Abstract
Bevacizumab (Bev) plus chemotherapy is a standard first-line treatment in metastatic colorectal cancer (mCRC), however to date no predictive factors of response have been identified. Results of our previous analysis on patients enrolled in a randomized prospective phase III multicenter study (ITACa study) showed a predictive value of Vascular Endothelial Growth Factor (VEGF) polymorphism (VEGF + 936), a 27-nucleotide variable number tandem repeat (VNTR) of the endothelial nitric oxide synthase (eNOS) gene and eNOS + 894 polymorphism. mCRC patients, treated with Bev plus chemotherapy, were included in this prospective validation trial. eNOS + 894G > T was analyzed by Real time PCR, while the eNOS VNTR and VEGF + 936C > T were determined by standard PCR and direct sequencing analysis. These polymorphisms were assessed in relation to progression-free survival (PFS), overall survival (OS) and objective response rate (ORR). These three polymorphisms were not predictive of PFS (p 0.91, 0.59 and 0.09, respectively), and OS (p 0.95, 0.32 and 0.46, respectively). Moreover, the haplotype analyses did not confirm what was found in our previous study; patients bearing a specific haplotype of eNOS had not significantly improved outcomes. This prospective study failed to validate the predictive impact of eNOS and VEGF polymorphisms for response to Bev plus first-line chemotherapy in mCRC patients.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is one of the most common malignancies, globally repre-senting the fourth most diagnosed cancer in the world, third in incidence in men and second in women. CRC is the third leading cause of cancer death in the world, consider-ing both men and women globally1,2.
In about 25% of cases, it occurs as a metastatic disease already at diagnosis. In this setting, systemic treatments are considered the gold standard. Associations of fluoropy-rimidines with oxaliplatin and/or irinotecan and a biologic agent are commonly used in the first-line setting. Bevacizumab (Bev), a humanised monoclonal antibody directed against the Vascular Endothelial Growth Factor (VEGF), was the first drug with a molec-ular target established in the first-line treatment of mCRC3. A recent meta-analysis showed no benefit in overall survival (OS) from Bev-containing regimens, while adding Bev to chemotherapy significantly improved progression free survival (PFS)3. Despite efforts in clinical and translational research covering more than ten years, no biomarkers of response or resistance to Bev treatment have been found to date. Therefore, Bev is com-monly used in association with any chemotherapy regimen and independently of any se-lection factor.
VEGF represents the main pro-angiogenic factor, and acts binding to specific recep-tors present on the membrane of endothelial cells, VEGFR-1 and VEGFR-2. This led to the variation of the structure of the cytoskeleton of the endothelial cells, with the consequent modification of the cell motility. VEGF expression is related to blood vessel formation and metastasization in various cancers.
Several researchers assessed the role of VEGF Single Nucleotide Polymorphisms (SNPs) in relation to Bev responsiveness with contradictory findings4,5,6,7. Our team analyzed VEGF and endothelial nitric oxide synthase (eNOS) SNPs in relation to clinical out-come (PFS, OS and overall response rate, ORR) in mCRC patients undergoing Bev-based first-line chemotherapy in the phase III prospective multicenter randomized “Italian Trial in Advanced Colorectal Cancer (ITACa)” study8. This study demonstrated that VEGF 936C/T, eNOS + 894 G/T, and VNTR were linked with prognosis only in Bev plus first-line chemotherapy patients. Moreover, patients with a particular haplotype combination of the two eNOS polymorphisms (defined as eNOS Haplo1/Haplo1 and eNOS Haplo 2/Haplo2), in comparison to those with the other genotypes, demonstrated significantly longer PFS and OS, as well as a higher ORR8.
The results of the abovementioned work needed to be confirmed in an independent case series, thus the present trial was designed to validate the role of these polymorphisms and haplotypes. If confirmed, these data could represent a valid selection criterion for candidates for treatment with Bev.
Results
From January 2016 to October 2019, 182 patients were enrolled into this trial. Thirteen patients were excluded from the analysis due to eligibility criteria violation. Table 1 summarizes the patient characteristics. Fifty-five percent of patients were men and the median age was 69 years. ECOG PS was equal to 0 in 75% of cases, 1 in 23% of cases and equal to 2 in a minority (2%). As regards the localization of the primary tumor, 69 patients (42%) had a neoplasm originating from the left colon, 64 patients (39%) from the right colon and 32 patients (19%) from the rectum. Analyzing the site of metastases, 58% of patients had me-tastases to the liver, 37% to the lungs and 24% to the peritoneum. Grade 3 adenocarcinomas were the most represented (38%). Prior surgery for primary tumor and adjuvant therapy occurred in 74% and 30% of cases, respectively. Most patients (88%) received a first-line chemotherapy treatment containing intravenous fluoropyrimidine and oxaliplatin or irinotecan (specifically, 107 patients received FOLFOX and 41 FOLFIRI). As regards mutational analyses performed on tissue, KRAS, NRAS e BRAF mutations were detected in 58%, 7% and 13% of cases, respectively. Table 2 shows the distribution of the polymorphisms VEGF + 936, eNOS + 894G > T and eNOS VNTR in the study population. At a median follow-up of 52.6 months, there had been 161 (95%) progressions and 134 (79%) deaths in the cohort under analysis. Median PFS and OS were 12.2 months (95% CI 11.2–13.4) and 22.6 months (95% 18.6–29.1), respectively. During treatment, 83 patients (72%) reached a CR or PR as best overall response.
Association between polymorphisms and clinical outcomes
We evaluated the correlation between PFS and VEGF + 936, eNOS + 894 and eNOS VNTR polymorphisms. The analysis carried out did not confirm what was found in the previous study, as none of the polymorphisms correlated significantly with PFS (Table 3, Supplementary Fig. S1).
With regard to OS, no statistically significant difference was observed between polymorphism genotypes (Table 3, Supplementary Fig. S1).
We also investigated eNOS + 894 and eNOS VNTR polymorphisms in relation to ORR. VEGF + 936 was not considered in this analysis due to the small frequencies of CT and TT genotypes. eNOS VNTR was the only polymorphism significantly associated with ORR in univariate analysis, but this result was not confirmed in multivariate analysis (Supplemetary Table S1).
eNOS haplotype
In our previous analysis, two haplotypes based on eNOS VNTR 4a/b and eNOS + 894 G/T were described: eNOS Haplo 1 (4b-G), eNOS Haplo 2 (4b-T).
Homozygosity in eNOS Haplo1 (Haplo1/Haplo1), in eNOS Haplo2 (Haplo2/Haplo2) and their combination (Haplo1/Haplo1 + Haplo2/Haplo2) were considered for correlation with patient outcomes.
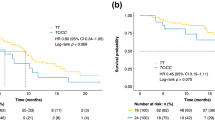
The analyses performed did not confirm what was found in the previous study, in particular patients bearing eNOS Haplo1/Haplo1 or the combination of eNOS Haplo1/Haplo1 and eNOS Haplo2/Haplo2 had not significantly improved outcomes in terms of PFS, OS and ORR (Table 4, Fig. 1, Supplementary Table S2). A significant effect on PFS was instead observed in the group eNOS Haplo2/Haplo2. In particular eNOS Haplo2/Haplo2 showed worse PFS than other geno-types (HR 2.25, 95% CI 1.26–4.03, p = 0.006). The result obtained was confirmed in multivariate analysis (adjusted-HR 2.83, 95% CI 1.33–5.99, p = 0.006) (Table 4 and Fig. 1).
Discussion
The present study investigated VEGF and eNOS polymorphisms in relation to clinical outcome in patients with mCRC treated with first line Bev-based chemotherapy.
The inhibition of angiogenesis, through blockade of the VEGF/VEGFR pathway, is an effective strategy in the treatment of mCRC. Positive results from randomised phase III clinical trials are available not only with Bev9, but also with the multikinase inhibitor regorafenib10 and aflibercept, a fully human fusion protein that highly specifically and potently blocks all VEGF isoforms and placental growth factor (PlGF)11. From a clinical perspective, the relatively small absolute benefit provided by these new agents, as well as the availability of an increasing number of therapeutic options, make the identification of predictive biomarkers an essential requirement for optimizing the use of antiangiogenic agents. Unfortunately, studies in this context, characterized by different designs, have shown contradictory results. In fact, although several studies have reported significant results regarding VEGF polymorphisms, others have failed to confirm their predictive role12.
For example, the retrospective study by Loupakis and colleagues hypothesized a role of the VEGF-1498 C/T polymorphism as a predictive biomarker of Bev efficacy, showing that patients with this genotype had a worse prognosis13. However, the same authors did not confirm this hypothesis in a subsequent prospective validation study7. In another retrospective study, VEGF-1498 C/T and -2578 A/C were correlated with outcome6. In other prospective studies, VEGF-1154 G/A and VEGF-634 G/C were significantly corre-lated with patient response to treatment14.
One of the most compelling reasons for the failure of predictive marker studies is the biological complexity of tumor angiogenesis. Data in the literature suggest multiple pathways in the growth of new tumor vessels, leading to study the simultaneous inhibi-tion of multiple angiogenic targets as a potentially effective strategy15. The role of different cell types in the development of such an intricate network of signals has also emerged and the contribution of both stromal cells and bone marrow-derived vascular progenitors recruited and stimulated by hypoxic conditions has been highlighted16. The relevance of the tumor microenvironment as a crucial player in the growth and stabiliza-tion of new vessels and the critical implication of the extracellular matrix in supporting angiogenesis are now well established, thus confirming the contribution of both host and tumor factors to the so called “angiogenic balance”.
Mechanisms of action of anti-VEGF therapy remain unclear since blockade of circu-lating VEGF can affect not only tumors, but also stroma and endothelial cell proliferation and maturation. While the antiangiogenic properties of Bev were initially attributed to the inhibition of endothelial cell proliferation, several biological effects are now recognized, such as the inhibition of bone marrow-derived progenitors, the normalization of vessel structure, the vascular “constriction”, the destruction of the cancer stem cell niche, the di-rect effect on cancer cells and the interaction with the host immune system. Because of these multiple mechanisms of action, the translation of in vitro and in vivo results into the human model is not immediate, and several difficulties make the development of effective preclinical models extremely complicated.
The ITACA study secondary analysis has shown that specific polymorphisms of eNOS were significantly correlated with patient survival and response to treatment. Nota-bly, patients with a specific combination of eNOS Haplo1/Haplo1 and eNOS Haplo2/Haplo2 had significantly longer PFS and OS and a higher ORR than those with the other genotypes8. The correlation was significant in the Bev-treated group and not in the chemotherapy-only group, highlighting the potential role of eNOS polymorphisms as pre-dictive biomarkers of Bev. Nitric oxide (NO), which is produced by the endothelium through the constitutively expressed gene known as eNOS, is essential for preserving the functional integrity of endothelial cells, controlling hemodynamics, and developing collateral circulation17. For the prevention of thrombotic and atherogenic processes, appropriate eNOS expression and activity, which results in enough NO generation, are crucial18. It has been demonstrated that VEGF inhibition causes a reduction in eNOS expression, which in turn results in a reduction in NO production19, and that this phenomenon is connected to the induction of hypertension, one of the most frequent dose-limiting toxicities of VEGF inhibitors20. Intron 4 eNOS VNTR polymorphism plays a role in regulating eNOS expression by acting as an enhancer/repressor and by coding for a 27-nt small RNA, which appears to inhibit eNOS expression at the transcriptional level21,22,23,24. The higher the number of 27-nt repeats, the more 27nt sir-RNA is produced, inhibiting eNOS expression. However, the association between eNOS VNTR in intron 4 and eNOS expression is still a much-debated issue25,26,27.
The purpose of the current study was to validate our previous findings, which, if verified, might have served as a reliable criterion for choosing individuals for Bev therapy.
Unfortunately, the study did not confirm the earlier findings and demonstrated the failure of both VEGF and eNOS polymorphisms as a potential predictor of benefit from Bev. The validation of haplotypes predictive role was thus compromised by the negative results on eNOS polymorphisms.
The lack of correlation evidence in the study could be due to the reduced power caused by fewer patients’ enrolled respect to the planned sample size. Although eNOS VNTR showed a significant association with ORR in univariate analysis the result was not confirmed in multivariate analysis probably because of the further reduction of sample to non-missing values.
The present study confirms the absolute importance of prospective validation as an essential step on the road of biomarkers towards clinical application. Furthermore, based on the complexity of the biology of tumor angiogenesis and the involvement of multiple actors in this process, it seems rather unlikely that a single germline SNP alone could explain the efficacy of Bev. The failure of the “candidate SNP strategy” certainly opens the way to new questions on the possibility of effectively exploiting the pharmacogenetic ap-proach to identify predictors of benefit from Bev.
This prospective study failed to validate the hypothesized predictive impact of eNOS and VEGF polymorphisms for response to Bev plus first-line chemotherapy for mCRC. The results of the previous exploratory analysis performed on patients enrolled in a ran-domized and prospective phase III multicenter study (ITACa study) were not confirmed. While on the one hand the reduction in the sample size might have played a role in the negative result, we believe that the most probable cause is biological. Furthermore, since eNOS is not the direct target of Bev, other factors could definitely be involved in the relationship between eNOS activity and Bev efficacy. Therefore the main weakness of our trial is to have hypothesized that a single polymorphism could predict such a complex event as the inhibition of angiogenesis.We believe that future directions in this research field must necessarily include more comprehensive approaches, also considering the complexity of the mechanism of angiogenesis and the mecha-nisms of action of anti-VEGF therapy.
Materials and methods
Patients and treatment
This is a prospective non-pharmacological trial aimed at validating VEGF and eNOS polymorphisms as predictors of efficacy of Bev plus first-line chemotherapy in patients with mCRC. The primary objective of the study was to assess the correlation of the selected polymorphisms with PFS. Secondary objectives were the correlations with OS and ORR. The Local Ethics Committee (Comitato Etico Area Vasta Romagna and IRST) approved the study and informed consent was obtained from all patients before blood samples were obtained for genotype testing.
This study involved 182 patients with mCRC treated with first-line chemotherapy plus Bev at the IRCCS Istituto Romagnolo per lo Studio dei Tumori (IRST) “Dino Amadori” in Meldola. Patients ≥ 18 years of age, with histologically confirmed mCRC, one or more unidimensional measurable lesions according to Response Evaluation Criteria in Solid Tumours (RECIST) 1.0, Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2 (≤ 1 if age ≥ 70 years), and an estimated life expectancy of at least 12 weeks were included. Patients were required to have adequate hematological, hepatic and renal func-tion. Prior adjuvant chemotherapy for CRC or neoadjuvant/adjuvant chemo-radiotherapy for rectal cancer were permitted, if completed at least 6 months before recurrence; conversely, prior chemotherapy or immunotherapy for advanced or metastatic disease were not permitted. Patients received one of the following chemotherapy regimens in combina-tion with Bev: FOLFOX4, CAPOX, FOLFIRI, CAPIRI.
Treatment was to continue until disease progression (PD), withdrawal of consent, or unacceptable toxicity, whichever came first. If a patient became eligible for curative resection of metastatic disease, Bev was discontinued at least 6–8 weeks prior to the scheduled surgery date. After surgery, the choice of treatment was at the physician's discretion, and patients could resume treatment at least 28 days after surgery or complete wound healing, until PD. We analyzed KRAS gene (codons 12, 13, 59, 61, 117, 146), BRAF gene (codons 594, 600, 601) and NRAS gene (codons 12, 13, 18, 59, 61, 117, 146) as for clinical practice.
Polymorphisms and genotyping
VEGF SNP (VEGF + 936C > T), eNOS SNP (eNOS + 894G > T) and a 27-nucleotide variable number tandem repeat (VNTR) of eNOS were analyzed.
VEGF + 936C > T (re3025039) is located in 3’UTR region, eNOS + 894G > T (rs1799983) is located within exon 7, while eNOS VNTR 27 bp 4a/b is located in intron 4 of the gene. eNOS VNTR 27 bp 4a/b has 2 common alleles: “4°” with 4 repeats of 27 nucleotides and “4b” with 5 repeats.
The analysis of the selected polymorphisms was performed on peripheral blood samples. Genomic DNA was extracted from 200 μl of whole blood using the QIAamp DNA Mini Kit (Qiagen SPA, Milan, Italy) and quantified with the Nanodrop instrument (Celbio Spa, Milan, Italy).
eNOS + 894 were analyzed by Real time PCR (7500 Applied Biosystems) with TaqMan SNP Genotyping assays (Assay ID C_3219460_20) (Applied Biosystems, Foster City, CA, USA) and starting from 10 ng of DNA. The eNOS VNTR and VEGF + 936 polymorphisms were instead determined by standard PCR and direct sequencing analysis on the ABI 3130 sequencer (Applied Biosystems). PCRs were performed starting from 50 ng of genomic DNA. PCR conditions and primer sequences for VEGF + 936 and eNOS VNTR were report-ed in our previous study8.
All samples were analyzed at the Biosciences Laboratory of IRST IRCCS (Meldola, FC).
Statistical analysis
Based on the results obtained from the ITACa study, we assumed a prevalence of the haplotype to be validated (homozygosity in eNOS Haplo 1 and homozygosity in eNOS Haplo2, Haplo1: 4b-G, Haplo2: 4b-T based on eNOS VNTR 4a/b and eNOS polymor-phisms + 894 G/T) of 0.5. By establishing a significance threshold of 5%, a type II error (2-tailed) of 20%, a median PFS of 9 months for the haplotype other than Hap-lo1/Haplo1 + Haplo2/Haplo2, and a hazard ratio (HR) of 0.65, we estimated a total of 200 patients to be recruited.
Patient characteristics were summarized using the median and range for the contin-uous variables and frequencies and percentages for the categorical variables. PFS was de-fined as the time between the date of diagnosis and that of progression or death; OS was defined as the time between the date of diagnosis and death; ORR was defined as the per-centage of patients achieving a complete (CR) or partial response (PR).
The Kaplan–Meier method was used to estimate the time-to-event curves and the log-rank test was applied for their comparison. HRs for each polymorphism and haplo-types and relative 95% CI were calculated using the Cox proportional hazards model. The association between polymorphisms or haplotypes and ORR was analyzed by a logistic regression model and the effect of polymorphisms on ORR was estimated by odds ratio (OR) and 95% CI.
Multivariate analyses were performed after univariate analyses revealed a significant effect of the polymorphism/haplotype. Models were adjusted for chemotherapy regimen (FOLFOX/CAPOX vs FOLFIRI/CAPIRI vs other), gender, age, KRAS status, NRAS status, BRAF status, tumour location (rectum vs colon) as probable confounders and prognostic factors8.
All p-values were based on two-sided testing and p < 0.05 were considered statistically significant. Analyses were performed using R (version 4.1.0).
Ethical approval
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Ethics Committee of IRCCS Istituto Romagnolo per lo Studio dei Tumori (IRST) “Dino Amadori”, Meldola, Italy (reference number IRSTB038 and date of approval 08/07/2015).
Consent for publication
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data availability
The data supporting the fndings of this study could be obtained from the corresponding author.
References
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Siegel, R. L., Wagle, N. S., Cercek, A., Smith, R. A. & Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. (2023).
Hurwitz, H. et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 350, 2335–2342 (2004).
Formica, V. et al. Predictive value of VEGF gene polymorphisms for metastatic colorectal cancer patients receiving first-line treatment including fluorouracil, irinotecan, and bevacizumab. Int. J. Colorectal Dis. 26, 143–151 (2011).
Koutras, A. K. et al. Vascular endothelial growth factor polymorphisms and clinical outcome in colorectal cancer patients treated with irinotecan-based chemotherapy and bevacizumab. Pharmacogenomics J. 12, 468–475 (2012).
Morita, S. et al. Association between bevacizumab-related hypertension and vascular endothelial growth factor (VEGF) gene polymorphisms in Japanese patients with metastatic colorectal cancer. Cancer Chemother. Pharmacol. 71, 405–411 (2013).
Loupakis, F. et al. Prospective validation of candidate SNPs of VEGF/VEGFR Pathway in metastatic colorectal cancer patients treated with first-line FOLFIRI plus bevacizumab. PLoS One 8, e66774 (2013).
Ulivi, P. et al. eNOS polymorphisms as predictors of efficacy of bevacizumab-based chemotherapy in metastatic colorectal cancer: Data from a randomized clinical trial. J. Transl. Med. 13, 258 (2015).
Giantonio, B. J. et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J. Clin. Oncol. 25, 1539–1544 (2007).
Grothey, A. et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381, 303–312 (2013).
Van Cutsem, E. et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J. Clin. Oncol. 30, 3499–3506 (2012).
Schneider, B. P. et al. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J. Clin. Oncol. 26, 4672–4678 (2008).
Loupakis, F. et al. Retrospective exploratory analysis of VEGF polymorphisms in the prediction of benefit from first-line FOLFIRI plus bevacizumab in metastatic colorectal cancer. BMC Cancer 11, 247 (2011).
do Espírito Santo, G. F., Galera, B. B., Duarte, E. C., Chen, E. S., Azis, L., Damazo, A. S. et al. Prognostic significance of vascular endothelial growth factor polymorphisms in colorectal cancer patients. World J. Gastrointest. Oncol. 9, 78 (2017)
Tejpar, S., Prenen, H. & Mazzone, M. Overcoming resistance to antiangiogenic therapies. Oncologist 17, 1039–1050 (2012).
Bottsford-Miller, J. N., Coleman, R. L. & Sood, A. K. Resistance and escape from antiangiogenesis therapy: Clinical implications and future strategies. J. Clin. Oncol. 30, 4026–4034 (2012).
Epstein, F. H., Gibbons, G. H. & Dzau, V. J. The emerging concept of vascular remodeling. N. Engl. J. Med. 330, 1431–1438 (1994).
Oemar, B. S. et al. Reduced endothelial nitric oxide synthase expression and production in human atherosclerosis. Circulation 97, 2494–2498 (1998).
Winnik, S. et al. Systemic VEGF inhibition accelerates experimental atherosclerosis and disrupts endothelial homeostasis—Implications for cardiovascular safety. Int. J. Cardiol. 168, 2453–2461 (2013).
Facemire, C. S., Nixon, A. B., Griffiths, R., Hurwitz, H. & Coffman, T. M. Vascular endothelial growth factor receptor 2 controls blood pressure by regulating nitric oxide synthase expression. Hypertension 54, 652–658 (2009).
Wang, J., Dudley, D. & Wang, X. L. Haplotype-specific effects on endothelial NO synthase promoter efficiency. Arterioscler. Thromb. Vasc. Biol. 22 (2002).
Senthil, D. et al. Genotype-dependent expression of endothelial nitric oxide synthase (eNOS) and its regulatory proteins in cultured endothelial cells. DNA Cell Biol. 24, 218–224 (2005).
Zhang, M.-X. et al. Regulation of endothelial nitric oxide synthase by small RNA. Proc. Natl. Acad. Sci. 102, 16967–16972 (2005).
Ou, H. et al. Effect of nuclear actin on endothelial nitric oxide synthase expression. Arterioscler. Thromb. Vasc. Biol. 25, 2509–2514 (2005).
Tsukada, T. et al. Evidence of association of the ecNOS gene polymorphism with plasma NO metabolite levels in humans. Biochem. Biophys. Res. Commun. 245, 190–193 (1998).
Wang, X. L. et al. Genotype dependent and cigarette specific effects on endothelial nitric oxide synthase gene expression and enzyme activity. FEBS Lett. 471, 45–50 (2000).
Yoon, Y., Song, J., Hong, S. H. & Kim, J. Q. Plasma nitric oxide concentrations and nitric oxide synthase gene polymorphisms in coronary artery disease. Clin. Chem. 46, 1626–1630 (2000).
Acknowledgements
This work was partly supported thanks to the contribution of Ricerca Corrente by the Italian Ministry of Health within the research line "Precision, gender and ethnicity-based medicine and geroscience: genetic-molecular mechanisms in the development, characterization and treatment of tumors.
Author information
Authors and Affiliations
Contributions
Conceptualization, A.P. and P.U.; methodology, I.A.; validation, G.M., I.A., A.P, P.U.; formal analysis, I.A.; investigation, G.M., F.R., G.B., M.U., M.C., C.M., S.A.D.; resources, A.P., G.B., L.M., F.G.S., S.A.D., C.G., G.G., G.L.F.; data curation, G.M., A.P., P.U.; writing-original draft preparation, G.M, I.A, A.P, P.U.; writing-review and editing, G.M., I.A, A.P; visualization, G.M., I.A.; supervision, A.P., P.U.; project administration, A.P., P.U. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marisi, G., Azzali, I., Passardi, A. et al. Prospective validation of VEGF and eNOS polymorphisms as predictors of first-line bevacizumab efficacy in patients with metastatic colorectal cancer. Sci Rep 13, 12921 (2023). https://doi.org/10.1038/s41598-023-40220-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-40220-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.