Abstract
A randomized complete block with a 2 × 3 factorial arrangement was used to design a nutrition experiment conducted for the evaluation of the relation between walnut meal (WM—6% inclusion rate) and cranberry leaves (CL—1% and 2% inclusion rate) supplements and their effects on tissue lipid profile, lipid metabolism indices and oxidative stability of meat. Semi-intensive system conditions were simulated for 240 Ross 308 broilers and the animals were reared on permanent shave litter in boxes of 3 m2 (40 broilers / each group, housed in a single box). The current study results showed that the diets enriched in linolenic acid (LNA) (WM diets) led to broilers meat enriched in LNA, but the synthesis of long-chain omega-3 polyunsaturated fatty acids (LC n-3 PUFA) was stimulated when the diets were supplemented with a natural antioxidants source (CL diets). The CL diet also exhibited the most powerful effect in counteracting the oxidative processes of meat.
Similar content being viewed by others
Introduction
The n-3 and n-6 series of polyunsaturated fatty acids (PUFA) are essential for the human diet, together with their ratio (n-6/n-3) being a marker of lifestyle. Alpha-linolenic (C18:3n3, LNA), eicosapentaenoic (C20:5n3, EPA), and docosahexaenoic acids (C22:6n3, DHA) are the main three PUFA n-3 demanded in the human diet known as beneficial nutrients for human health1. Long-chain PUFA n-3 (LC-PUFA n-3), EPA, and DHA are associated with beneficial effects on cardiovascular and autoimmune disease, cancer, diabetes, and others2. Taking into account that terrestrial plants do not contain LC-PUFA3, animal-origin food represents a valuable source of these nutrients. Evolution steps and adaptation capacities led to different bioconversion rates of PUFA to LC-PUFA specific for each species. It seems that the LC-PUFA biosynthesizing ability respects the order of fish > birds > mammalians4. In this context, marine sources were put under great pressure but the poultry industry can be an alternative for human nutrition5. Different nutritional strategies were applied to obtain PUFA-enriched animal-origin food. PUFA meat enrichment through animal nutrition strategy was mostly based on diets supplemented with linseed (oil, seeds, or meal)6,7,8, fish oil9, camelina (oil, cakes, meal), and microalgae10. Using this strategy, meat products showed an enhancement of LNA concentrations, and a better n-6/n-3 ratio but LC-PUFA was not much positively influenced. Studies on the conversion efficiency of LNA to LC-PUFA in the human body showed a rate below 1%11, but the same observation is valid for most livestock species12. Apart from this, sensory and lipid peroxidation negative effects related to the acceptability of meat on market, have to be considered when the lipid profile of meat is improved by animal feeding. To counter these negative effects, dietary antioxidants are used in animal nutrition, the synthetic forms being popular for many years. Naturally occurring antioxidants become an interesting option with proven benefits on performance and endogenous antioxidant systems of animals13. Herbs and spices (clove, cinnamon, pepper, ginger, thyme, etc.) or by-products derived from the food industry (grapes, tomato, orange, olives, etc.) are the most common phyto-antioxidants studied in poultry nutrition14,15,16.
Walnut (Juglans regia L.) is considered a strategic plant for human nutrition and a valuable source of antioxidants, PUFA, LA, and LNA. The polyphenols content in walnuts depends largely on genetic factors and environmental growing conditions, the majority being those of the non-flavonoid type belonging to ellagitannins, or hydrolyzable tannins17, but also has small quantities of catechins and proanthocyanins monomers18. Up to 47% of the processed mass represents meal after oil extraction and depending on the method used the obtained walnut meal can become a natural source of n-3 PUFA and antioxidants for animal nutrition19. Recent studies have reported that walnut meal as a by-product20,21 is rich in many nutrients and is a promising dietary source that can contribute to the development of functional foods22. It has been shown23, that using walnut seed meal in broiler diets decreased feed intake, serum total cholesterol, and abdominal and muscle fat. Also, there was an improvement in n-3 PUFA content in breast and thigh meat without affecting their organoleptic properties and oxidative stability.
Cranberry (Vaccinium oxycoccos L.) is a valuable source of polyphenols, organic acids, anthocyanins, lipids, fiber, and nutritive minerals, therefore representing a rich source of bioactive compounds and phytonutrients for both human and animal diets24. Although cranberries have traditionally been used for medicinal purposes, recent advances in nutritional science have shown that all components of the fruit, as well as their by-products resulting from processing, can be used in animal nutrition. Cranberry leaves are a good source of microelements, especially Fe, Mo, Mn, Cu, and B, depending on several factors25 and represent a potential source of health-promoting compounds. Due to their high vitamin content, cranberry fruits and their products exert antimicrobial, antifungal, anticancer, and detoxifying properties. Oszmianski et al.26 conducted a comparative study between cranberry fruits and leaves to evaluate the bioactive potential of the leaves due to the limited existing studies. Research results indicated that cranberry leaves was not only a valuable source of selected antioxidants, but they also contained significantly more polyphenols than the fruit and fruit-based products, flavanols and flavan-3-ols being the major polyphenol group identified. Therefore, cranberry leaves can be considered a promising alternative to the use of natural bioactive compounds in animal feed to improve their quality.
This paper aims to elucidate the relationship between the effects of walnut meal (WM) and cranberry leaves (CL) on tissue lipid profile, lipid metabolism indices, and oxidative stability of broilers’ meat.
Results
According to the fatty acids (FA) profile presented in Table 1, it can be noticed that the n-3 FA concentrations are approximately double in the 6% WM diets. LNA was the only n-3 FA presents in the profile of the feed and even though the sum of n-3 FA in the structure of nutritional supplements was similar, the differences in the sum of n-3 FA noticed in the feeds are explained by the different inclusion rates of plants in the diets.
The lipid quality indices belonging to breast meat are presented in Table 2 and the lipid structure of tissues is detailed in Table 3. SFA belonging to experimental groups registered lower values compared to control, while all PUFA increased under studied feed additives influence (Table 2). MUFA concentrations decreased significantly for all experimental groups compared to C, of which only C24 registered higher values for the 2% CL-supplemented group. Regarding n-3 FA, both CL and WM supplements had a significant effect on increasing their concentrations, especially LNA (C18:3n3) being almost double that of other groups under WM influence. Although n-3 PUFA concentration was higher in the WM group, the synthesis of LC-FA did not meet this tendency. LA (C18:2n6) recorded for experimental groups higher concentrations than the C group, tendency respected for all n-6 FA.
The ratio between n-6 and n-3 FA significantly decreased for groups that included WM supplements proving its beneficial effect on the quality of lipids (Table 2). The presence of CL (2%) in broilers’ diets (individual or mixed) increased the desaturase index specific for n-3. The n-6 desaturase index significantly decreased for all experimental groups except in the 2% CL group. The elongation index for n-3 also increased under CL (2%) influence and decreased in WM groups, n-6 EI being uninfluenced by treatments. The efficiency of conversion from precursor to product revealed that for n-3, 1% CL stimulated the desaturation process on C18 and C22 chains and WM influenced C20 desaturation. Overall, the ratio DHA/LNA (final product/precursor) recorded the major value for CL (1%) group, while the WM-supplemented groups registered significantly decreased values compared to other groups. In the n-6 series, only CL (2%) stimulated the desaturation process for C20. WM supplements led to decreasing values of the conversion rate on the C22 series compared to the C group. Overall, the ratio AA/LA (final product/precursor) recorded the major value for the 2% CL group. WM supplements affected LC-PUFA synthesis.
The lipid quality indices belonging to the liver are presented in Table 4 and the lipid structure of tissues is detailed in Table 5. In liver tissue, C14 and C16 SFA decreased under 2% CL influence, while C17 and C23 increased compared to the C group (Table 4). All MUFA decreased for experimental groups with one exception (2% CL—C24:1n9). Similar to the breast profile, in liver samples, LNA registered almost double values for WM-supplemented groups, but the tendency was not respected for others n-3 FA. The LA concentrations also increased for experimental groups compared with control and the tendency also was not respected for all n-6 FA. The total concentrations of n-3 and LC n-3 registered higher values for WM-supplemented groups (Table 4). The n-6/n-3 ratio was significantly influenced by WM presence in diets, resulting in a significantly lower n-6/n-3 ratio, for groups supplemented with WM compared to those without WM in their diets. The lipid quality indices regarding desaturases and elongases activity were decreased compared with the C group with few exceptions: for 1% CL and WM (separate supplemented groups) the n-6 desaturase index was increased and for 1% CL, the n-3 elongase index recorded increased values compared to other groups.
Principal component analysis (PCA) was conducted to explore the relationships between nutritional supplements used in the experiment and the FA metabolism parameters specific for liver and breast tissues. Figure 1a presents the correlation circle corresponding to the first three factors with eigenvalues > 1, which represent 78.89% of total variation (F1—36.43%; F2—28.83%; F3—13.63%). Figure 1b presents the distribution of the samples in the factor space. In the first diagram, F1 is related to the liver parameters and F2 is related to desaturase and elongase indices. Long-chain FA from the breast is negatively correlated with liver parameters but the conversion ratio of DHA from LNA is directly correlated with liver data. If the variables presented short vector length (like L_LC n-6; B_EPA/LNA or B_n-6 EI) the information might be better represented in other PCA like F1/F3. In the second diagram, it can be noticed that in breast n-3 and n-6 LC and AA/LA are very good correlated and negatively correlated with liver parameters. The distribution of the samples in the factor space showed that C and 1% CL were characterized by the well-correlated parameters of the liver and 2% CL was related to breast n-6 parameters (B_n-6 DI, B_LC n-6, B_AA/LA).
(a) The correlation circle represents the projection of considered variables in the factors space. (b) The distribution of the samples in the factor space. The variables considered were: elongation and desaturation indices for n-3 and n-6 FA series, long-chain FA n-3 and n-6 series, and efficiency of conversion: DHA/LNA, EPA/LNA, and AA/LA. The parameters notation was preceded by “L” or “B” corresponding to “liver” or “breast” tissue.
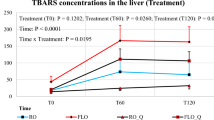
Data presented in Table 6 showed a positive influence of dietary supplements on lipid peroxidation parameters and myoglobin fractions. Primary oxidation products like peroxide value and conjugated dienes were decreased after 7 days of meat storage at refrigerated temperature when WM was included in diets, and the products formed in the latest stage of peroxidation were significantly decreased for all experimental groups. These achievements proved that the antioxidant compounds of vegetal materials included in the diets were very efficient in delaying the peroxidation process. Both phytogenic additives used in this experiment led to increased concentrations of Oxy Mb, by retarding the oxidation of iron from the second oxidation state to the third.
Discussions
The manipulation of meat FA profile has been carried out using different sources of dietary PUFA. Also, some of the reported results proved that by using different nutritional supplements in animal diets, the essential FA from chicken meat can be transferred into human diets3. It seems that the PUFA metabolism of broilers is affected by dietary lipid sources and the bioconversion rate is limited by dietary supply11. The FAs with a higher number of carbon atoms called long-chain PUFA (LC-PUFA) are metabolically converted from LA and LNA through enzymatic pathways27,28. The same desaturase enzymes promote the desaturation of n-3 and n-6 C18 FA occurring in a permanent competition for the formation of LC-PUFA. By using diets enriched in LNA, it can be presumed that the transformation of LA into AA will be inhibited while EPA and DHA conversion will be stimulated.
The liver is the dominant player in the synthesis, oxidation, and deposition of FA29. The Δ5 and Δ6 are catalyzing enzymes (rate-limiting) that are responsible for LC-PUFA biosynthesis from their precursors5. These enzymes responsible for FA metabolism are expressed mainly in the liver and they are regulated by diet, hormones, and other factors29. As other reported results have shown, desaturase activity in non-hepatic tissues is very low, the liver is the fatty acids site production for peripheral tissues and in the present study, the desaturation and elongation activity were expressed by indices calculated as the ratio between the product and precursor 30. According to Gregory et al.31, a better understanding of LNA conversion into LC-PUFA n-3 is related to the status of elongase enzymes. It seems that ELOVL2 and ELOVL5 liver enzymes can elongate more efficient DPA to C24:5n3 in chickens compared to mammals and it led to a better synthesis and deposition of DHA.
In the current study, the most abundant FA in the lipid structure of the breast is oleic acid (C18:1n9) followed by linoleic (C18:2n6) and palmitic (C 16:0) acid, and in the lipid structure of the liver, palmitic (C16:0), stearic (C18:0), oleic (C18:1n-9) and linoleic (C18:2n-6) acids possessed similar ratios. The main n-3 LC-PUFA in breast meat and liver tissue was eicosatrienoic acid (C20:3n-3), a fatty acid produced by elongation from LNA. The bioconversion of LNA to stearidonic acid (18:4n-3) follows two metabolic pathways32: a desaturation step to C18:4n-3 and elongation to C20:4n-3 or elongation to C20:3n-3 and desaturation to C20:4n-3, the second pathway being a route not present in humans. Some researchers considered that the conversion of LNA to stearidonic acid is the rate-limiting step in the metabolism of LNA to DHA, but other studies proved that the elongation and desaturation steps involved in the conversion of DPA to DHA are also controlled points33.
For breast samples, in the control group, the conversion efficiency in the n-3 C20 carbon chain showed that WM-supplemented groups registered higher values. The minimum yield of conversion rate was noticed for the 2% CL supplemented group while in the next step of FA synthesis, the same group registered the higher value for the ratio between product and precursor (C22:5n-3: C20:5n-3), the elongation index presenting a maximum value for 2% CL group. All experimental groups presented significantly higher concentrations of EPA in breast tissues compared to the control group and all WM-supplemented groups and the 2% CL group had significantly higher concentrations of DPA. Finally, only groups supplemented with 1% CL and 6% WM individually, recorded higher concentrations of DHA in the breast (Table 3), with 1% CL recording the higher values for efficiency of conversion (DHA/LNA, EPA/LNA) (Table 2).
As in the case of n-3 PUFA, the LA (C18:2n-6) concentrations in the breast reflects the dietary n-6 supply, and all experimental groups recorded significantly increased concentrations compared to the control. The main pathway of LA (C18:2n-6) conversion to C20:3n-6 was by elongation to C20:2n-6 and desaturation to C20:3n-6. The highest concentrations of C20:3n-6 were noticed for 2% CL, WM + 1% CL and WM + 2% CL supplemented groups. The desaturation to arachidonic acid (AA) was most efficient for the 2% CL group. Arachidonic acid is the most important metabolite of LA and it was noticed that its concentrations are inversely proportional to n-3 LC-PUFA34. In the present study, the 1% CL and 6% WM individual inclusion groups recorded the higher DHA and the lowest AA concentrations.
Some authors suggested that a lower fat deposition in tissues is correlated with a higher rate of PUFA ingestion due to lipid metabolism pathways, like increased lipolysis or decreased lipogenesis and preferential oxidation of PUFA compared to SFA35. In birds, unlike mammals, fat from the tissues originates from liver lipid synthesis36. Compared to other tissues, the liver FA profile is not correlated with diet. The liver is the main site for de novo synthesis of FA but is not a storage organ35.
Jing et al.29 noticed that feeding diets with different LA: LNA ratios, the decreased values of the ratio are specific to the diets with an increased rate of n-3 PUFA incorporated in tissues. Our results showed that WM-supplemented diets had almost double LNA concentrations in the breast and liver and almost the same LA concentrations compared with other groups and in consequence, LA: LNA ratio had lower values than C or CL-supplemented diets. The competition between PUFA classes for the same desaturating and elongating enzymes may explain the effects observed, the most important values for desaturation and elongation index specific to the n-6 class of lipids in the breast, were recorded for the 2% CL supplemented group. As was expected, the conversion rate of LA to AA was decreased for WM groups compared to others, but the most important concentrations of AA were noticed for the 2% CL-supplemented group.
But the desaturase and elongase enzymes in breast samples led to a specific index for n-3 PUFA increased for 2% CL supplemented groups too. The enzymatic activity expressed as Δ5 + Δ6 desaturase index was more pronounced in 2% CL supplemented groups (without WM) for n-3 series accompanied by the major value for elongation index, proving that supplementation of broilers diets with 2% CL stimulated the synthesis of n-3 LC-PUFA in breast tissue.
The major values of efficiency of conversion for n-3 C18 and C22 were recorded for CL (1%) group. When the efficiency was calculated, the type of desaturase enzyme involved in the bioconversion process was not considered an influential factor. For example, in the C22 desaturation process, the Δ4 enzyme is involved32. The efficiency of bioconversion for n-3 (C22) recorded the major values for the 1% CL group and also the ratio DHA/LNA and EPA/LNA, calculated as an end product to precursor ratio, sustained the previous observations, CL (1%) being significantly different from other groups. Even if the desaturation index for n-3 and n-6 series indicated the most pronounced activity for the 2% CL group, the most efficient group for bioconversion of n-3 FA (DHA/LNA and EPA/LNA) was 1% CL. The efficiency of conversion specific only for n3 C20 showed a positive effect of WM in broilers’ diets. For n-6 conversion, 2% CL was the most efficient group.
A possible explanation for the facts presented before may be the increased endogenous oxidation process occurrence when n-3 PUFA-enriched diets are used. Research studies revealed significant changes occur in the expression of the oxidative stress response in the liver when PUFA diets are administrated. It was demonstrated the existence of a preferential order of oxidation (PUFA vs SFA) and the oxidation process is directed to PUFA provided in surplus by the diet. It was suggested that n-3 PUFAs surplus depress hepatic de novo lipogenesis and enhances FA oxidation capacity in the liver37. On the other hand, the presence of a dietary supplement that brings smaller quantities of n-3 PUFA, but is scientifically recognized as a powerful source of antioxidants, like CL, led to increased endogenous bioconversion of LC-PUFA from their precursors.
In Fig. 1a the considered variables were plotted as points in the factor space and they were represented by their correlations. The liver parameters (desaturase indices, LC-PUFA, elongase index for n-3 FA, and conversion efficiency of product to precursor) and breast parameters (DHA/LNA) are far from the center and close to each other, so they are positively correlated. All those parameters are negatively correlated with LC-PUFA n-3 from the breast, which is also far from the center but on the opposite side. These observations are confirmed by the squared cosines of the variables. On the vertical axis, breast n-6 parameters (DI, LC, and AA/LA) are negatively correlated with breast n-3 parameters (DI and LC). In the second diagram, it can be noticed that n-3 DI and EPA/LNA are correlated in breast tissue.
In Fig. 1b the observations are represented by their projections. According to the observation graphs and the squared cosines of the observations, the liver parameters are the dominant variables for C and 1% CL groups. The mixed group with 1% CL and 6% WM supplements is associated with LC-PUFA n-3. The group with 2% CL supplements was characterized by n-6 FA parameters of the breast (DI, LC, and AA/LA) while the dominant variable for WM single group is breast n-3 parameters (DI and LC). From the last diagram, we can conclude that the 2% CL plus 6% WM group is related to n-3 DI and EPA/LNA variables.
Based on statistical data and the relationships between liver and breast parameters it can be considered that the liver is the main production site for LC FA but the distribution and deposition of FA in peripheral tissues are not directly correlated with the production rate.
Fatty acid oxidation in meats is a process whereby PUFA reacts with reactive oxygen species (ROS) leading to a series of secondary reactions which in turn lead to the degradation of lipids. Dietary antioxidants can solve this problem by inhibiting the oxidation of double bonds in the PUFA chain via directly scavenging ROS or by delaying it via stalling the propagation phase38. In our study, the diet supplemented with WM had a powerful effect on primary oxidation products (PV and CD), decreasing their concentration, while the secondary products of oxidation (P-anisidine and TBARS) were reduced by the CL and WM supplementation. Taking into account the antioxidant potential of both supplements, a more pronounced effect was observed in delaying the oxidative process by decreasing levels of P-anisidine and TBARS in meat when both WM and CL were supplemented in the diet than these alone. The phenomenon of synergy between the antioxidant compounds contained in supplements may be the explanation for the protective effect of WM + CL against lipid peroxidation of meat. It was reported that some bioactive compounds express antioxidant effects in the initial phase of oxidation and others have an effect in the final phase of oxidation8, but no clear mechanism has been described. The effects observed may be attributed to important concentrations of polyphenols such as flavonols and flavan-3-ols26 from CL and tocopherol and catechins from WM17. Other authors found that cranberry leaves are also valuable sources of vitamin E, β-carotene, lutein, and zeaxanthin39 which may explain the radical scavenging activities with effect on the inhibition of lipid peroxidation.
Similar to lipids, proteins are attacked by ROS but also by lipid oxidation secondary products. The lipid oxidizing system includes some lipid oxidation products like MDA are ROS for protein oxidation40. Myoglobin is a heme protein from the muscle that determines the quality of meat. The relation between myoglobin and lipids oxidation can provide information regarding the efficiency of antioxidants present in diets on oxidative process delaying. Our findings showed a retard oxidation of MbFe2+ state (OxiMb) to Mb Fe3+ state (MetMb) in experimental groups. The WM groups provide increased concentrations of LC-PUFA, delayed lipid oxidation in the first and final stages, and also were effective in counteracting the oxidation of myoglobin. The presence of CL in broilers’ diets stimulated the LC-PUFA synthesis and also exhibited the main effect in delaying the oxidation process in meat, with a significant influence on myoglobin oxidation.
Material and methods
The study was approved before the initiation of research, by the Ethical Committee of the National and Development Institute for Biology and Animal Nutrition (INCDBNA-IBNA), Balotesti according to experimental protocol no. 1375/ 2020 and in compliance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. Also, the study complied with the principles of Romanian Law 43/2014 ordinance 28/31.08.2011 and Directive 2010/63/EU concerning the protection of animals used for scientific purposes.
Experimental design
A randomized complete block with a 2 × 3 factorial arrangement was used to design a nutrition experiment conducted on mixed-sex broiler chicken aged 10 days. In the first 10 days (starter phase) all broilers received a conventional diet based on corn, wheat, and soybean meal, with 3008.00 kcal/kg metabolizable energy and 22% crude protein. Experimental diets were calculated according to the feeding requirements recommended by the National Research Council41 and the nutritional requirements of the Ross 308 hybrid42. Six dietary ratios which included three concentrations of cranberry leaves (0.1 and 2%) and two concentrations of walnut meal (0 and 6%) were developed as follows: (C) a control diet (corn-soybean diet with no nutritional supplements added); two experimental diets with 1 and 2% CL and no WM added; one experimental diet supplemented with 6% WM in CL absence and two mixed diets with 6%WM and 1 respective 2% CL (Table 7).
Semi-intensive system conditions were simulated for 240 Ross 308 broilers and the animals were reared on permanent shave litter (10–12 cm thick) in boxes of 3 m2 (40 broilers / each group, housed in a single box), fed ad libitum, and free access to the water. The experiment lasted 32 days and environmental conditions were monitored throughout the experimental period. For the first 3 days of age, the room temperature was maintained at 33 °C and then gradually reduced to 22 °C, with an average temperature of 26.84 ± 3.45 °C throughout the experimental period. The light program was set according to the management breeding guide (23 h light / 1 h dark).
At the end of the experiment, on the last day of the finishing phase (42 days), 6 broilers/groups were randomly selected and slaughtered by cervical dislocation. Tissue samples (breast and liver) were collected for further analysis. The productive parameters (average daily gain, feed conversion) were calculated from the weekly records of the body weights and daily feed intake. For the study of oxidative parameters, breast samples were constituted and stored at refrigerated temperature (4 °C) for 7 days. After the storage period, oxidative stability parameters were performed.
Chemical analysis
Fatty acids determination
The fatty acid content of the walnut meal, cranberry leaves, feeds and organic tissue samples (breast and liver) was performed using a Perkin Elmer Clarus 500 (Massachusetts, United States) gas chromatography. The method used aims to transform the fatty acids from the analyzed sample into methyl esters, separate the compounds on the chromatographic column, and identify them by reference to standard chromatograms. The gas chromatograph was equipped with a flame ionization detector (FID) and capillary separation column with high stationary polar phase TRACE TR-Fame, (Thermo Electron, Massachusetts, United States), measuring 60 m × 0.25 mm × 0.25 μm film43.
Lipid metabolism indices were calculated as follows:
The activities of desaturating and elongating enzymes converting PUFA in LC-PUFA were reported as indices, the efficiency of precursor conversion into product corresponding to each carbon chain length and the efficiency of precursor conversion into product expressed as DHA/LNA, EPA/LNA, and AA/LA, was calculated by relating the product to a precursor for each n-3 and n-6 fatty acids series. For a rigorous determination of the PUFA desaturation and elongation steps, it was considered the metabolic pathways described by29.
Oxidative stability parameters
Folch procedure modified was applied for the extractions of lipids. The minced breast sample reacts with a chloroform/methanol mixture (2:1, v/v). The homogenate was filtered in a separation funnel and the lower organic layer was collected, evaporated and stored for further analysis. The determination of peroxide value (PV) was performed by the ferric thiocyanate method, by dissolving the lipid extract in a chloroform/methanol (7:3, v/v) mixture. After the addition of xylenol orange (10 mmol/l) and FeCl2 (1000 mg/kg), the sample solution obtained was left at rest for 5 min and the absorbance was recorded at 560 nm. The value of conjugated dienes (CD) and trienes (CT) was determined by dissolving the lipid extract in 2,2,4-trimethylpentane (iso-octane) and recording the spectra at 233 nm (CD) and 268 nm (CT). The aldehydic compounds formed as peroxidation products were determined as p-anisidine values by measuring the absorbance at 350 nm of sample lipid solution dissolved in 2,2,4-trimethylpentane (iso-octane) before and after reaction with the p-anisidine reagent. The TBARS values (thiobarbituric acid reactive substances) were determined by the third derivative spectrophotometry modified method first described by44. The minced breast sample was treated with TCA (7.5%) /BHT (0.8%) mixture (2:1; v:v) and centrifuged at 3000 × g for 3 min. The filtered solution (2.5 mL) reacts with 1.5 mL 0.8% aqueous thiobarbituric acid solution at 80 °C for 50 min. The third spectra were recorded at 540 nm. The fractions of myoglobin were determined spectrophotometric according to the description of45 and the equation used for quantification was proposed by40.
The oxidative stability methods were previously described by Untea et al.8 and all determinations were performed using a UV VIS spectrophotometer (Jasco V-530, Japan Servo Co. Ltd., Japan).
Statistics
The comparison for a randomized complete block design with a 2 × 3 factorial arrangement of treatments was performed using tested by two-way ANOVA (XLStat, Addinsoft, New York, USA) was used for performing statistical analysis) followed by Tukey's HSD test (P < 0.05). The statistical model included the fixed effects of WM (0 and 6%) and CL (0, 1% and 2%) and their interactions. Data were analyzed considering all broilers in a cage as an experimental unit.
To determine the relationships between lipid metabolisms parameters corresponding to each tissue sample considered, a principal component analysis (PCA) was performed using XLStat (Addinsoft, New York, USA).
The chemical compounds studied in this article
Docosahexaenoic acid (PubChem CID:445580); Linolenic acid (PubChem CID:5280934); Linoleic acid (PubChem CID:5280450); Arachidonic acid (PubChem CID:444899); Eicosapentaenoic acid (PubChem CID:446284); Malondialdehyde (PubChem CID:10964); Thiobarbituric acid (PubChem CID:2723628).
Conclusions
The current study results showed that the diets enriched in LNA (WM supplemented diets) led to animal origin food (broiler meat) enriched in LNA, but the synthesis of LC-PUFA n-3 was stimulated and the efficiency of conversion recorded maximum values when the diets were supplemented with a natural antioxidants source (CL supplemented diets) and also exhibited the most powerful effect in counteracting the oxidative processes occurred during the storage of meat.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Turchini, G. M. & Francis, D. S. Fatty acid metabolism (desaturation, elongation and β-oxidation) in rainbow trout fed fish oil-or linseed oil-based diets. Brit. J. Nutr. 102(1), 69–81 (2009).
O’Neill, C. M. & Minihane, A. M. The impact of fatty acid desaturase genotype on fatty acid status and cardiovascular health in adults. Proc. Nutr. Soc. 76(1), 64–75 (2017).
Kumar, F. et al. Dietary flaxseed and turmeric is a novel strategy to enrich chicken meat with long chain ω-3 polyunsaturated fatty acids with better oxidative stability and functional properties. Food Chem. 305, 125458 (2020).
Emery, J. A., Hermon, K., Hamid, N. K., Donald, J. A. & Turchini, G. M. Δ-6 desaturase substrate competition: dietary linoleic acid (18∶2n-6) has only trivial effects on α-linolenic acid (18∶3n-3) bioconversion in the teleost rainbow trout. PLoS ONE 8(2), e57463 (2013).
Kumar, F. et al. Role of flaxseed meal feeding for different durations in the lipid deposition and meat quality in broiler chickens. J Am. Oil Chem. Soc 96(3), 261–271 (2019).
Orczewska-Dudek, S. & Pietras, M. The effect of dietary Camelina sativa oil or cake in the diets of broiler chickens on growth performance, fatty acid profile, and sensory quality of meat. Animals 9(10), 734 (2019).
Panaite, T. D. et al. Flaxseed and dried tomato waste used together in laying hens diet. Arch. Anim. Nutr. 73(3), 222–238 (2019).
Untea, A. E. et al. Effect of dietary chromium supplementation on meat nutritional quality and antioxidant status from broilers fed with Camelina-meal-supplemented diets. Animal 13(12), 2939–2947 (2019).
Konieczka, P., Czauderna, M. & Smulikowska, S. The enrichment of chicken meat with omega-3 fatty acids by dietary fish oil or its mixture with rapeseed or flaxseed—Effect of feeding duration: Dietary fish oil, flaxseed, and rapeseed and n-3 enriched broiler meat. Anim. Feed Sci. Technol. 223, 42–52 (2017).
Liu, B., Jiang, J., Yu, D., Lin, G. & Xiong, Y. L. Effects of supplementation of microalgae (aurantiochytrium sp.) to laying hen diets on fatty acid content, health lipid indices, oxidative stability, and quality attributes of meat. Foods 9(9), 1271 (2020).
Poureslami, R., Raes, K., Turchini, G. M., Huyghebaert, G. & De Smet, S. Effect of diet, sex and age on fatty acid metabolism in broiler chickens: n-3 and n-6 PUFA. Brit. J. Nutr. 104(2), 189–197 (2010).
Pérez-Palacios, T., Ruiz-Carrascal, J., Solomando, J. C. & Antequera, T. Strategies for enrichment in ω-3 fatty acids aiming for healthier meat products. Food Rev. Int. 35(5), 485–503 (2019).
Lee, M. T., Lin, W. C., Yu, B. & Lee, T. T. Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals—A review. Asian Australas. J. Anim. Sci. 30(3), 299 (2017).
Vlaicu, P. A., Panaite, T. D. & Turcu, R. P. Enriching laying hens eggs by feeding diets with different fatty acid composition and antioxidants. Sci. Rep. 11, 20707. https://doi.org/10.1038/s41598-021-00343-1 (2021).
Madhupriya, V. et al. Phyto feed additives in poultry nutrition–A review. Int. J. Sci. Environ. Technol. 7(3), 815–822 (2018).
Oluwafemi, R. A., Olawale, I. & Alagbe, J. O. Recent trends in the utilization of medicinal plants as growth promoters in poultry nutrition-A review. Res. Agric. Vet. Sci. 4(1), 5–11 (2020).
Haddad, E. H., Gaban-Chong, N., Oda, K. & Sabaté, J. Effect of a walnut meal on postprandial oxidative stress and antioxidants in healthy individuals. Nutr. J. 13(1), 1–9 (2014).
Gomez-Caravaca, A. M., Verardo, V., Segura-Carretero, A., Caboni, M. F. & Fernandez-Gutierrez, A. Development of a rapid method to determine phenolic and other polar compounds in walnut by capillary electrophoresis-electrospray ionization time-of-flight mass spectrometry. J. Chromatogr. A 1209, 238–245 (2008).
Sandulachi, E. & Chiriţa, E. Walnut meal composition and its use. J. Food Pack. Sci. 2013, 89–93 (2013).
Li, Y. et al. Preventive effect of pressed degreased walnut meal extracts on T2DM rats by regulating glucolipid metabolism and modulating gut bacteria flora. J. Funct. Foods 64, 103694 (2020).
Carolina, G., Rodica, S., Turcanu, D., Eugenia, C. & Olga, G. Walnut meal whitening and the impact of whitening factors on its quality. Food Nutr. Sci. 12, 1–12 (2021).
Yang, X.-Y. et al. Effect of walnut meal peptides on hyperlipidemia and hepatic lipid metabolism in rats fed a high-fat diet. Nutrients 13, 1410. https://doi.org/10.3390/nu13051410 (2021).
Adeyemi, K. D. et al. Dietary supplementation of tetracarpidium conophorum (African Walnut) seed enhances muscle n-3 fatty acids in broiler chickens. Eur. J. Lipid Sci. Technol. https://doi.org/10.1002/ejlt.201900418 (2020).
Das, Q. et al. Organic cranberry pomace and its ethanolic extractives as feed supplement in broiler: impacts on serum Ig titers, liver and bursal immunity. Poult. Sci. 100(2), 517–526 (2021).
Karlsons, A., Osvalde, A., Čekstere, G. & Pormale, J. Research on the mineral composition of cultivated and wild blueberries and cranberries. Agro. Rese. 16(2), 454–464 (2018).
Oszmiański, J., Wojdyło, A., Lachowicz, S., Gorzelany, J. & Matłok, N. Comparison of bioactive potential of cranberry fruit and fruit-based products versus leaves. J. Funct. Foods 22, 232–242 (2016).
Smink, W. et al. Effect of dietary fat sources on fatty acid deposition and lipid metabolism in broiler chickens. Poult. Sci. 89(11), 2432–2440 (2010).
da Silva Filardi, R. et al. nfluence of different fat sources on the performance, egg quality, and lipid profile of egg yolks of commercial layers in the second laying cycle. J. Appl. Poult. Res. 14(2), 258–264 (2005).
Jing, M., Gakhar, N., Gibson, R. A. & House, J. D. Dietary and ontogenic regulation of fatty acid desaturase and elongase expression in broiler chickens. Prostaglandins Leukot. Essent. Fatty Acids 89(2–3), 107–113 (2013).
Boschetti, E. et al. Fatty acid composition of chicken breast meat is dependent on genotype-related variation of FADS1 and FADS2 gene expression and desaturating activity. Animal 10(4), 700–708 (2016).
Gregory, M. K., Geier, M. S., Gibson, R. A. & James, M. J. Functional characterization of the chicken fatty acid elongases. J. Nutr. 143(1), 12–16 (2013).
Moltó-Puigmartí, C. et al. FADS1 FADS2 gene variants modify the association between fish intake and the docosahexaenoic acid proportions in human milk. Am. J. Clin. Nutr. 91(5), 1368–1376 (2010).
Wu, B. et al. Comprehensive lipidomics analysis reveals the effects of different omega-3 polyunsaturated fatty acid-rich diets on egg yolk lipids. J Agric. Food Chem. 68(50), 15048–15060 (2020).
Betti, M., Perez, T. I., Zuidhof, M. J. & Renema, R. A. Omega-3-enriched broiler meat: 3. Fatty acid distribution between triacylglycerol and phospholipid classes. Poult. Sci. 88, 1740–1754 (2009).
González-Ortiz, G., Sala, R., Cánovas, E., Abed, N. & Barroeta, A. C. Consumption of dietary n-3 fatty acids decreases fat deposition and adipocyte size, but increases oxidative susceptibility in broiler chickens. Lipids 48(7), 705–717 (2013).
Skřivan, M. et al. Effect of dietary fat type on intestinal digestibility of fatty acids, fatty acid profiles of breast meat and abdominal fat, and mRNA expression of lipid-related genes in broiler chickens. PLoS ONE 13(4), e0196035 (2018).
Palou, A., & Bonet, M. L. Controlling lipogenesis and thermogenesis and the use of ergogenic aids for weight control. Novel Food Ingredient for Weight Control. 58–103 (Woodhead Publishing Limited, New York, Washington, DC 2007).
Laguerre, M., Lecomte, J. & Villeneuve, P. Evaluation of the ability of antioxidants to counteract lipid oxidation: Existing methods, new trends and challenges. Prog. Lipid. Res. 46, 244–282 (2007).
Varzaru, I., Untea, A. E. & Saracila, M. In vitro antioxidant properties of berry leaves and their inhibitory effect on lipid peroxidation of thigh meat from broiler chickens. Eur. J. Lipid Sci. Technol. 122(4), 1900384 (2020).
Wang, Z., He, Z., Emara, A. M., Gan, X. & Li, H. Effects of malondialdehyde as a byproduct of lipid oxidation on protein oxidation in rabbit meat. Food Chem. 288, 405–412 (2019).
National Research Council. Nutrient Requirements of Poultry 9th edn. (National Academy Press, Washington, DC, 1994).
Broiler Nutrition Specification Cobb-508, 2018, Home page address: www.cobbvantress.com, pp. 1–24. (2018).
Turcu, R. P. et al. Effects of grape seed oil supplementation to broilers diets on growth performance, meat fatty acids, health lipid indices and lipid oxidation parameters. Agriculture 11(5), 404 (2021).
Botsoglou, N. A. et al. Rapid, sensitive, and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food, and feedstuff samples. J. Agric. Food Chem. 42, 1931–1937 (1994).
Viriyarattanasak, C., Hamada-Sato, N., Watanabe, M., Kajiwara, K. & Suzuki, T. Equations for spectrophotometric determination of relative concentrations of myoglobin derivatives in aqueous tuna meat extracts. Food Chem. 127, 656–661 (2011).
Acknowledgements
This research was funded by the Ministry of Research, Innovation, and Digitalization, Project PN 19.09-0102, and National Research Development Project to Finance Excellence (PFE)—8/2021.
Author information
Authors and Affiliations
Contributions
A.E.U.: Conceptualization, Methodology, Writing—original draft, Data curation, Writing—review and editing, Software. R.P.T.: Methodology, Formal analysis, Data curation. T.D.P.: Methodology, Formal analysis. P.A.V.: Writing—review, and editing. M.S.: Data curation, Writing—original draft preparation. A.G.O.: Formal analysis, Software.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Untea, A.E., Turcu, R.P., Saracila, M. et al. Broiler meat fatty acids composition, lipid metabolism, and oxidative stability parameters as affected by cranberry leaves and walnut meal supplemented diets. Sci Rep 12, 21618 (2022). https://doi.org/10.1038/s41598-022-25866-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-25866-z
This article is cited by
-
Effect of DFIG control parameters on small signal stability in power systems
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.