associations of low handgrip strength with cancer mortality: A multicentre observational study. J. Cachexia Sarcopenia Muscle 11, 1476–1486. https://doi.org/10.1002/jcsm.12614 (2020).
Kilgour, R. D. et al. Handgrip strength predicts survival and is associated with markers of clinical and functional outcomes in advanced cancer patients. Support Care Cancer 21, 3261–3270. https://doi.org/10.1007/s00520-013-1894-4 (2013).
Hadzibegovic, S. et al. Hand grip strength in patients with advanced cancer: A prospective study. J. Cachexia Sarcopenia Muscle https://doi.org/10.1002/jcsm.13248 (2023).
Funding
Open Access funding enabled and organized by Projekt DEAL. We acknowledge support by the Open Access Publication Funds of the Göttingen University. This study was partly funded by the German Center for Cardiovascular Research (CD, SH, MSA, SvH).
Author information
Authors and Affiliations
Contributions
Conceptualization: R.E., M.S.A. and S.v.H.; methodology: R.E., M.S.A. and S.v.H.; formal analysis: R.E. and T.G.V.; investigation: C.D. and K.G.; writing—original draft preparation: R.E. and S.v.H.; writing—review and editing: R.E., C.D., K.G., M.V., T.G.V., T.R.O., F.B., A.L., S.H., A.B., U.K., U.L., A.O.K., G.H., A.S., M.S.A., S.v.H. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
MSA reports personal fees from Servier, outside the submitted work. All authors declare no conflict of interest with respect to this work.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Evertz, R., Diehl, C., Gödde, K. et al. Predictors of lower exercise capacity in patients with cancer. Sci Rep 13, 14861 (2023). https://doi.org/10.1038/s41598-023-41390-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-41390-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.