Abstract
The aim of this study is to evaluate the beneficial effects of coconut essential oil on growth performance, carcass criteria, antioxidant status, and immune response of broiler chicks. A total of 192 un-sexed 7-days broiler chicks were divided into six treatment sets with four copies of 8 chicks per set. The groups were as follows: (1) basal diet (without additive), (2) basal diet plus 0.5 ml coconut essential oil/kg, (3) basal diet plus 1 ml coconut essential oil/kg, (4) basal diet plus 1.5 ml coconut essential oil/kg, (5) basal diet plus 2 ml coconut essential oil/kg and (6) basal diet plus 2.5 ml coconut essential oil/kg. The results showed that the most prevalent compound in coconut oil is 6-Octadecenoic acid (oleic acid) representing 46.44% followed 2(3H)-Furanone, dihydro-5-pentyl- (CAS) (11.36%), Hexadecanoic acid (CAS) (4.71%), and vanillin (2.53%). Dietary 1 and 1.5 ml of coconut oil improved significantly the body weight and gain of broiler chickens. Dietary supplementation of 1 ml of coconut oil improved significantly liver function compared to control and other treatment groups. The supplementation with 1 ml coconut oil significantly reduced TG and VLDL compared to control and other treatment groups, while no significant differences in TC, HDL, and LDL due to dietary coconut oil. The present findings showed that dietary coconut oil with 1 and 1.5 ml/kg feed improved significantly antioxidants status through increased antioxidant enzymes like SOD and GSH while decreasing significantly MDA levels compared to control and other treatment groups. Therefore, it was concluded that the diets of broiler chickens could be fortified with coconut oil with 1 or 1.5 ml to improve the growth, feed utilization, and antioxidant status of broiler chickens.
Similar content being viewed by others
Introduction
In recent years, there is great attention to the use of natural feed additives in poultry production as a global demand. The successful application of herbal growth promoters increases the profitability of the poultry industry by enhancing feed efficiencies and health conditions1,2. The secondary metabolites of herbal plants, including phenolic compounds, saponins, and essential oils, are linked to some of the health benefits3,4.
Coconut oil is saturated oil, and medium-chain fatty acid (MCFA) represents about 60% of its total fatty acid composition that contains a chain length of 6 to 12 carbon atoms, which are absorbed directly into the portal circulation without re-esterification in the intestinal cells5. The antibacterial properties, antioxidant activity, and anti-inflammatory effect of coconut oil make it a valuable feed additive in poultry feed6,7. The main natural antioxidants found in coconut oil include capric acid, tocotrienols, and lauric acid.
In broiler chickens, coconut oil significantly increased the growth through the 1–21-days period (9.9%) compared the fish oil-diet8. The supplementation with coconut oil significantly increased superoxide dismutase (SOD) activity compared to the fish oil group. Besides, coconut oil-supplemented rations significantly decreased plasma malondialdehyde (MDA) compared to the fish oil diet8. While, Wang et al.9 stated that a coconut oil -supplemented diet has no impact on body weight gain (BWG), feed intake (FI) and feed conversion ratio (FCR). In addition, coconut oil improves the digestion of fats and the performance index during coccidiosis infection9.
It is hypothesized that the dietary addition of coconut oil is expected to exert beneficial effects on broiler chicks. Thus, this study aimed to determine the impact of coconut essential oil on growth performance, carcass criteria, liver and kidney function, antioxidant and immunity, and lipid profile of broiler chicks.
Material and methods
Ethical statement
The experiment was accepted by the Ethics Committee of the Local Experimental Animals Care Committee and performed under the guidelines of the Department of Poultry, Faculty of Agriculture, Zagazig University, Egypt (ZU-IACUC/2/F/56/2021). The study was conducted following ARRIVE guidelines.
Animals, design and diets
One hundred ninety-two broiler chicks were divided into six groups sets with four replicates of 8 chicks per set. The groups were as follows: (1) basal diet (control), (2) basal diet plus 0.5 ml coconut essential oil/kg, (3) basal diet plus 1 ml coconut essential oil/kg, (4) basal diet plus 1.5 ml coconut essential oil/kg, (5) basal diet plus 2 ml coconut essential oil/kg and (6) basal diet plus 2.5 ml coconut essential oil/kg. We provided standard broiler rations (National Research Council, 1994). Water and food were available freely. Two phases of commercial diets were administered: the beginning (0–3 weeks) and the finishing phase (4–5 weeks). Table 1 indicates the form and structure of trade broiler rations.
Gas chromatography/ mass spectrometry examination of coconut essential oil
According to Adams10, the components (major and minor) of coconut essential oil were identified and measured by gas chromatography (Agilent Technologies 6890 series, Wilmington, DE, USA).
Growth parameters, blood sampling and carcass properties
To determine weight changes and body weight growth, chick weights at ages 0, 3, and 5 weeks were recorded. At ages 1, 3, and 5 weeks, feed consumption and conversion were also measured. At the end of the experiment, chickens (twenty four birds; 4 birds randomly possessed per group) were weighed and then were anesthetized by using intramuscular injection with 1 ml/kg of ketamine xylazine mixture (2:1) and slaughtered by sharp knife to complete bleeding, and their blood was taken in sterile tubes. The samples were then centrifuged after clotting at 3500 rpm (2328.24 G) for 15 min, and serum was kept at -20 °C until biochemical analysis. Weights of the heart, gizzard, and liver were measured and given as grams per kilogramme of killing weighing (KW). Weighing measurements were taken for the carcass, dressed, and giblet; (Carcass weight + Giblet weight)/Lives body weight was used to calculate the dressed weight.
Biochemical parameters
The collected sera were used to determine total protein (TP), albumin (ALB), liver function represent as aspartate aminotransferase (AST), alanine aminotransferase (ALT), kidney function represent as creatinine, urea grades, lipid profile like total cholesterol (TC), high-density lipoprotein (HDL), cholesterol, triglyceride (TG). Also immune response such as immunoglobulin G (IgG) and immunoglobulin M (IgM) and antioxidants (superoxide dismutase; SOD, reduced glutathione; GSH and malondialdehyde; MDA) were measured using commercial diagnostic tools from Biodiagnostic Co. (Giza, Egypt). Low-density lipoprotein (LDL) cholesterol was studied via the Friedewald et al.11 model: \(LDL = TC - HDL - TG/5\).
Statistical analysis
One-way ANOVA was used to statistically examine the variances between sets. SPSS® (2008) statistical software v.11.0 was used for all analyses. Duncan's Multiple Range Test was used to determine the significant intergroup differences12.
Results
Bioactive components and essential oils in coconut oil
Table 2 displays the results of the analyses for coconut oil using gas chromatography and mass spectrometry, along with the retention times and peak area percentages. The most prevalent compound in coconut oil is 6-Octadecenoic acid (oleic acid) representing 46.44% followed 2(3H)-Furanone, dihydro-5-pentyl- (CAS) (11.36%), Hexadecanoic acid (CAS) (4.71%), and vanillin (2.53%).
Growth parameters
Live body weight and weight gain data are presented in Table 3. The supplementation with 1 and 1.5 ml of coconut oil improved significantly body weight and gain (quadratic p < 0.01). In contrast, 2.5 ml of coconut oil impaired significantly body weight and gain. With the same trend, dietary supplementation of 1 ml of coconut oil improved significantly FCR (Table 4) while 2.5 ml level increased FCR compared to other levels and control groups. Feed intake increased significantly due to dietary supplementation of 2.5 ml of coconut oil (Table 4).
Carcass measurements
No significant changes were seen among all the carcass parameters examined (dressing, liver, gizzard, and giblets) in response to coconut oil (Table 5).
Liver and kidney functions
Regarding liver function (Table 6), dietary supplementation of 1 ml of coconut oil improved significantly (linear p < 0.01) liver function in comparison to the control and other treatment groups. The addition of coconut oil to broiler diets has no significant effect on total protein, albumin, globulin, and creatinine. The supplementation with coconut oil linearly increased ALT compared to the control group (p = 0.002). Dietary coconut oil with 0.5, 1, 2 and 2.5 ml /kg significantly reduced AST compared to the control group (p = 0.007).
Lipid profile
The supplementation with 1 ml coconut oil significantly reduced TG and VLDL compared to control and other treatment groups (Table 7) while no significant differences in TC, HDL, and LDL due to dietary coconut oil.
Antioxidant status and immunity
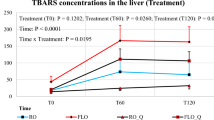
Regarding immune response and antioxidants status (Table 8), the present findings showed that dietary coconut oil with 1 and 1.5 ml/kg improved significantly antioxidants status through increased antioxidant enzymes like SOD and GSH while decreasing significantly MDA levels compared to control and other treatment groups. However, coconut supplementation has no significant effect on immune response.
Discussion
Our findings showed that dietary coconut oils up to 1.5 ml/kg significantly improved body weight and weight gain compared to a control group, this might be due to the bioactive compounds in coconut oil13,14. On the same context, use of coconut oil in broiler diets increased the growth rate during the 1–21-days period (9.9%) compared the fish oil-diet8. Moreover, it is suggested that that plant-derived essential oils can increase appetite. The digestion and absorption of nutrients in the gut are also improved by herbal supplements15. Several studies demonstrated the promotional effects of dietary coconut oil on growth performance in broilers13, and Japanese quail16. Moreover, dietary 2% coconut oil improved growth performance in broiler chicks challenged with coccidiosis17. However, dietary coconut has no effect on weight gain of broiler chickens18. In the same line dietary coconut at 1% had not negative effect in European quail performance19. These discrepancies in the results may be due the dietary level or the extraction process. The present findings showed that the best FCR was obtained by 1 ml of coconut , while the worst level was 2.5 ml level, the reason for that might due to increase the level of caprylic acid supplementation that affect feed conversion in broiler chickens20, however the mode of action of caprylic acid in affecting FCR is not clear .
Inclusion of 1 ml coconut oil in to broiler diets improved liver function. It could be due to the medium-chain fatty acids (MCFA), particularly lauric acid, in coconut oil's components, which have antioxidant and anti-inflammatory characteristics21. These findings point to a protective effect of PUFA on the integrity of hepatic cell membranes, which may be caused by increased phospholipids, which are a crucial component of cell membrane integrity and contain two hydrophobic long-chain fatty acids LCFA22,23.
Regarding to lipid profile, our findings showed that coconut oil inclusion at 1 ml coconut oil reduced significantly TG and VLDL. Our results agree with Attia et al.8 who found that dietary coconut oil has a positive effect on lipid profile in broiler chickens. Our finding showed that the lack of an impact on HDL cholesterol, demonstrated that MCFA in the form of coconut oil didn't lower cholesterol by bringing LDL cholesterol to the liver where it could be processed again, but rather by some other mechanisms18. Due to their effect on raising HDL-C while lowering LDL-C, the hazardous lipoprotein segment, UFA and beneficial PUFA have been shown in previous research to have desirable and healthful effects on plasma lipids24,25,26.
The present findings showed that dietary coconut oil with 1 and 1.5 ml /kg improved significantly antioxidants status through increased antioxidant enzymes like SOD and GSH while decreasing significantly MDA levels in comparison to the control and other groups. These results agree with Attia et al.8 who found that dietary supplementation of coconut oil decreased plasma MDA compared to the fish oil diet in broiler chickens. The main natural antioxidants found in coconut oil include capric acid, tocotrienols, and lauric acid. It has been reported that coconut oil as a source of lauric acid has an antioxidant activity in broilers diet27. Moreover, dietary coconut oil in rabbit diet improved GSH and SOD12, improved the antioxidant status in coconut oil supplemented diets might due to the bioactive ingredients that existing in coconut like higher saturated fatty acids and polyphenolic compounds, that have antioxidant properties28. Boosting the secretion of digestive enzymes, endocrine function, immune function, and antioxidant status are just a few of the multiple ways that phytogenic supplements may perform29,30. Our results indicated that broilers' growth performance and health condition could be improved in the future by using coconut oil as a natural antioxidant.
In the present study, dietary supplementation with coconut oil has not significant effect on immune response; these results disagree with previous one in broilers24 they found that the coconut-enriched diets in broiler chickens significantly increased α1-globulin. Moreover, El-Abasy et al.21 suggested that including dietary coconut (2%) in rabbit diets may have a positive impact on the animals' health and immunological functions. With the same trend, El Kholy et al.31 found that dietary supplementation of 1.5 or 2% of coconut oil in Domyati ducklings diets increased plasma immunoglobulin levels, which collectively suggested that the immunological response had improved. The non- significant effect of coconut oil as immune stimulator in our study may be due to unstressed condition that birds reared in.
Conclusion
Our findings showed that dietary coconut oil with 1 and 1.5 ml improved significantly body weight, gain, and FCR in broiler chickens. Also, the antioxidant status of broiler chickens was improved due to dietary coconut oil. However, the immune response has not affected by coconut oil supplementation. The supplementation with 1 ml coconut oil reduced TG and VLDL compared to control and other treatment groups. Therefore, the diets of broiler chickens could be fortified with coconut oil with 1 or 1.5 ml to improve growth, feed utilization, and antioxidant status of broiler chickens.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- TG:
-
Triglyceride
- HDL:
-
High density lipoprotein
- LDL:
-
Low density lipoprotein
- VLDL:
-
Very low density lipoprotein
- SOD:
-
Superoxide dismutase
- GSH:
-
Reduced glutathione
- MDA:
-
Malondialdehyde
- BWG:
-
Body weight gain
- FI:
-
Feed intake
- FCR:
-
Feed conversion ratio
- MCFA:
-
Medium-chain fatty acid
References
Alagawany, M. et al. Use of lemongrass essential oil as a feed additive in quail’s nutrition: its effect on growth, carcass, blood biochemistry, antioxidant and immunological indices, digestive enzymes and intestinal microbiota. Poult. Sci. 100(6), 101172 (2021).
Rafeeq, M. et al. Application of herbs and their derivatives in broiler chickens: A review. World Poult. Sci. J. 79(1), 95–117 (2023).
Dhama, K. et al. Multiple beneficial applications and modes of action of herbs in poultry health and production-a review. World. Poult. Sci. J. 11(3), 152–176 (2015).
Dhama, K. et al. Medicinal and therapeutic potential of herbs and plant metabolites/extracts countering viral pathogens-current knowledge and future prospects. Curr. Drug Meta. 19(3), 236–263 (2018).
Bhatnagar, A. S., Prasanth Kumar, P., Hemavathy, J. & Gopala Krishna, S. Fatty acid composition, oxidative stability, and radical scavenging activity of vegetable oil blends with coconut oil. J. Am. Oil Chem. Soc. 86(10), 991–999 (2009).
Nevin, K. & Rajamohan, T. J. C. B. Beneficial effects of virgin coconut oil on lipid parameters and in vitro LDL oxidation. Clin. Biochem. 37(9), 830–835 (2004).
Vysakh, A. et al. Polyphenolics isolated from virgin coconut oil inhibits adjuvant induced arthritis in rats through antioxidant and anti-inflammatory action. Int. Immunopharmacol. 20(1), 124–130 (2014).
Attia, Y. A., Al-Harthi, M. A. & Abo El-Maaty, V. S. The effects of different oil sources on performance, digestive enzymes, carcass traits, biochemical, immunological, antioxidant, and morphometric responses of broiler chicks. Front. Vet. Sci. 7, 181 (2020).
Wang, J. et al. Effects of dietary coconut oil as a medium-chain fatty acid source on performance, carcass composition and serum lipids in male broilers. Asian-Austral. J. Anim. Sci. 28(2), 223 (2015).
Adams, B. Y. C., Vahl, H. A. & Veldman, A. Interaction between nutrition and Eimeria acervulina infection in broiler chickens: Diet compositions that improve fat digestion during Eimeria acervulina infection. Br. J. Nutr. 75, 875–880 (1996).
Friedewald, W. T., Levy, R. I. & Fredrickson, D. S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18(6), 499–502 (1972).
Duncan, D. B. Multiple range and multiple F tests. Biometrics 11, 1–42 (1955).
Alagawany, M. et al. Dietary cold pressed watercress and coconut oil mixture enhances growth performance, intestinal microbiota, antioxidant status, and immunity of growing rabbits. Animals 8(11), 212 (2018).
Sundu, B., Hatta, U., Mozin, S., Toana, N. & Sarjuni, S. Coconut meal as a feed ingredient and source of prebiotic for poultry. In IOP Conference Series: Earth and Environmental Science (IOP Publishing, 2020).
Khan, R., Naz, S., Nikousefat, Z., Tufarelli, V. & Laudadio, V. Thymus vulgaris: alternative to antibiotics in poultry feed. World Poult. Sci. J. 68(3), 401–408 (2012).
Abeysekara, T. & Atapattu, N. Effects of dietary coconut oil meal with or without an enzyme mixture on laying performance and physical parameters of eggs of Japanese quail (Coturnix coturnix). Trop. Agri. Res. 27, 414–419 (2016).
Abdul Hafeez, A., Ullah, Z., Khan, R. U., Ullah, Q. & Naz, S. Effect of diet supplemented with coconut essential oil on performance and villus histomorphology in broiler exposed to avian coccidiosis. Trop. Anim. Health Prod. 52, 2499–2504 (2020).
Demirci, M., Evci, Ş., Karsli, M.A. & Şenol, A. Effects of free capric acid, lauric acid, and coconut oil supplementation on performance, carcass, and some blood biochemical parameters of broiler chickens. Turkish J. Vet. Anim. Sci 47, 138–145 (2023).
Veras, A. G. et al. Canola and coconut oils in the feed of European quails (Coturnix coturnix). Rev. Bras. Zootec. 48(48), e20180123 (2019).
de Los Santos, F. S. et al. Caprylic acid supplemented in feed reduces enteric Campylobacter jejuni colonization in ten-day-old broiler chickens. Poult. Sci. 87(4), 800–804 (2008).
El-Abasy, M. A., Abdelhady, D. H., Kamel, T. & Shukry, M. Ameliorative effect of coconut oil on hematological, immunological and serum biochemical parameters in experimentally infected rabbits. Alex. J. Vet. Sci. 50(1), 36–48 (2016).
Poorghasemi, M. et al. Influence of dietary fat source on growth performance responses and carcass traits of broiler chicks. Asian-Aust. J. anim. Sci. 26(5), 705–710 (2013).
Poorghasemi, M. et al. Effect of dietary fat source on humoral immunity response of broiler chickens. Eur. Poult. Sci. https://doi.org/10.1399/eps.2015.92 (2015).
Wignjosoesastro, N., Brooks, C. C. & Herrick, R. The effect of coconut meal and coconut oil in poultry rations on the performance of laying hens. Poult. Sci. 51(4), 1126–1132 (1972).
Aggoor, F., Attia, Y. & Qota, E. M. A. A study on the energetic efficiency of different fat sources and levels in broiler chick vegetable diets. J. Agric. Sci. Mansoura Univ. 25(2), 801–820 (2000).
Özdoǧan, M. & Akşit, M. Effects of feeds containing different fats on carcass and blood parameters of broilers. J. Appl. Poult. Res. 12(3), 251–256 (2003).
Londok, J. J. (Peer Review) Growth Performance, Carcass Characteristics and Fatty Acids Profile of Broilers Supplemented with Lauric Acid and Natural Antioxidant from Areca vestiaria Giseke (2017)
Shan, B., Cai, Y. Z., Sun, M., Corke, H. & Chemistry, F. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 53(20), 7749–7759 (2005).
Lee, K.-W. et al. Effects of dietary essential oil components on growth performance, digestive enzymes and lipid metabolism in female broiler chickens. Br. Poult. Sc. 44(3), 450–457 (2003).
Daader, A., Al-Sagheer, A., Gabr, H. A. & Abd El-Moniem, E. A. Alleviation of heat-stress-related physiological perturbations in growing rabbits using natural antioxidants. Span. J. Agri. Res. 16(3), e0610 (2018).
El-Kholy, K., Gad, H. A., Atef, M., Ali, R. A. M. & Ghazal, M. N. Physiological and immunological performance of Domyati ducklings fed different levels of coconut oil. Egypt. Poult. Sci. J. 38(3), 847–860 (2018).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Conceptualization, Methodology, Formal analysis M.S.E., D.E.A.-K., M.M.E.-H., and M.A., Supervision, Data curation D.E.A.-K., M.M.E.-H., and M.A. Conceptualization, Resources, Software. M.S.E., D.E.A.-K., M.M.E.-H., and M.A. Investigation, M.S.E., and M.A. Writing - review & editing. M.M.; M.S.E., Mo.A. and M.A., Writing an original draft, Writing - review & editing, Investigation. All authors have drafted, reviewed, revised, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elewa, M.S., Abou-Kassem, D.E., El-Hindawy, M.M. et al. Effect of coconut oil on growth performance, carcass criteria, liver and kidney functions, antioxidants and immunity, and lipid profile of broilers. Sci Rep 13, 13974 (2023). https://doi.org/10.1038/s41598-023-41018-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-41018-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.