Abstract
Lakes are significant players for the global climate since they sequester terrestrially derived dissolved organic carbon (DOC), and emit greenhouse gases like CO2 to the atmosphere. However, the differences in environmental drivers of CO2 concentrations are not well constrained along latitudinal and thus climate gradients. Our aim here is to provide a better understanding of net heterotrophy and gas balance at the catchment scale in a set of boreal, sub-Arctic and high-Arctic lakes. We assessed water chemistry and concentrations of dissolved O2 and CO2, as well as the CO2:O2 ratio in three groups of lakes separated by steps of approximately 10 degrees latitude in South-Eastern Norway (near 60° N), sub-Arctic lakes in the northernmost part of the Norwegian mainland (near 70° N) and high-Arctic lakes on Svalbard (near 80° N). Across all regions, CO2 saturation levels varied more (6–1374%) than O2 saturation levels (85–148%) and hence CO2 saturation governed the CO2:O2 ratio. The boreal lakes were generally undersaturated with O2, while the sub-Arctic and high-Arctic lakes ranged from O2 saturated to oversaturated. Regardless of location, the majority of the lakes were CO2 supersaturated. In the boreal lakes the CO2:O2 ratio was mainly related to DOC concentration, in contrast to the sub-Arctic and high-Arctic localities, where conductivity was the major statistical determinant. While the southern part is dominated by granitic and metamorphic bedrock, the sub-Arctic sites are scattered across a range of granitic to sedimentary bed rocks, and the majority of the high-Arctic lakes are situated on limestone, resulting in contrasting lake alkalinities between the regions. DOC dependency of the CO2:O2 ratio in the boreal region together with low alkalinity suggests that in-lake heterotrophic respiration was a major source of lake CO2. Contrastingly, the conductivity dependency indicates that CO2 saturation in the sub-Arctic and high-Arctic lakes was to a large part explained by DIC input from catchment respiration and carbonate weathering.
Similar content being viewed by others
Introduction
Oxygen (O2) and carbon dioxide (CO2) are key gases for life on Earth. Their ratio is mainly determined by the balance between heterotrophic and autotrophic processes in the biosphere. Under net autotrophic conditions, CO2 is sequestrated in both terrestrial and aquatic ecosystems, thus decreasing atmospheric CO2 concentrations and greenhouse effects on global climate. Lakes, and notably boreal lakes, are key players in this context since they convert a significant part of terrestrially derived organic carbon to CO2 and CH41,2. A large share of CH4 may also be converted to CO2 by methanotrophs in the water column3.
Simultaneous and complementary biological processes thus drive variations in O2 and CO2 concentrations4,5 and this coupling has led to the assumption that O2 and CO2 can be used interchangeably when studying metabolism of aquatic ecosystems. O2 is advantageous over CO2 because it can be measured directly in the aqueous phase without gas extraction. Therefore, indirect assessments of CO2 based on dissolved O2 are often favored over direct CO2 measurements6,7. However, combined CO2 and O2 measurements have shown decoupling over time8,9,10. This may in part be explained by decoupling of production and consumption of these gases across the aquatic-terrestrial interface and thus reflect the catchment type of the aquatic ecosystem9. Other explanations for the deviations in CO2:O2 coupling could be inputs of CO2-rich water and anaerobic CO2 cycling11. Simultaneous measurements of CO2 and O2 concentrations thus increase the understanding of lake ecosystem functioning. Ratios of CO2:O2 may in turn give a better indication of lake net heterotrophy than CO2 or O2 alone, because production and decomposition affect the nominator and the denominator of the ratio with opposite signs. Thus we would expect a stronger response to increased heterotrophy in the CO2:O2 ratio than in CO2 alone. It will also provide insight in spatial drivers of CO2 emissions that could allow a space-for-time approach to address future changes in e.g. climate, hydrology, forest cover and other catchment properties.
Biological oxidation of dissolved organic matter (DOM) is a main source of autochthonous aquatic CO212,13,14 while photochemical oxidation plays a smaller role14,15. Other sources of CO2 may be allochthonous, e.g. from catchment processes like root exudation or supersaturated groundwater (Raymond et al., 2013). Depending on the pH and buffering capacity of the water, inorganic carbon is either present as CO2 or reacts with water forming bicarbonate or carbonate. Lake chemistry and notably pH thus plays a key role in determining the CO2 concentration in the water. Similarly, elevated temperatures will reduce concentrations while not necessarily the level of saturation or the CO2:O2 ratio, as the dissolution of both gases depends similarly on temperature.
The Boreal zone is characterized by extensive land–water interfaces. Forests with large above- and below-ground C-pools as well as abundant bogs and wetlands, export terrestrial DOM and dissolved inorganic carbon (DIC) to waters, making boreal aquatic ecosystems an especially important component of the global carbon cycle2. In certain areas also agricultural areas may be important sources of organic C. Climate change, changes in land use, most notably afforestation, and the recovery from acidification16 have led to increased export of terrestrial DOM and a shift in water color towards brown in many boreal freshwaters17,18. This “browning” may affect lake metabolism in various ways. Terrestrially derived DOM provides energy and nutrients to heterotrophs, stimulating bacterial metabolism and CO2 production19,20. Nutrients associated to DOM may also benefit autotrophs. However, the beneficial effect of enhanced nutrient loadings on autotrophs is overridden by enhanced light attenuation inhibiting primary production as DOM concentration increases20,21. This trade-off between positive and negative effects of increasing DOM inputs on photosynthesis may yield a unimodal response in primary production with a maximum around 5–10 mg DOC l−122,23.
In contrast to boreal lakes, Arctic lakes have generally sparsely forested, or unforested catchments with less developed soils, yet there is a gradient from sub-Arctic to high-Arctic sites. Still they are generally supersaturated with and net emitters of CO2 to the atmosphere24. Lake abundancy is higher in the Arctic than in any other region of the world with high complexity in lake type ranging from clear, pristine, mountain lakes to brown, DOM rich thermokarst lakes formed by thawing permafrost25,26,27. Climate warming in the Arctic has led to increased biomass and production of terrestrial plants and schrubs, so called Arctic greening, leading to increased loads of DOM to Arctic lakes28,29,30. At the same time, thawing permafrost results in inputs of old organic carbon to Arctic lakes that may undergo microbial oxidation31,32,33. Increased DOM loadings from thawing permafrost are thus expected to result in enhanced CO2 production and emission from Arctic lakes30,34,35.
Autochthonous (in-lake) production is however not the only source of DIC. Lateral flux of inorganic carbon produced in the catchment may account for a sizeable share of lake CO2, especially in small lakes with short retention times and long ice-free seasons36. In-lake DOM mineralization together with catchment derived (allochthonous) DIC inputs make most lakes worldwide supersaturated with- and net emitters of CO2 to the atmosphere1,37.There are conflicting reports of whether CO2 produced in aquatic environments via DOM mineralization or exported from terrestrial environments is the main regulator of lake CO2 flux36,38,39,40. Some of these contradictions likely depend on climate, local hydrology, catchment slopes, water retention time, and not the least catchment properties like lake size or fraction and type of forest, bogs and wetlands41,42.
In this study we assess the patterns and drivers of CO2:O2 ratios in lakes along a latitudinal gradient to see if these drivers vary between the distinctly different sets of lakes. More specifically we aim to gain understanding of lake CO2 sources, whether they are dominated by in-lake processes or by allochthonous inputs and whether there is a latitudinal difference in drivers of lake CO2:O2 ratio, notably related to forested or unforested catchments. To do so, we couple surface CO2 and O2 concentrations in 103 Norwegian lakes to environmental variables along a geographical gradient ranging from the boreal zone in southern Norway (58° N) through sub-Arctic northern Norway (69 o N) to the high Arctic at Svalbard (79° N). The gradient reflects different catchment properties varying from dense spruce forest, via open birch forest to totally unforested catchments with shallow soils in the high Arctic.
This wide spatial gradient across climatic regions and catchment properties should provide insights relevant to larger parts of both the boreal and the arctic biome, yet with the proviso that there clearly may be pronounced regional differences within this vast area.
Materials and methods
Study lakes
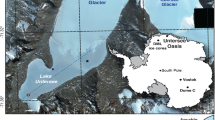
During fall 2019, 73 lakes in South-Eastern Norway (Boreal) and 22 Arctic lakes on Svalbard (high-Arctic) were sampled and a number of water chemistry parameters and dissolved gases were measured. In fall 2020, additionally 14 sub-Arctic lakes in the Finnmark county (Northern Norway; sub-Arctic) were sampled (Fig. 1). The boreal, southern cluster of lakes is within the coniferous forest zone but covers locations differing in size and altitude. The sub-Arctic lakes have sparsely forested catchments with birch, less topographic variation and larger areas of bogs and wetlands, while the high Arctic sites are treeless and also with very sparsely developed soils. Granitic and metamorphic bedrock dominate in the southern zone, yielding low alkalinity lakes (Fig. S1). The catchments of these lakes are also characterized by well-developed soils. The sub-Arctic lakes are situated on granitic to sedimentary rocks (slate) giving rise to a wide range in alkalinity. The sub-Arctic lakes were chosen to span a geographical gradient from the southern inland to the costal northeast, reflecting a gradient in biome domination from taiga to Arctic tundra. A high proportion of the high-Arctic lakes are situated on limestone bedrock, with elevated alkalinity (Fig. S1). The high-Arctic lakes are mostly small with catchments devoid of soil and vegetation beyond scrubs and mosses, covering a gradient from rocky terrain from glacier fronts to shore sites, the latter influenced by birds and with some vegetation43.
Map of the site locations. The map is generated by R (version 3.4.1): rnaturalearth (https://cran.r-project.org/web/packages/rnaturalearth/README.html) kombined with sf (https://cran.r-project.org/web/packages/sf/index.html).
Sampling and field sample preparations
Sampling was performed at, or shortly after, fall overturn, to secure a maximum vertical homogenization of the water masses and gases. Since onset of fall differs with latitude, the samplings were performed during October, September and August for the three clusters of lakes (Boreal, sub-Arctic and high-Arctic, respectively), reflecting their geographical position from south to north. This procedure was based on the procedures adopted in the national surveys on water chemistry in Northern Europe, initiated to address the impact of acidification on lake water chemistry44. The rationale for this is primarily that following mixing, the water masses are homogenized and thus the sampled water volume should be more representative than a surface sample during stratification. No doubt the dissolved gases may differ with season and depth. Potentially autumn samples could yield higher CO2:O2 ratios than summer samples (reduced photosynthesis relative to respiration), but since a full seasonal and vertical inventory of all lakes for obvious reasons could not be performed, this post-overturn sampling would serve as an integrated fingerprint of lake metabolism. Since only few localities were accessible from road, sampling from boat was problematic. Hence the sampling was performed from the shore using a designed 4 m sampling rod with a holder and beaker at the end. All localities were collected during daytime (11 a.m to 2 pm) at three different sites, preferably representing different substratum and shoreside properties, yet in line with the designed, survey of North European lakes, near the outlet wherever this could be identified, and the three sites were pooled into one sample. Again, the benefit of sampling after fall overturn is that local impacts would, if not cancelled out, at least be minimized by mixing. Also, most sites were rather small headwater lakes, and the high Arctic sites mostly ponds, meaning that sampling 4 m from the shore should be representative of the mixed water masses.
At the sampling sites, in situ measurements of temperature (T), pH and electrical conductivity (EC) were taken using a portable multimeter (Hach HQ40D). Samples were processed in situ for further analysis of lake chemistry: 50 ml of unfiltered water was taken for the analysis of total organic carbon (TOC), total nitrogen (TN) and total phosphorus (TP). For analysis of DOC, dissolved nitrogen (DN) and phosphorus (DP), surface water from the bucket was filtered in the field through Sterivex 0.22 µm pore size filters (Millipore). All samples for water analysis were kept cool (4 °C) until back from the field (the same evening), where they were frozen at − 20 °C until further analysis at the University of Oslo, generally within 2 to 4 weeks. Dissolved CO2, O2 and argon (Ar; all sites except Svalbard) were analyzed using the headspace method after acidification45. The samples were prepared in the field by filling 30 ml water in a syringe, creating an in-situ headspace with air (20 ml) and adding 0.6 ml of 3% HCl (≈ 1 M). Syringes were closed and equilibrated by shaking the syringes for 3 min. In situ lake temperature was used as equilibration temperature and caution was exercised to not warm the syringes during shaking. The equilibrated headspace gas was then injected into He-washed and pre-evacuated glass vials crimp sealed with butyl rubber septa, and then kept at room temperature until analysis by gas chromatography.
Laboratory analysis
TOC and DOC were measured by infrared CO2 detection after catalytic high temperature combustion (Shimadzu TOC-VWP analyser). TP was measured on a SEAL AA3 HR AutoAnalyzer (SEAL Analytical Inc., Mequon, Wisconsin 53092, USA) as phosphate after wet oxidation with peroxodisulfate. TN was measured on unfiltered samples by detecting nitrogen monoxide by chemiluminescence using a TNM-1 unit attached to the Shimadzu TOC-VWP analyser. Concentrations of CO2, O2 and Ar were determined by automated gas chromatography (GC) analysis with He back-flushing as described by Yang et al.13. The level of saturation of each gas relative to equilibrium with ambient air was calculated from measured concentrations using Henry’s law with temperature-dependent solubility constants. In the sites where Ar was measured, O2 was normalized to Ar saturation for a more stable and accurate estimate of O2 saturation46.
Statistical analysis
All data analysis was performed using the open-source software R version 3.4.147. We used Spearman’s rank correlation as a measure of association between continuous variables. To calculate DIC from pH and CO2 at the in situ temperature, we used the AquaEnv package48. For the statistical modelling, we used the mgcv package49 to fit generalized additive models (gam) of the gaussian family to the dependent variables. To test the dependency of CO2:O2 ratio and O2 saturation, we used a gam model with smoothers on each of the explanatory variables DOC (mg l−1), TP (µg l−1), TN (mg l−1), and conductivity (EC; µS cm−1). Predictive variable selection was done by applying additional shrinkage on the null space of the penalty with the select = TRUE argument in the mgcv::gam function, as recommended by Marra and Wood50. The resulting models have all smoothers that are not necessary for the fit as close to zero as possible.
Results
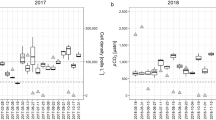
Saturation levels of O2 varied with geographical location (Fig. 2). By contrast, CO2 saturation covered a much wider range with both undersaturated and supersaturated lakes in the sub-Arctic and saturation or supersaturation in the boreal and the high-Arctic lakes (Table 1; Fig. 2).
The majority of the boreal lakes were supersaturated with CO2 and undersaturated with O2, with a weak but significantly negative correlation between CO2 and O2 saturation levels (ρ = − 0.42, p < 0.001). The sub-Arctic and high-Arctic lakes, which were all saturated or supersaturated with O2, showed deviating patterns of CO2 saturation with no significant correlation between CO2 and O2 saturation levels (Fig. S2). Across all three latitudinal zones, lake O2 saturation levels showed lower variation than CO2 saturation levels. Hence, the variation in CO2:O2 ratio was mainly governed by CO2 saturation (Fig. 2).
General patterns among nutrients and conductivity
While high Arctic lakes had lower DOC concentrations than boreal lakes on the mainland, there were no major differences in DOC concentrations among the boreal and the sub-Arctic mainland lakes (Table 1). In the boreal lakes, DOC concentrations correlated positively with TN and total conductivity (ρ = 0.48 and ρ = 0.45, respectively; Fig. S3). The relation between DOC and TP was also positive but weak. Conductivity ranged between 6 and 243 µS cm−1 with a median value at 24 µS cm−1 (Table 1) and correlated best with TN (ρ = 0.72; Fig. S3).
Similar to the boreal lakes, DOC was positively correlated to TN in both the sub-Arctic and in the high-Arctic lakes (ρ = 0.42 and ρ = 0.65, respectively). However, there was no significant relation between DOC and conductivity in the high-Arctic while in the sub-Arctic, the relation was weak but negative (Fig. S3). DOC and pH correlated positively in the high-Artic (ρ = 0.65) and in the sub-Arctic lakes there was a positive correlation between conductivity and pH (ρ = 0.59). TP was generally lower in the high-Arctic than in the boreal and sub-Arctic lakes and 12 of the 22 lakes in the high-Arctic had TP concentrations below detection limit. Conductivity in the high-Artic lakes was generally higher and had a wider range than in the boreal lakes (Table 1).
In all lakes, there was a strong positive correlation between conductivity and DIC concentrations (Fig. S3). In the boreal lakes, DIC concentrations were generally low. Contrastingly, in the Arctic the relationship between DOC and DIC was either negative (sub-Arctic) or non-existing (high-Arctic) and thus inorganic compounds are the main sources of alkalinity in the arctic lakes. Including all lakes in the full model with DOC, TP, TN, and conductivity as independent variables, the model explained 42% of the deviance, with conductivity, TP and DOC concentration as significant predictors (Table 2). Since the variables predicting the CO2:O2 ratio differed with geographic location, the models were also run for each zone separately.
Boreal zone
As stated, the CO2 and O2 saturation were negatively related with each other in the boreal lakes. A model with DOC as the only explanatory variable explained 35% of the observed deviance. The full model, including TN, TP, and conductivity, improved the explained deviance to 73% (Table 2; Fig. 3a). DOC and conductivity were the strongest predictors, both having positive effects on the CO2:O2 ratio. O2 saturation in the boreal lakes was negatively related to DOC concentrations and positively related to TN (Fig. S3).
Result plots of the generalized additive models (gams) predicting CO2:O2 saturation ratio for the three regions. In the Boreal lakes (a), the best predictor was DOC concentration followed by conductivity. In both the sub-Arctic (b) and high-Arctic (c), conductivity was the best predictor for CO2:O2 saturation ratio.
High-and sub-Arctic lakes
CO2:O2 ratios in the Arctic lakes were primarily associated with conductivity (positive) and TN (negative) with the models explaining 75% and 98% of the deviance for the sub-Arctic and the high-Arctic, respectively (Table 2; Fig. 3b,c). In the high-Arctic, DOC was a weak (p = 0.04) and mainly negative predictor of CO2:O2 ratio while TP was below detection limit in most lakes and excluded from the analysis. In the sub-Arctic, neither DOC nor TP were significant predictors of CO2:O2 ratio.
All lakes in the Arctic were saturated with O2. In the sub-Arctic, O2 saturation was at 100% in all lakes without variation among lakes and, hence, no correlation was found between O2 saturation and any other variable. In the high-Arctic, O2 saturation spanned a wider range from saturation to oversaturation. The only variable that correlated with O2 saturation was conductivity (ρ = − 0.48). Modelling the CO2:O2 ratio in the high-Arctic lakes with conductivity as the only independent variable explained 69% of the deviance. In the sub-Arctic, 70% of the deviance was explained with conductivity as the only explanatory variable.
Discussion
O2 and CO2 saturation
Our study revealed striking differences between boreal lakes on the one hand, and sub-Arctic and high-Arctic sites on the other. The boreal lakes (n = 73) all had catchments dominated by coniferous forests, primarily spruce and pine and the terrestrial C-fixation by forests enrich also lake water with DOC17,18,51, which in turn promote the biogenic production of CO2 from bacterial respiration1,2,14,38. The positive relationship between CO2 saturation and DOC concentration indicates in-lake CO2 production by microbial DOC mineralization. Still CO2 saturation can be affected by retention time, as under low retention times DOC and CO2 flushed in from the catchment into systems might increase saturation. Short retention times are also associated with inputs of highly reactive DOC52, yielding enhanced CO2 production rates. As such the positive relationship between CO2 saturation and DOC concentration needs to be interpreted with caution in systems with low retention time as it is likely impacted by complex interactions of allochthonous and autochthonous processes.
Besides biological processes and air–water exchange, lateral input via groundwater and surface run-off contribute to lake CO253,54. Groundwater input has been shown to correlate well with conductivity, as water in contact with bedrock is likely to become enriched in ions and minerals. The positive relationship between CO2 saturation and conductivity may thus indicate lateral input of CO255, in particular in the studied subarctic and arctic systems. Further, lake pH may regulate the CO2 concentration with a higher proportion of the DIC staying as free CO2 at low pH values while at higher pH values, more CO2 enters the carbonate cycle, forming carbonate and bicarbonate. Boreal lakes are low in alkalinity (Fig. S1) and sensitive to changes in pH while Arctic systems due to glacial influence are well buffered. Therefore, besides serving as a substratum for heterotrophic bacteria, enhanced input of humic acids may result in a decline in pH and thus an additional increase in CO2 saturation in boreal lakes56 but hardly in well buffered arctic lakes.
The corresponding negative relationship between DOC concentration and O2 saturation suggests enhanced microbial O2 consumption, which may go along with a decrease in primary production when DOC inputs and thus light attenuation increases21,57. The net heterotrophy that is prevailing in boreal DOC-rich lakes is attributed both to reduced photosynthesis in the water column and enhanced microbial respiration58, implying that the degree of net heterotrophy increases with increasing DOC concentration in the boreal lakes.
The role of nutrients
Phosphorus is the major limiting nutrient of bacterioplankton and has been previously shown to be a strong driver of lake CO2 supersaturation13,59,60. The influence of TP on the CO2:O2 ratio in the boreal lakes was positive up to about 15 µg DOC l−1, above which the effect declined and became negative. However, there were only few observations with high TP concentrations resulting in wide confidence interval in the second half of the curve (Fig. 3a). The ratio of CO2:O2 could also be affected by the bacterial carbon use efficiency which depends on the nutrient to C ratio of the substrate with high ratios allowing to allocate more C to growth while with low ratios, bacteria may dispose excess C as enhanced respiration61,62. Accordingly, CO2 saturation was negatively related to the TP:DOC ratio in boreal lakes (Fig. S4), suggesting enhanced rates of CO2 production in lakes where DOC concentrations were high in relation to TP concentrations.
Primary production in boreal lakes has been shown to be strongly affected by nitrogen availability in areas receiving low input of N63 and the positive effect of TN on O2 saturation likely reflects increased primary production with increased TN concentrations.
Different dynamics in high-latitude lakes
A main result of our study was the difference between boreal and sub-Arctic and high-Arctic catchments in terms of CO2 and thus CO2:O2-ratio. In contrast to the boreal lakes, DOC concentration did not appear to drive the CO2:O2 ratio in Arctic lakes, and there was no significant trend in CO2 saturation in response to DOC concentrations. This was consistent both for sub-Arctic and high-Arctic sites despite the difference in catchment characteristics of the systems (birch forest/bog vs barren or glacier dominated catchments), and is in support of a recent comparison between boreal (forested) and Arctic sites41. The majority of the studied Arctic lakes were saturated or oversaturated with both O2 and CO2 without any relation between O2 and CO2 saturation level. These lakes are generally shallow and low in pelagic productivity, but may have a vigorous benthic production64. Also, the high-Arctic shallow sites are continuously wind-mixed, which could override internal metabolic drivers of CO2 and O2. In the cases of oversaturation, this can be attributed to the high contribution from dense mats of mosses and benthic algae in these localities. Hence, O2 saturation is most likely a result of both wind-induced mixing with support from benthic primary production in the Arctic sites.
Among the significant predictors of the CO2:O2 ratio in the Arctic, conductivity was clearly predominant (Table 2; Fig. 3b,c). This suggests that the majority of lake CO2 in the Arctic entered from the surrounding mineral soils65 and was not produced through in-lake DOC mineralization. For some high-Arctic sites, conductivity can also be coupled to their proximity to the sea. The lakes closest to the sea are also the most productive lakes since they are influenced by vegetation and most frequently visited by birds, yielding enhanced DOC and nutrients43.
While CO2 saturation increased with conductivity, we observed no correlation between conductivity and DOC concentration in neither the sub-Arctic nor the high-Arctic. Instead, conductivity was closely correlated with DIC concentrations and total alkalinity in both Arctic regions. In the sub-Arctic, CO2 saturation spanned from undersaturated to supersaturated. Saturation level was closely related to conductivity and thus also to total alkalinity. Alkalinity in turn could be coupled to bedrock, indicating weathering to be a source of lake DIC. While weathering of Si-holding rocks typically consumes CO2 via the Urey-reaction, others can generate DIC, e.g. via pyrite oxidation66. Likewise, the relatively high alkalinity together with the lime-rich bedrock suggests carbonate weathering to be a DIC source also in the high-Arctic lakes41,67.
In the high-Arctic lakes, O2 saturation correlated negatively with conductivity. While all lakes were supersaturated with O2, there was more variation in CO2 saturation with 7 out of 22 lakes being undersaturated or close to saturation and the rest supersaturated. The seven CO2 undersaturated lakes were all high in DOC and TN and highly influenced by birds. Many high latitude lakes are naturally poor in nutrients and although there was no significant relation between TN and O2 saturation, additions of N (and P) via birds may stimulate primary production, not the least of benthic autotrophs, with a concomitant drawdown of CO2. As bird feces also provide a source of organic C to lakes (geese feces typically has concentrations of 450, 11 and ca 3.2 mg per g dry weight for C, N and P respectively), and goose impacted lakes generally also have the highest levels of DOC68, also the heterotrophic bacterial production could to some extent be stimulated, yet we do not know how much of the fecal C eventually becomes accessible to pelagic bacteria as DOC.
The sub-Arctic lakes were all close to O2 saturation with little variation among lakes (95–99%), meaning that the variability in CO2:O2 ratio was primarily determined by CO2. Several studies suggest that Arctic lakes are net heterotrophic, as they are net emitters of CO2 to the atmosphere2,69. The majority of Arctic lakes in our study was indeed supersaturated with CO2 and net emitters of CO2 at the time of sampling. However, the uncoupling of CO2:O2 ratio from DOC and of CO2 and O2 saturation indicate decoupling of CO2 and O2 production. This decoupling could be the result of groundwater or glacial water CO2 inputs, or benthic primary production in surface sediments, while heterotrophic production dominates in deeper sediment layers. The high O2 saturation levels together with a dominance of allochthonous DIC suggest net system autotrophy rather than heterotrophy in these arctic lakes if only the autochthonous balance is considered. CO2 may also be produced from organic C mobilized by permafrost thaw, however, permafrost in the Scandinavian Arctic is generally only found in restricted palsas or in mountainous areas. Although allochthonous DIC may govern the CO2 in the arctic lakes in this study, heterotrophic mineralization of DOC may thus have an impact on CO2 saturation in arctic lakes affected by permafrost thawing.
Conclusion
Based on a large number of (mostly) boreal lakes, Larsen et al.38 claimed DOC to be a universal predictor of lake pCO2, while groundwater influx was a minor contributor. However, including also sub- and high-Arctic lakes, we found a clear distinction in drivers of CO2 saturation along a latitudinal gradient. The boreal lakes followed the expected pattern with both CO2 and O2 saturation being largely dependent on DOC concentrations and relating negatively to each other, suggesting enhanced net heterotrophy with increased DOC inputs. In the Arctic lakes, despite the differences between sub-Arctic and high-Arctic sites, there was no correlation between DOC and CO2, yet these sites were also to a large degree supersaturated with CO2 and thus could be considered net heterotrophic. However, most of these lakes were also saturated or supersaturated with O2, indicative of low respiratory activity in agreement with generally nutrient-poor conditions and low levels of primary production. This is supported by the positive correlation between CO2:O2 ratio and conductivity, while the influence of DOC concentration was weak or non-significant. This may suggest that the major share of CO2 in these lakes is of allochthonous origin, likely from organic carbon mineralization and carbonate weathering in the catchment soils, entering via groundwater flow41,55. This points to fundamentally different drivers of CO2 and O2 concentrations in boreal and Arctic lakes due to differences in vegetation, landscape structure, hydrology and lake bathymetry.
Data availability
All data will be made available upon request to Lina Allesson, or if demanded, stored in a UiO repository (Git-HUB).
References
Cole, J. J. et al. Plumbing the global carbon cycle: Integrating inland waters into the terrestrial carbon budget. Ecosystems 10(1), 172–185 (2007).
Tranvik, L. J. et al. Lakes and reservoirs as regulators of carbon cycling and climate. Limnol. Oceanogr. 54(6part2), 2298–2314 (2009).
Bastviken, D. et al. Fates of methane from different lake habitats: Connecting whole-lake budgets and CH4 emissions. J. Geophys. Res. https://doi.org/10.1029/2007JG000608 (2008).
Cole, J. J. & Caraco, N. F. Carbon in catchments: Connecting terrestrial carbon losses with aquatic metabolism. Mar. Freshw. Res. 52(1), 101–110 (2001).
Hanson, P. C. et al. Lake dissolved inorganic carbon and dissolved oxygen: Changing drivers from days to decades. Ecol. Monogr. 76(3), 343–363 (2006).
Marchand, D., Prairie, Y. T. & Del Giorgio, P. A. Linking forest fires to lake metabolism and carbon dioxide emissions in the boreal region of Northern Quebec. Glob. Change Biol. 15(12), 2861–2873 (2009).
Del Giorgio, P. A. et al. Coherent patterns in bacterial growth, growth efficiency, and leucine metabolism along a northeastern Pacific inshore-offshore transect. Limnol. Oceanogr. 56(1), 1–16 (2011).
Torgersen, T. & Branco, B. Carbon and oxygen fluxes from a small pond to the atmosphere: Temporal variability and the CO2/O2 imbalance. Water Resour. Res. https://doi.org/10.1029/2006WR005634 (2008).
Crawford, J. T. et al. CO2 and CH4 emissions from streams in a lake-rich landscape: Patterns, controls, and regional significance. Global Biogeochem. Cycles 28(3), 197–210 (2014).
Stets, E. G. et al. Carbonate buffering and metabolic controls on carbon dioxide in rivers. Glob. Biogeochem. Cycles 31(4), 663–677 (2017).
Vachon, D., Lapierre, J.-F. & del Giorgio, P. A. Seasonality of photochemical dissolved organic carbon mineralization and its relative contribution to pelagic CO2 production in northern lakes. J. Geophys. Res. Biogeosci. 121(3), 864–878 (2016).
Jansson, M. et al. Allochthonous organic carbon and phytoplankton/bacterioplankton production relationships in lakes. Ecology 81(11), 3250–3255 (2000).
Yang, H. et al. Greenhouse gas metabolism in Nordic boreal lakes. Biogeochemistry 126(1), 211–225 (2015).
Allesson, L. et al. The role of photomineralization for CO2 emissions in boreal lakes along a gradient of dissolved organic matter. Limnol. Oceanogr. 66, 158–170 (2020).
Koehler, B. et al. Sunlight-induced carbon dioxide emissions from inland waters. Glob. Biogeochem. Cycles 28(7), 696–711 (2014).
De Wit, H. A. et al. Long-term increase in dissolved organic carbon in streamwaters in Norway is response to reduced acid deposition. Environ. Sci. Technol. 41(22), 7706–7713 (2007).
Finstad, A. G. et al. From greening to browning: Catchment vegetation development and reduced S-deposition promote organic carbon load on decadal time scales in Nordic lakes. Sci. Rep. 6(1), 1–8 (2016).
Škerlep, M. et al. Afforestation driving long-term surface water browning. Glob. Change Biol. 26(3), 1390–1399 (2020).
Hessen, D., Andersen, T. & Lyehe, A. Carbon metabolism in a humic lake: Pool sires and cycling through zooplankton. Limnol. Oceanogr. 35(1), 84–99 (1990).
Karlsson, J., Jansson, M. & Jonsson, A. Respiration of allochthonous organic carbon in unproductive forest lakes determined by the Keeling plot method. Limnol. Oceanogr. 52(2), 603–608 (2007).
Karlsson, J. et al. Light limitation of nutrient-poor lake ecosystems. Nature 460, 506–509 (2009).
Seekell, D. A. et al. The influence of dissolved organic carbon on primary production in northern lakes. Limnol. Oceanogr. 60(4), 1276–1285 (2015).
Tanentzap, A. J. et al. Terrestrial support of lake food webs: Synthesis reveals controls over cross-ecosystem resource use. Sci. Adv. 3(3), e1601765 (2017).
Tan, Z. et al. Modeling CO 2 emissions from A rctic lakes: Model development and site-level study. J. Adv. Model. Earth Syst. 9(5), 2190–2213 (2017).
Downing, J. A. et al. The global abundance and size distribution of lakes, ponds, and impoundments. Limnol. Oceanogr. 51(5), 2388–2397 (2006).
Verpoorter, C. et al. A global inventory of lakes based on high-resolution satellite imagery. Geophys. Res. Lett. 41(18), 6396–6402 (2014).
Matveev, A., Laurion, I. & Vincent, W. F. Methane and carbon dioxide emissions from thermokarst lakes on mineral soils. Arctic Sci. 4(4), 584–604 (2018).
Myers-Smith, I. H. et al. Shrub expansion in tundra ecosystems: Dynamics, impacts and research priorities. Environ. Res. Lett. 6(4), 045509 (2011).
Kaiser, K. et al. Origins and transformations of dissolved organic matter in large Arctic rivers. Sci. Rep. 7(1), 1–11 (2017).
Elder, C. D. et al. Greenhouse gas emissions from diverse Arctic Alaskan lakes are dominated by young carbon. Nat. Clim. Chang. 8(2), 166–171 (2018).
Drake, T. W. et al. Ancient low–molecular-weight organic acids in permafrost fuel rapid carbon dioxide production upon thaw. Proc. Natl. Acad. Sci. USA 112(45), 13946–13951 (2015).
Vonk, J. E. et al. Reviews and syntheses: Effects of permafrost thaw on Arctic aquatic ecosystems. Biogeosciences 12(23), 7129–7167 (2015).
Wauthy, M. et al. Increasing dominance of terrigenous organic matter in circumpolar freshwaters due to permafrost thaw. Limnol. Oceanogr. Lett. 3(3), 186–198 (2018).
Tank, S. E. et al. Landscape matters: Predicting the biogeochemical effects of permafrost thaw on aquatic networks with a state factor approach. Permafrost Periglac. Process. 31(3), 358–370 (2020).
Waldrop, M. et al. Carbon fluxes and microbial activities from boreal peatlands experiencing permafrost thaw. J. Geophys. Res. 126(3), e2020JG005869 (2021).
Weyhenmeyer, G. et al. Significant fraction of CO2 emissions from boreal lakes derived from hydrologic inorganic carbon inputs. Nat. Geosci. 8, 933–936 (2015).
Raymond, P. A. et al. Global carbon dioxide emissions from inland waters. Nature 503(7476), 355–359 (2013).
Larsen, S., Andersen, T. & Hessen, D. The pCO2 in boreal lakes: Organic carbon as a universal predictor?. Glob. Biogeochem. Cycles https://doi.org/10.1029/2010GB003864 (2011).
Nydahl, A. C., Wallin, M. B. & Weyhenmeyer, G. A. Diverse drivers of long-term p CO2 increases across thirteen boreal lakes and streams. Inland Waters 10(3), 360–372 (2020).
Jonsson, A. et al. Whole-lake mineralization of allochthonous and autochthonous organic carbon in a large humic lake (örträsket, N. Sweden). Limnol. Oceanogr. 46(7), 1691–1700 (2001).
Puts, I. C. et al. Landscape determinants of pelagic and benthic primary production in northern lakes. Glob. Change Biol. https://doi.org/10.1111/gcb.16409 (2022).
Valiente, N. et al. Catchment properties as predictors of greenhouse gas concentrations across a gradient of boreal lakes. Front. Environ. Sci. https://doi.org/10.3389/fenvs.2022.880619 (2022).
Hessen, D. O. et al. Global change and ecosystem connectivity: How geese link fields of central Europe to eutrophication of Arctic freshwaters. Ambio 46(1), 40–47 (2017).
Henriksen, A. et al. Northern European lake survey, 1995: Finland, Norway, Sweden, Denmark, Russian Kola, Russian Karelia, Scotland and Wales. Ambio 1998, 80–91 (1995).
Åberg, J. & Wallin, B. Evaluating a fast headspace method for measuring DIC and subsequent calculation of p CO2 in freshwater systems. Inland Waters 4(2), 157–166 (2014).
Stanley, R. H. & Jenkins, W. J. Noble gases in seawater as tracers for physical and biogeochemical ocean processes. In The Noble Gases as Geochemical Tracers 55–79 (Springer, 2013).
R Core Team. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, 2017). http://www.R-project.org/.
Hofmann, A. F. et al. AquaEnv: An Aqua tic Acid-Base Modelling Env ironment in R. Aquat. Geochem. 16(4), 507–546 (2010).
Wood, S. N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. B 73(1), 3–36 (2011).
Marra, G. & Wood, S. N. Practical variable selection for generalized additive models. Comput. Stat. Data Anal. 55(7), 2372–2387 (2011).
Larsen, S., Andersen, T. & Hessen, D. O. Climate change predicted to cause severe increase of organic carbon in lakes. Glob. Change Biol. 17(2), 1186–1192 (2011).
Catalán, N. et al. Organic carbon decomposition rates controlled by water retention time across inland waters. Nat. Geosci. 9(7), 501–504 (2016).
Ojala, A. et al. Carbon gas fluxes from a brown-water and a clear-water lake in the boreal zone during a summer with extreme rain events. Limnol. Oceanogr. 56(1), 61–76 (2011).
Vachon, D. & Del Giorgio, P. A. Whole-lake CO2 dynamics in response to storm events in two morphologically different lakes. Ecosystems 17(8), 1338–1353 (2014).
Rodríguez-Rodríguez, M. et al. Using water temperature, electrical conductivity, and pH to Characterize surface–groundwater relations in a shallow ponds system (Doñana National Park, SW Spain). Water 10(10), 1406 (2018).
Nydahl, A. C. et al. Colored organic matter increases CO2 in meso-eutrophic lake water through altered light climate and acidity. Limnol. Oceanogr. 64(2), 744–756 (2019).
Thrane, J.-E., Hessen, D. O. & Andersen, T. The absorption of light in Lakes: Negative impact of dissolved organic carbon on primary productivity. Ecosystems 17(6), 1040–1052 (2014).
Ask, J., Karlsson, J. & Jansson, M. Net ecosystem production in clear-water and brown-water lakes. Glob. Biogeochem. Cycles https://doi.org/10.1029/2010gb003951 (2012).
Vadstein, O. Heterotrophic, planktonic bacteria and cycling of phosphorus. In Advances in Microbial Ecology 115–167 (Springer, 2000).
Allesson, L. et al. Phosphorus availability promotes bacterial DOC-mineralization, but not cumulative CO2-production. Front. Microbiol. 11, 2272 (2020).
Hessen, D. O. Dissolved organic carbon in a humic lake: Effects on bacterial production and respiration. Hydrobiologia 229(1), 115–123 (1992).
Hessen, D. O. & Anderson, T. R. Excess carbon in aquatic organisms and ecosystems: Physiological, ecological, and evolutionary implications. Limnol. Oceanogr. 53(4), 1685–1696 (2008).
Elser, J. J. et al. Shifts in lake N: P stoichiometry and nutrient limitation driven by atmospheric nitrogen deposition. Science 326(5954), 835–837 (2009).
Rautio, M. & Vincent, W. F. Benthic and pelagic food resources for zooplankton in shallow high-latitude lakes and ponds. Freshw. Biol. 51(6), 1038–1052 (2006).
Dean, J. F. et al. Biogeochemistry of “pristine” freshwater stream and lake systems in the western Canadian Arctic. Biogeochemistry 130(3), 191–213 (2016).
Klaminder, J. et al. Carbon mineralization and pyrite oxidation in groundwater: Importance for silicate weathering in boreal forest soils and stream base-flow chemistry. Appl. Geochem. 26(3), 319–325 (2011).
Marcé, R. et al. Carbonate weathering as a driver of CO2 supersaturation in lakes. Nat. Geosci. 8(2), 107–111 (2015).
Van Geest, G. et al. Goose-mediated nutrient enrichment and planktonic grazer control in arctic freshwater ponds. Oecologia 153(3), 653–662 (2007).
Kling, G. W., Kipphut, G. W. & Miller, M. C. The flux of CO2 and CH4 from lakes and rivers in arctic Alaska. Hydrobiologia 240(1), 23–36 (1992).
Acknowledgements
We are most grateful to our colleagues Camille Crapart, Jing Wei, Even Werner, and Laurent Fontaine for sampling assistance. The project has received grants from Centre for Biogeochemistry in the Anthropocene and the Belmont Forum project BiodivERsA, project no. 295367.
Funding
This work was supported by the Centre of Biogeochemistry in the Anthropocene (CBA) and Belmont Forum project ARCTIC-BIODIVER.
Author information
Authors and Affiliations
Contributions
L.A. and D.H. conceived the idea. L.A., P.D. and N.V. performed material preparation and data collection. All authors were involved in the analysis of data and final writing and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Allesson, L., Valiente, N., Dörsch, P. et al. Drivers and variability of CO2:O2 saturation along a gradient from boreal to Arctic lakes. Sci Rep 12, 18989 (2022). https://doi.org/10.1038/s41598-022-23705-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23705-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.