Abstract
Lakes located in the boreal region are generally supersaturated with carbon dioxide (CO2), which emerges from inflowing inorganic carbon from the surrounding watershed and from mineralization of allochthonous organic carbon. While these CO2 sources gained a lot of attention, processes that reduce the amount of CO2 have been less studied. We therefore examined the CO2 reduction capacity during times of phytoplankton blooms. We investigated partial pressure of CO2 (pCO2) in two lakes at times of blooms dominated by the cyanobacterium Gloeotrichia echinulata (Erken, Sweden) or by the nuisance alga Gonyostomum semen (Erssjön, Sweden) during two years. Our results showed that pCO2 and phytoplankton densities remained unrelated in the two lakes even during blooms. We suggest that physical factors, such as wind-induced water column mixing and import of inorganic carbon via inflowing waters suppressed the phytoplankton signal on pCO2. These results advance our understanding of carbon cycling in lakes and highlight the importance of detailed lake studies for more precise estimates of local, regional and global carbon budgets.
Similar content being viewed by others
Introduction
Lakes in the boreal region are generally supersaturated with carbon dioxide (CO2) and, thus, net sources of CO2 to the atmosphere1,2. We know that much of the supersaturation emerges from import of inorganic carbon from the surrounding catchment3, including groundwater inflow4,5, and from mineralization of imported organic carbon6,7,8,9. On the other hand, processes that reduce the amount of CO2 in the water, like photosynthesis, are much less studied in this context. However, recent studies suggest that CO2 uptake by algae can have a stronger influence on CO2 emissions from lakes, even boreal ones, than previously thought10,11. Therefore, determining the scale of algal CO2 fixation on lake CO2 concentrations is a crucial step towards understanding the carbon cycling in those lakes and predicting their contribution to climate change.
We aimed here to get a better understanding of the role of phytoplankton in lake carbon cycling in the boreal region by assessing relations between phytoplankton densities and the partial pressure of CO2 (pCO2) in two lakes in which two different types of phytoplankton blooms occurred, i.e. blooms of cyanobacteria and blooms of raphidophytes. Phytoplankton communities in boreal lakes are commonly dominated by cryptophytes, cyanobacteria, chrysophytes, diatoms and raphidophytes12,13. Cyanobacteria blooms mostly occur in eutrophic non-colored lakes14, while humic lakes are more susceptible to blooms of flagellates, especially from the nuisance alga Gonyostomum semen15,16,17. Both cyanobacteria and G. semen blooms are expected to increase as a result of higher temperatures and nutrient concentrations due to increased runoff in a changing climate15,18,19,20,21,22. That makes them perfect model organisms to assess the impact of algal blooms on lake carbon cycling.
To fulfill our aim, we used pCO2 monitoring data from two Swedish lakes within the Swedish Infrastructure for Ecosystem Science (SITES): a humic lake (Erssjön) in Västergötland (spring – autumn 2017 and 2018), and a mesotrophic non-humic lake (Erken) in Uppland (spring – autumn 2018). The former one is subject to blooms of the raphidophyte G. semen, and the latter to blooms of the cyanobacterium Gloeotrichia echinulata in summer and diatoms in spring and autumn. We identified phytoplankton blooms based on chlorophyll a measurements and G. semen and G. echinulata densities. This was done with the help of water chemistry and phytoplankton monitoring data, as well as quantitative PCR (qPCR) for G. semen specifically. We hypothesized that a) pCO2 in Erssjön decreases with increasing G. semen densities, and b) pCO2 in Erken decreases with increasing G. echinulata densities.
Results
Algal blooms and phytoplankton community composition
We confirmed the presence of the bloom-forming raphidophyte G. semen in Erssjön in all DNA samples (analysis: qPCR) taken in 2017 and 2018. Densities of G. semen (Fig. 1) varied from being almost absent (110 and 20 cells L−1 in 2017 and 2018 respectively) to bloom conditions (102,760–119,660 cells L−1 in May, June and August 2017, Fig. 1a), based on our phytoplankton bloom identification of cell abundances > 100,000 cells L−123. In 2018, no G. semen bloom was observed (maximum abundance 20,360 cells L−1 in 2018–05-31, Fig. 1b). In Erken, the bloom-forming cyanobacterium G. echinulata was present in phytoplankton samples from 2018–07-17 to 2018–08-14. Chlorophyll a concentration in 2018 ranged from 1.9 – 11.95 µg L−1 (Fig. 2) and was only once high enough to be considered as a potential phytoplankton bloom according to EU-monitoring standards23 (11.95 µg L−1, 2018–08-08). At this date, G. echinulata densities and biomass was high enough to be considered a bloom (81,588 cells L−1, biomass: 0.37 mm3 L−1) based on WHO guidelines24 (2000 cells mL−1 or 0.2 mm3 L−3).
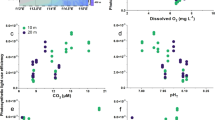
Lake pCO2 and G. semen densities based on qPCR results in Erssjön in 2017 (a) and 2018 (b). For each date, results from 3 sampling locations (pCO2 measurements, 4 replicates per sampling spot) or one sampling location (G. semen abundance) are shown. The boxes symbolize 50% of the data and the line is the median. Boxplots = pCO2, grey triangles = G. semen abundance, dashed line = pCO2 equilibrium (400 µatm), dotted line = cell number threshold indicating a phytoplankton bloom (100,000 cells L−1). Note the different scales in cell densities in (a) and (b).
Lake pCO2 and chlorophyll a concentration in Erken in 2018. For each date in the figure, results from 3 sampling locations (pCO2 measurements, 4 replicates per sampling spot) or one sampling location (chlorophyll a) are shown. The boxes symbolize 50% of the data and the line is the median. Chlorophyll a concentration was measured from an integrated water sample of either the epilimnion (2018–06-13 until 2018–09-09) or the whole water column after autumn stratification started (2018–09-28 – 2018–10-26). Boxplots = pCO2, grey triangles = chlorophyll a, dashed line = pCO2 equilibrium (400 µatm), dotted line = chlorophyll a threshold indicating an algal bloom (10 µg L−1).
In Erssjön we found a typical summer phytoplankton community for a humic, mesotrophic lake (based on four sampling occasions in 2018). Dominant phytoplankton groups were green algae, cryptophytes and colonial cyanobacteria (20–45% of the total phytoplankton biomass). Phytoplankton biomass was high (8.77–13.54 mm3 L−1), with G. semen making up for 0.5–10% of the total phytoplankton community biomass (Supplementary material, Table S1). The number of species ranged from 24 to 30 and Shannon diversity (H’) ranged from 0.5 to 1.1. No single species dominated the phytoplankton community during this time. The Cphyto:TOC ratio (= phytoplankton share in total organic carbon) in summer 2018 ranged from 5–9%.
The phytoplankton community in Erken from June to October in 2018 was mainly comprised of diatoms, cryptophytes, dinoflagellates and cyanobacteria. Cryptophytes and dinoflagellates dominated the community in June and July, while cyanobacteria took over in August. Once autumn mixing started in mid-September, diatoms dominated the phytoplankton community up to 79% (2018–10-23, Supplementary material, Table S2). Total phytoplankton biomass (maximum: 2.9 mm3 L−1) was 3–6 times lower compared to Erssjön. The number of species ranged from 20 to 43 and Shannon diversity was (H’) ranged from 0.47 to 0.92. The Cphyto:TOC ratio remained < 5% through all seasons.
pCO2 and its relation to phytoplankton blooms
Lake pCO2 in Erssjön ranged from 334 to 1737 µatm in 2017 (Fig. 1a) and from 527 to 1290 µatm in 2018 (Fig. 1b). Thus, Erssjön was supersaturated in CO2 (pCO2 > 400 µatm) during the sampling period, except for 2017–05-16 (Fig. 1a). In 2017, pCO2 decreased during spring, increased at the beginning of summer and then once at the beginning of autumn. In 2018, there was a different seasonal pattern: pCO2 remained constant during late spring and early summer, and started to increase from the middle of summer onwards. In early autumn, pCO2 decreased before increasing again in the middle of autumn. There was no significant relationship between G. semen densities and pCO2 in 2017 (linear regression, p = 0.52, R2 = 0, n = 29). In 2018, pCO2 did decrease slightly with increasing G. semen abundance (linear regression, R2 = 0.15, p = 0.02, n = 32).
Lake pCO2 in Erken ranged from 291 to 1569 µatm in summer and autumn of 2018, with most occasions showing a slight supersaturation (450 to 550 µatm, Fig. 2). The highest pCO2 was observed in the end of July (2018–07-25), though pCO2 varied considerably between replicates at those dates. Overall, there was no significant fluctuation in pCO2 between samplings (Kruskal–Wallis test, chi-squared = 3.51, df = 7, p = 0.84). While the second highest chlorophyll a concentration (9.56 µg L−1) was observed during the pCO2 maximum (2018–07-25), there was no significant relationship between chlorophyll a concentration and pCO2 (linear regression, R2 = 0.1, p = 0.13, n = 23) over the year. Phytoplankton total biomass and pCO2 (linear regression, R2 < 0.01, p = 0.7, n = 23) and total phytoplankton density and pCO2 (linear regression, R2 < 0.01, p = 0.4, n = 23) were not significantly correlated with each other either.
Since a relationship between phytoplankton and pCO2 might be non-linear, we also used an additional approach where we took a closer look at the three periods when algal blooms occurred: May/June and August 2017 (Erssjön) and August 2018 (Erken). Using the Kruskal–Wallis test, we compared pCO2 during bloom periods (G. semen abundance and chlorophyll a maximum respectively) to pCO2 sampled two weeks before (= pre-bloom) and after (= post-bloom) the blooms were detected. Applying this approach for Erssjön, we found that pCO2 was similar before, during and after the G. semen bloom in May/June 2017 (Kruskal–Wallis test, chi-squared = 7.62, df = 3, p value = 0.06, Fig. 3a). During the bloom in August 2017, pCO2 appeared to be significantly higher after the G. semen bloom compared to before and during the bloom was observed (Kruskal–Wallis test, chi-squared = 8.74, df = 3, p value = 0.03, Fig. 3b). However, the post-hoc test revealed that pCO2 during this bloom was no different from before and after the bloom (Dunn-test with Bonferroni correction for p values, all p values > 0.05, Fig. 3b), indicating that the significant result from the Kruskal–Wallis test was due to a type-I error.
Lake pCO2 before, during and after observed and potential blooms of G. semen (Erssjön) and G. echinulata (Erken). In May 2017 in Erssjön, no G. semen density data is available for the potential pre- and post-bloom periods. Letters signify the results of the post-hoc Test (Dunn-test, p values adjusted with the Bonferroni method) for the Kruskal–Wallis test results. bloomGS = G. semen bloom, bloomGL = G. echinulata bloom.
Also, in Erken, pCO2 remained the same before, during and after the G. echinulata bloom in August 2018 (Kruskal–Wallis test, chi-squared = 0.62, df = 2, p value = 0.72, Fig. 3c). Since chlorophyll a concentration was quite high two weeks before the G. echinulata bloom (2018–07-25, “pre-bloom” in Fig. 3c), we also ran a Kruskal–Wallis test including 2018–07-11 as the pre-bloom period, to account for either a longer bloom period or two separate blooms happening on 2018–07-11 and 2018–07-25. Still, neither the pre-bloom nor the post-bloom pCO2 was higher or lower than pCO2 during the blooms (Kruskal–Wallis test, chi-squared = 1.05, df = 3, p value = 0.79).
pCO2 and its relation physical lake variables
During sampling, water temperatures at 1-m depth in Erssjön ranged from 5 to 20 °C in 2017 and from 5 to 24 °C in 2018, with summer temperatures (June – August) > 16 °C (Supplementary material, Table S3). Air temperatures ranged from − 1 to 21 °C (2017) and − 1 to 28 °C (2018). Wind speed ranged from 0.2 to 8.4 m1 s−1 (2017) and 0.2 to 6.4 m1 s−1 (2018), the average wind speeds being 2.3 and 2.4 m1 s−1 for 2017 and 2018 respectively. In Erken, water temperatures ranged from 6 to 24 °C (18 – 24 °C between June–August), air temperatures from 2 to 27 °C and wind speed from 0.4 to 8.3 m1 s−1 (average: 3.7 m1 s−1).
We observed no significant correlations between pCO2 and any of the described above physical variables in the two lakes (Supplementary material, Table S4). In 2018, there was a trend towards decreasing pCO2 with increasing wind speed in Erssjön, but very little of the overall variation in pCO2 was explained by this (R2 = 0.06). Furthermore, water column stability (expressed as the Schmidt number) and gas transfer velocity for water–air gas exchange (expressed as k600) were not significantly correlated with pCO2 either.
Discussion
In this study we investigated whether the occurrence of phytoplankton blooms can suppress pCO2 in lakes. We studied two different lakes, one in which cyanobacterial (G. echinulata) blooms occurred (Erken), and another one in which we observed blooms of the raphidophyte G. semen (Erssjön). Contrary to what we expected the phytoplankton signal was not detectable as short-term fluctuations in pCO2 during those times, which indicates that other factors are more important for pCO2 in those lakes.
One criterium for phytoplankton to impact lake pCO2 is a high enough biomass so that photosynthesis dominates over bacterial respiration and mineralization of carbon10. Available Cphyto:TOC ratios were > 5% in Erssjön during summer of 2018. Since this is a threshold value for when phytoplankton biomass potentially can have a detectable effect on CO210, we would have expected that a phytoplankton bloom in this lake caused a decrease in pCO2. Since our hypothesis could not be supported, an interesting question in this context is the identity of the bloom forming phytoplankton species, especially the proportion of mixotrophic species. Erssjön’s phytoplankton community is typical for a boreal brown water lake with a low pH, with cryptophytes, chrysophytes, euglenids and chlorophytes dominating in the summer of 2018. Some of these are mixotrophic species (Uroglena sp., Dinobryon sp., Gymnodium sp., Perdinium sp. and Ceratium sp.25, see Supplementary material, Table S5) and could potentially significantly contribute to heterotrophic respiration instead of autotrophic carbon fixation in boreal lakes26, especially in a warming climate27. This could be one contributing factor as to why CO2 in many boreal brown water lakes is seemingly not impacted by photosynthetic processes and remains supersaturated throughout the year, even though theses lakes demonstrate a high phytoplankton biomass with bloom conditions.
In Erken, the total phytoplankton biomass remained relatively low (< 2 mm3 L−1) during most of the year, which is below the Cphyto:TOC ratio of 5% that Engel et al.10 identified as a threshold for a detectable phytoplankton influence on lake CO2. However, a phytoplankton bloom still occurred (G. echinulata in August), where also chlorophyll a concentrations were higher than 10 µg L−1 (total phytoplankton biomass: 1.12 mm3 L−1). However, again, these bloom conditions did not leave a detectable signal on the pCO2. The lack of a direct relation between phytoplankton and pCO2 in Erken might be a result of measuring phytoplankton and pCO2 at different sites. Erken is a relatively big lake that is strongly wind exposed with frequent lake water mixing, causing a patchy phytoplankton distribution, especially regarding large floating G. echinulata colonies.
Wind exposure is not only affecting the phytoplankton distribution in a lake but also pCO2. Previous studies have shown that wind-induced upwelling and vertical mixing affect the transport of CO2 through the water column28,29, as well as the CO2 flux to the atmosphere30. It is possible that wind suppresses the phytoplankton signal on lake pCO2, because CO2 at the lake surface, where phytoplankton are photosynthesizing and, thus, consuming CO2, is mixed with CO2 from waters from below. This may be especially true at Erken, which is highly susceptible to wind mixing, even causing perturbations in the thermal stratification during summer31. Furthermore, wind exposure may explain why lake pCO2 does not increase in autumn after mixing starts in this lake, even though this is typically observed in eutrophic lakes when deep-water, CO2 water mixes with surface oxygen-rich water32.
However, the G. echinulata bloom in Erken occurred mostly at low wind speeds (1.2–5.4 m1 s−1, mean: 2.9 m1 s−1) when water mixing is low, as did the G. semen blooms in Erssjön (0.5–5.1 and 0.4 – 2.8 m1 s−1 in May and June respectively). Consequently, CO2 import from deeper waters should be low. On the other hand, the gas transfer velocity of CO2 from water to the atmosphere is lower at low wind speeds, which could lead to higher observed pCO2 than would be expected during periods of high photosynthetic activity. Thus, the interaction between wind-controlled processes in the water column may be why pCO2 and physical lake variables do not correlate in our study.
Besides mixing, inorganic carbon import via groundwater and river inflow could have affected pCO2 in both lakes, which is common for lakes in the studied geographical region3. This could for example explain the increase of pCO2 in Erssjön in autumn in 2017 and 2018, as this is when autumn mixing starts, precipitation events become more common and water in- and outflow from the lake increase. Furthermore, Erssjön is a rather small lake with a potentially shorter water retention time than Erken (7 years), so hydrological factors contributing to CO2 supersaturation might be dominating over biological processes.
Even if blooms caused by G. semen or other phytoplankton species lead to a decrease in lake pCO2, it is unclear whether this would have a short-term or long-term effect on lake CO2 emissions. Autochthonous carbon is preferably used by bacteria and often gets re-mineralized before it can sink to the lake bottom and contribute to the fixed carbon in the sediment33,34. Erssjön’s pCO2 increased in autumn in 2017 and 2018, indicating that phytoplankton biomass may contribute to internal lake carbon respiration and thus CO2 emissions from the lake when being degraded. In order to understand the ecosystem effects, we would therefore need to monitor G. semen blooms and phytoplankton in general more closely, both regarding their biomass and the sedimentation of organic material before, during and after a bloom.
Sampling frequency is also critical for capturing times when G. semen migrates vertically through the water column during the day35. During migration, it easy to underestimate G. semen densities by sampling too early or too late during the day. It is also known that the carbon flux has high diel and seasonal variability32,36, with diel variations being especially pronounced during mixing events32. Even though the floating chambers were deployed for around 24 h, deployment and measurement times were not consistent between samplings (ranging from ~ 8:00 to 20:00, see Supplementary material, Table S6). This means that changes in pCO2 during the day may not always have been captured completely. Thus, it is likely that the pCO2 measurements at the surface of the lakes caused an under- or overestimation of pCO2 for our sampling dates due to the time of sampling.
In summary, our results show that neither G. semen nor G. echinulata blooms were associated with decreases in pCO2 in two Swedish boreal lakes. It is likely that physical factors, such as wind induced water column mixing and import of inorganic carbon via groundwater inflow and runoff, suppress the phytoplankton signal on pCO2. A higher sampling frequency is necessary to further study the dynamics between phytoplankton blooms and lake CO2 concentrations, as phytoplankton blooms can occur rapidly and disappear within days. Further studies should focus on monitoring phytoplankton biomass and pCO2 at higher frequency to capture diel variations, and include sedimentation rates of autochthonous organic matter and daily CO2 fluctuations to determine if there is a long-term effect of phytoplankton on lake carbon fluxes. Another interesting aspect to study is the species composition of phytoplankton blooms, since the degree of mixotrophy could affect the relationship between phytoplankton and pCO2.
Methods
Sampling sites
Sampling was performed in a mesotrophic brown water lake (Erssjön) in the Bäveån catchment in Västergötland, Sweden (58°22′16.5"N, 12°09′40.3"E) and in a mesotrophic non-humic lake (Erken) in the Broströmmen catchment in Uppland, Sweden (59°50′26.35"N, 18°37′39.75"E). Background information about the two lakes are publicly available at the SITES website (https://www.fieldsites.se/en-GB/sites-thematic-programs/lakes-in-sites-44856978). Erssjön is situated 73 m above sea level, has an area of 0.061 km2 and a maximum depth of 4.5–5 m. The surrounding area is mostly forest and some agricultural land. The lake is dimictic, with anoxic periods during summer stratification. Samples were taken from March to October in 2017 (16 CO2 samplings, 11 DNA samplings) and from May to November in 2018 (13 CO2 samplings, 11 DNA samplings) every 2–4 weeks.
Erken is 13 m above sea level, has an area of 24 km2 and a maximum depth of 21 m. The surrounding area is mostly forest, water and some agricultural land and pasture. The lake is dimictic. Samples were taken from June to October in 2018 (8 CO2 samplings, every 2–4 weeks). Phytoplankton densities and chlorophyll a are monitored through weekly samplings conducted by the Erken Laboratory.
CO 2 measurements
Carbon dioxide concentrations were measured with floating chambers designed according to Bastviken et al.37. In each lake, 4 floating chambers were set along 3 transects from the lake shore towards the center (Fig. 4, check Supplementary material, Table S7 for coordinates). The lake water depths below the floating chambers ranged from 0.3 to 1.9 m (Erssjön) and 0.6 – 4.7 m (Erken). After a deployment of 18–28 h (Supplementary material, Table S6) a subsample of the headspace was taken with a syringe (3 × 60 mL) and transferred into a gas-tight, sealed glass vial through a needle (0.5 mm; 25 gauge). Samples were measured on a gas chromatograph autosampler. These headspace CO2 concentrations should be close to equilibrium and pCO2 was calculated according to Henry’s Law. This data is publicly available upon request on the SITES website (www.fieldsites.se).
Floating chamber and phytoplankton sampling locations in Erssjön (a) and Erken (b). Numbers in Erssjön (a) refer to 1 = phytoplankton and DNA sampling location, 2 = chambers A1 – A4, 3 = chambers B5 – B8, 4 = chambers C9 – C12, numbers in Erken (b) to 1 = phytoplankton and chlorophyll sampling location, 2 = NJÄ_01 – NJÄ_04, 3 = KHM_01 – KHM_04, 4 = SBA_01 – SBA_04. For individual chamber coordinates, refer to Supplementary material, Table S7. This map was generated with the R software (R 4.1.2, package: ggmap, ggspatial) with shapefiles created in Google Earth.
Phytoplankton bloom identification
Phytoplankton blooms in lake surface waters were identified by analyzing chlorophyll a concentration, phytoplankton biomass and phytoplankton densities38,39. Sampling for those variables took place between 9:00 and 12:00 at the two lakes. A chlorophyll a threshold of 10 µg L−1 is often used to indicate a phytoplankton bloom23,40. In addition, phytoplankton biomass is often monitored to identify blooms of harmful algal species (HABs), e.g. toxic cyanobacteria40,41. To identify phytoplankton blooms in the two study lakes, we decided to use a chlorophyll a threshold of 10 µg L−1 to screen for phytoplankton blooms, as is recommended for moderately deep lakes in Europe23. For Erken, chlorophyll a data was available from the weekly monitoring program upon request (Erken Laboratory, Uppsala University, Sweden, https://www.ieg.uu.se/erken-laboratory/lake-monitoring-programme/), and is now also available on the SITES Data portal:
(https://meta.fieldsites.se/objects/NT59BPT70Ce7h1huzA0xSc1c). For chlorophyll analysis, water was filtered through a Whatman GF/C filter. Chlorophyll was extracted with 90% acetone and absorbance measured with a spectrophotometer at 750, 664, 647 and 630 nm according to the Swedish standard SS028146.
Since reliable chlorophyll a data from Erssjön was not available, we also identified blooms by determining densities of G. semen (qPCR) and G. echinulata (phytoplankton monitoring data). For densities the following thresholds for a bloom identification were used: 2,000 cells mL−1 or 0.2 mm3 L−1 for G. echinulata which is the lowest WHO threshold for dangerous cyanobacteria blooms40, based on the fact that these species are potentially toxic42,43, and 100,000 cells L−1 for G. semen since this species has an unusual big cell size44.
DNA analysis for the identification of G. semen densities
In Erssjön, a DNA sample was taken every 2–4 weeks from the Southern side of the mesocosm platform (N 58.371322°, E 12.161074°, see Fig. 4a) during 2017–05-15 – 2017–10-16 and 2018–05-15 – 2018–11-01. An integrated water sample (0–1 m) of 10 L was taken and a subsample of 2 L filtered through a membrane filter (0.2 µm pore size, 47 mm diameter). The filter was then folded, put into a 2 mL cryotube and stored at − 80 °C. DNA was extracted with the Qiagen DNeasy PowerSoil Kit according to the kit’s instruction manual. The DNA concentrations were quantified using PicoGreen, measured on a Tecan Spark plate reader (Tecan, Switzerland) and found to be high enough for further analysis (~ 3–20 µg DNA µL−1).
For determining the G. semen abundance, we used qPCR with the specific primers developed by Johansson et al.45. The DNA was diluted 20 × and the master mix done with SYBR Green. The qPCR was run on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad, USA) with the following cycle conditions: 2 min at 90 °C, 40 cycles at 95 °C for 15 s, 40 cycles at 60 °C for 15 s and 40 cycles at 72 °C for 15 s. For each sample, three technical replicates were measured and the average copy number per vegetative cell was used for quantification of G. semen cells. To determine the copy number per vegetative cell, we measured the extracted and 10 × diluted DNA of three samples of isolated and washed G. semen vegetative cells (40 cells per sample) from a G. semen culture established from a lake in Northern Uppland (Siggefora, culture SF1A8, established by Ingrid Sassenhagen). More details on this procedure is available in Münzner et al.46. The copy number per cell ranged from 171,500 to 304,770, with an average copy number of 243,596 (standard deviation: 54,605) per G. semen vegetative cell.
Phytoplankton community composition
To get a better understanding on phytoplankton dynamics we also determined the phytoplankton biomass and community composition. In Erssjön we sampled during four dates in 2018 (July 10, July 24, August 14, September 1). Phytoplankton samples were taken from surface water samples (~ 0.5 m depth) and a subsample 100 mL was fixed with Lugol. Samples were stored at 4 °C in the dark before analysis. Phytoplankton samples were acclimatized to room temperature (24 h) and were counted with the Utermöhl method with an inverted microscope (Leica DMIL LED fluo) after settling for at least 12 h in a 10 mL chamber. We did a whole chamber count at 40 × magnification for phytoplankton > 100 µm (e.g. colonial algae), counts of diagonals (1 or 2) at 200 × magnification until a cell count of at least 200 cells (> 20 µm) was reached, and 10 random field counts at 400 × magnification. For each identified species, cell length and width were measured for one individual and used for biomass calculations. Phytoplankton shapes and biovolume formulas were determined according to Hillebrand et al.47. Phytoplankton diversity (H’) and evenness (eh) were calculated using the Shannon–Wiener Index.
For Erken, we used publicly available plankton data from the Erken Laboratory (Uppsala University, Sweden, https://www.ieg.uu.se/erken-laboratory/lake-monitoring-programme/, data available upon request). Phytoplankton samples used in this data set were collected the following way: phytoplankton samples were collected at the deepest point of the lake with an integrated water sampler (“Ramberg tube”) in 2 m intervals. The samples were mixed together according to their proportion to the total lake volume (all samples if the lake was fully mixed, epilimnion and hypolimnion samples mixed separately if the lake was stratified). Then, a 100 mL subsample of the integrated sample (only epilimnion sample if the lake was stratified) was taken, fixated with a few drops of Lugol and stored at 4 °C before further analysis. Phytoplankton were identified and quantified with an inverted light microscope according to the quality standards (SS-EN 15,204:2006) approved by the Swedish Environmental Protection Agency (EPA).
From the phytoplankton data we calculated the Cphyto:TOC ratio to identify a previously described threshold when phytoplankton dynamics become detectable in lake waters10. For Cphyto a conversion factor of 0.15 was chosen according to Engel et al.10. For TOC (total organic carbon) we used data on dissolved organic carbon (DOC). DOC has been shown to stand for 97% of the TOC in boreal lakes48, thus we assumed DOC to be equal to TOC in our study lakes. Since carbon monitoring data were not available for Erssjön, we used an average of previously reported values (22.33 mg DOC L−1) by Groeneveld et al.49.
Lake physical variables
Publicly available data collected by SITES (SITES Data portal: https://data.fieldsites.se/portal/) of air temperature, water temperature at 1-m depth, wind direction and wind speed were used. For analyses, average values of those variables over the total deployment time of the chambers at each date were calculated. Schmidt number Sc was calculated according to Wanninkhof et al.50, and for piston velocity k600, the formula for low wind speeds by Cole and Caraco51 was used.
Statistics
All data were analyzed with R 4.1.2. Group comparisons of CO2 measurements within transects with the Kruskal–Wallis test (post-hoc test: Dunn-test with Bonferroni correction for p values) showed that pCO2 was significantly higher in the chambers closest to the shore compared to the chambers further away from the shore, both in Erssjön (chi-square = 9.18, df = 3, p = 0.03) and Erken (chi-square = 8.43, df = 3, p = 0.04). However, even though pCO2 decreased with increasing distance to the shore (linear regression analysis) in Erssjön (p < 0.001, R2 = 0.02, n = 316) and Erken (p < 0.05, R2 = 0.05, n = 104), almost none of the overall variation in pCO2 was explained by that. Following this, we used the average pCO2 of each transect for further analyses (n = 3 per lake per sampling).
Shown regressions and correlations are based on linear relationships. Since the distribution of all variables was skewed, they were log-transformed. Changes in pCO2 over sampling dates in each lake for each year were assessed with the Kruskal–Wallis test. The Kruskal–Wallis test was also used for comparisons of pCO2 before, during and after phytoplankton blooms. For post-hoc analysis of significant results, the Dunn test was used and p values were adjusted with the Bonferroni method (package: FSA).
Data availability
The datasets generated during and/or analysed during the current study are available in the DiVA (Digitala Vetenskapliga Arkivet) portal, [urn:nbn:se:uu:diva-479668].
References
Cole, J. J., Caraco, N. F., Kling, G. W. & Kratz, T. K. Carbon-dioxide supersaturation in the surface waters of lakes. Science 265, 1568–1570 (1994).
Raymond, P. A. et al. Global carbon dioxide emissions from inland waters. Nature 503, 355–359 (2013).
Weyhenmeyer, G. A. et al. Significant fraction of CO2 emissions from boreal lakes derived from hydrologic inorganic carbon inputs. Nat. Geosci. 8, 933–936 (2015).
Einarsdottir, K., Wallin, M. B. & Sobek, S. High terrestrial carbon load via groundwater to a boreal lake dominated by surface water inflow. J. Geophys. Res. Biogeosci. 122, 15–29 (2016).
Nydahl, A. C., Wallin, M. B., Laudon, H. & Weyhenmeyer, G. A. Groundwater carbon within a boreal catchment: Spatiotemporal variability of a hidden aquatic carbon pool. J. Geophys. Res. Biogeosci. 125, e2019JG005244. https://doi.org/10.1029/2019JG005244 (2019).
del Giorgo, P. A., Cole, J. J., Caraco, N. F. & Peters, R. H. Linking planktonic biomass and metabolism to net gas fluxes in Northern temperature lakes. Ecology 80, 1422–1431 (1999).
Jonsson, A., Karlsson, J. & Jansson, M. Sources of carbon dioxide supersaturation in clearwater and humic lakes in northern Sweden. Ecosystems 6, 224–235 (2003).
Sobek, S., Algesten, G., Bergström, A.-K., Jansson, M. & Tranvik, L. J. The catchment and climate regulation of pCO2 in boreal lakes. Glob. Chang. Biol. 9, 630–641 (2003).
Cole, J. J. et al. Plumbing the global carbon cycle: Integrating inland waters into the terrestrial carbon budget. Ecosystems 10, 172–185 (2007).
Engel, F., Drakare, S. & Weyhenmeyer, G. A. Environmental conditions for phytoplankton influenced carbon dynamics in boreal lakes. Aquat. Sci. 81, 35. https://doi.org/10.1007/s00027-019-0631-6 (2019).
Rohrlack, T., Frostad, P., Riise, G. & Hagman, C. H. C. Motile phytoplankton species such as Gonyostomum semen can significantly reduce CO2 emissions from boreal lakes. Limnologica 84, 125810. https://doi.org/10.1016/j.limno.2020.125810 (2020).
Lepistö, L. & Rosenström, U. The most typical phytoplankton taxa in four types of boreal lakes. Hydrobiologia 369, 89–97 (1998).
Maileht, K. et al. Water colour, phosphorus and alkalinity are the major determinants of the dominant phytoplankton species in European lakes. Hydrobiologia 704, 115–126 (2013).
Ptacnik, R. et al. Quantitative responses of lake phytoplankton to eutrophication in Northern Europe. Aquat. Ecol. 42, 227–236 (2008).
Cronberg, G., Lindmark, G. & Björk, S. Mass development of the flagellate Gonyostomum semen (Raphidophyta) in Swedish forest lakes—An effect of acidification?. Hydrobiologia 161, 217–236 (1988).
Lepistö, L., Antikainen, S. & Kivinen, J. The occurrence of Gonyostomum semen (Ehr) diesing in finnish lakes. Hydrobiologia 273, 1–8 (1994).
Hagman, C. H. C. et al. The occurrence and spread of Gonyostomum semen (Her.) Diesing (Raphidophyceae) in Norwegian lakes. Hydrobiologia 744, 1–14 (2015).
Paerl, H. W. & Huisman, J. Blooms like it hot. Science 320, 57–58 (2008).
Jeppesen, E. et al. Climate change effects on runoff, catchment phosphorus loading and lake ecological state, and potential adaptations. J. Environ. Qual. 38, 1930–1941 (2009).
Hehmann, A., Krienitz, L. & Koschel, R. Long-term phytoplankton changes in an artificially divided, top-down manipulated humic lake. Hydrobiologia 448, 83–96 (2001).
Findlay, D. L., Paterson, M. J., Hendzell, L. L. & Kling, H. J. Factors influencing Gonyostomum semen blooms in a small boreal reservoir lake. Hydrobiologia 533, 243–252 (2005).
Rengefors, K., Weyhenmeyer, G. A. & Bloch, I. Temperature as a driver for the expansion of the microalga Gonyostomum semen in Swedish lakes. Harmful Algae 18, 65–73 (2012).
Poikane, S. et al. Defining ecologically relevant water quality targets for lakes in Europe. J. Appl. Ecol. 51, 592–602 (2014).
World Health Organization (WHO). Cyanobacterial toxins: microcystins. Background document for development of WHO guidelines for drinking-water quality and guidelines for safe recreational water environments. World Health Organization. (2020) https://apps.who.int/iris/handle/10665/338066.
Sanders, R. W. & Porter, K. G. Phagotrophic phytoflagellates In: Advances in Microbial Ecology (ed. Nelson, K. E.), 167–192 (Springer, 1988).
Hansson, T. H., Grossart, H.-P., Beisner, B. E., del Giorgio, P. A. & St-Gelais, N. F. Environmental drivers of mixotrophs in boreal lakes. Limnol. Oceanogr. 64, 1688–1705 (2019).
Wilken, S., Huisman, J., Naus-Wiezer, S. & van Donk, E. Mixotrophic organisms become more heterotrophic with rising temperature. Ecol. Lett. 16, 225–233 (2012).
MacIntyre, S., Flynn, K. M., Jellison, R. & Romero, J. R. Boundary mixing and nutrient fluxes in Mono lake. California. Limnol. Oceanogr. 44, 512–529 (1999).
Polsenaere, P. et al. Thermal enhancement of gas transfer velocity of CO2 in an Amazon floodplain lake revealed by eddy covariance measurements. Geophys. Res. Lett. 40, 1734–1740 (2013).
Czikowsky, M. J., MacIntyre, S., Tedford, E. W., Vidal, J. & Miller, S. D. Effects of wind and buoyancy on carbon dioxide distribution and air-water flux of a stratified temperate lake. J. Geophys. Res. Biogeosci. 123, 2305–2322 (2018).
Yang, Y., Colom, W., Pierson, D. & Pettersson, K. Water column stability and summer phytoplankton dynamics in a temperate lake (Lake Erken, Sweden). Inland Waters 6, 499–508 (2016).
Rudberg, D. et al. Diel variability of CO2 emissions from northern lakes. J. Geophys. Res. Biogeosci. 126, 2021006246. https://doi.org/10.1029/2021JG006246 (2021).
Kritzberg, E. S. et al. Autochthonous versus allochthonous carbon sources of bacteria: Results from whole-lake 13C addition experiments. Limnol. Oceanogr. 49, 588–596 (2004).
Guillemette, F., McCallister, S. L. & Del Giorgio, P. A. Selective consumption and metabolic allocation of terrestrial and algal carbon determine allochthony in lake bacteria. ISME J. 10, 1373–1382 (2015).
Rohrlack, T. The diel vertical migration of the nuisance alga Gonyostomum semen is controlled by temperature and by a circadian clock. Limnologica 80, 125746. https://doi.org/10.1016/j.limno.2019.125746 (2020).
Spafford, L. & Risk, D. Spatiotemporal variability in lake-atmosphere net CO2 exchange in the littoral zone of an oligotrophic lake. J. Geophys. Res. Biogeosci. 123, 1260–1276 (2018).
Bastviken, D., Sundgren, I., Natchimuthu, S., Reyier, H. & Gålfalk, M. Technical note: cost-efficient approaches to measure carbon dioxide (CO2) fluxes and concentrations in terrestrial and aquatic environments using mini loggers. Biogeosciences 12, 3849–3859 (2015).
Armon, R. H. & Starosvetsky, J. Algal bloom indicators in Environmental indicators (eds. Armon, R. H. & Hänninen, O.), 635–640 (Springer, 2015).
Zhang, F., Hu, C., Shum, C. K., Liang, S. & Lee, J. Satellite remote sensing of drinking water intakes in Lake Erie for cyanobacteria populations using two MODIS-based indicators as a potential tool for toxin tracking. Front. Mar. Sci. 4, 124. https://doi.org/10.3389/fmars.2017.00124 (2017).
Binding, C. E., Greenberg, T. A., McCullough, G., Watson, S. B. & Page, E. An analysis of satellite-derived chlorophyll and algal bloom indices on Lake Winnipeg. J. Great Lakes Res. 44, 436–446 (2018).
Sellner, K. G., Doucette, G. J. & Kirkpatrick, G. J. Harmful algal blooms: causes, impacts and detection. J. Ind. Microbiol. Biotechnol. 30, 383–406 (2003).
Carey, C. C., Haney, J. F. & Cottingham, K. L. First report of microcystin-LR in the cyanobacterium Gloeotrichia echinulata. Environ. Toxicol. 22, 337–339 (2007).
Carey, C. C. et al. Occurrence and toxicity of the cyanobacterium Gloeotrichia echinulata in low-nutrient lakes in the northeastern United States. Aquat. Ecol. 46, 395–409 (2012).
Carletti, A. & Heiskanen, A.-S. Water framework directive intercalibration technical report – part 3: coastal and transitional waters. https://publications.jrc.ec.europa.eu/repository/handle/JRC51341 (2009).
Johansson, K. S. L., Lührig, K., Klaminder, J. & Rengefors, K. Development of a quantitative PCR method to explore the historical occurrence of a nuisance microalga under expansion. Harmful Algae 56, 67–76 (2016).
Münzner, K., Gauthier, V.A., Sassenhagen, I., Tranvik, L.J. & Lindström, E.S. (2023) Effects of a dominant algal species (Gonyostomum semen) on the carbon balance of brown water lakes. In preparation.
Hillebrand, H., Dürselen, C.-D., Kirschtel, D., Pollingher, U. & Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 35, 403–424 (1999).
von Wachenfeldt, E. & Tranvik, L. J. Sedimentation in boreal lakes—The role of flocculation of allochthonous dissolved organic matter in the water column. Ecosystems 11, 803–814 (2008).
Groeneveld, M., Tranvik, L. J. & Koehler, B. Photochemical mineralisation in a humic boreal lake: Temporal variability and contribution to carbon dioxide production. Biogeosci. Discuss. 12, 17125–17152 (2016).
Wanninkhof, R. Relationship between wind speed and gas exchange over the ocean. J. Geophys. Res. 97(C5), 7373–7382 (1992).
Cole, J. J. & Caraco, N. F. Atmospheric exchange of carbon dioxide in a low-wind oligotrophic lake measured by the addition of SF6. Limnol. Oceanogr. 43(4), 647–656 (1998).
Acknowledgements
This has study been made possible by the Swedish Infrastructure for Ecosystem Science (SITES), in this case at the Skogaryd Research Catchment and the Erken Laboratory. SITES receives funding through the Swedish Research Council under the grant no 2017-00635. We want to especially thank David Bastviken from the Department of Thematic Studies (TEMA) at Linköping University for sharing the SITES greenhouse gas data and his advice on how to use it. Many thanks also go to Pia Larsson at the Erken Laboratory for providing the phytoplankton community data for Erken, and Stina Drakare from the Section for Ecology and Biodiversity at the Department of Aquatic Sciences and Assessment at the Swedish University of Agricultural Sciences (SLU) for her help with phytoplankton species identification. Thank you to Ingrid Sassenhagen for providing samples for determining the copy number of vegetative G. semen cells, as well as her advice on how to interpret the qPCR results. This study was financed by grants from Stiftelsen Oscar och Lili Lamms minne, the Åforsk foundation, and Carl Tryggers Stiftelse för Vetenskaplig forskning to ESL, and the Malméns Stiftelse for KM. GAW received financially support from the Swedish Research Council (Grant No. 2020-03222) and the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS; Grant No. 2020-01091).
Funding
Open access funding provided by Uppsala University.
Author information
Authors and Affiliations
Contributions
S.L., K.M., G.W. and E.S.L. designed the study. K.M. and B.C. conducted the laboratory analyses. K.M. analyzed the data and prepared the figures. K.M. and S.L. wrote the initial manuscript draft. All authors reviewed the manuscript. E.S.L., S.L. and G.W. acted as supervisors. E.S.L. and S.L. provided the resources for the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Münzner, K., Langenheder, S., Weyhenmeyer, G.A. et al. Carbon dioxide reduction by photosynthesis undetectable even during phytoplankton blooms in two lakes. Sci Rep 13, 13503 (2023). https://doi.org/10.1038/s41598-023-40596-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-40596-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.