Abstract
In the context of the generalized fractional derivative, novel solutions to the D-dimensional Schrödinger equation are investigated via the improved Rosen-Morse potential (IRMP). By applying the Pekeris-type approximation to the centrifugal term, the generalized fractional Nikiforov-Uvarov method has been used to derive the analytical formulations of the energy eigenvalues and wave functions in terms of the fractional parameters in D-dimensions. The resulting solutions are employed for a variety of diatomic molecules (DMs), which have numerous uses in many fields of physics. With the use of molecular parameters, the IRMP is utilized to reproduce potential energy curves for numerous DMs. The pure vibrational energy spectra for several DMs are determined using both the fractional and the ordinary forms to demonstrate the effectiveness of the method utilized in this work. As compared to earlier investigations, it has been found that our estimated vibrational energies correspond with the observed Rydberg-Klein-Rees (RKR) data much more closely. Moreover, it is observed that the vibrational energy spectra of different DMs computed in the existence of fractional parameters are superior to those computed in the ordinary case for fitting the observed RKR data. Thus, it may be inferred that fractional order significantly affects the vibrational energy levels of DMs. Both the mean absolute percentage deviation (MAPD) and average absolute deviation (AAD) are evaluated as the goodness of fit indicators. According to the estimated AAD and MAPD outcomes, the IRMP is an appropriate model for simulating the RKR data for all of the DMs under investigation.
Similar content being viewed by others
Introduction
In recent decades, numerous works on the solutions to the Klein-Gordon, Dirac and Schrödinger equations were reported1,2,3,4,5,6. This is owing to the reality that the solutions to these wave equations include all of the data required for the quantum system under investigation. In this context, the vibrational energy spectra of diatomic molecules (DMs) were investigated using several potential functions, such as the Morse7, Kratzer8, Deng-Fan9, Hulthén10, Tietz-Hua11 and others.
In 1932, Rosen and Morse12 suggested a diatomic molecular function
where C, B and d are changeable parameters. The Rosen-Morse potential (RMP) was used to explore polyatomic vibrational states of the NH\({_3}\) molecule12. It was also employed to characterize the diatomic molecular vibrations13. By utilizing the equilibrium bond length (\({r_e}\)) and the dissociation energy (\({D_e}\)) for a DM as explicit parameters, Jia et al.14 presented an improved expression of the RMP based on the original form of the RMP.
where the screening parameter \({\alpha }\) is defined as follow15:
where \({w_e}\) is the equilibrium harmonic vibrational frequency and W is the Lambert W function16 that fulfils \({z=W(z)e^{W(z)}}\). The improved Rosen-Morse potential (IRMP) was extensively employed to depict the diatomic molecular vibrations by solving the relativistic and non-relativistic wave equations.
Wang et al.17 demonstrated that for diatomic molecules, one form of the Schiöberg potential is identical to the IRMP. Chen et al.18 used the supersymmetric shape invariance method to find the solutions of the Klein-Gordon equation (KGE) with the IRMP and determined the relativistic vibrational transition frequencies for the \({3^3\Sigma _g^+}\) state of the Cs\({}_{2}\) molecule. The ro-vibrational energy levels for the \({5^1\Delta _g^+}\) state of the Na\({}_{2}\) molecule and the \({3^3\Sigma _g^+}\) state of the Cs\({}_{2}\) molecule were calculated with the IRMP in D-dimensions using different techniques19,20,21. By using the parametric Nikiforov‑Uvarov (NU) method, Akanni and Kazeem22 derived the approximate solutions of the KGE with the IRMP. The thermodynamics properties for the Na\(_{2}\) dimer were discussed using the IRMP23. The authors in Ref.15 examined the solutions of the Dirac equation with the IRMP and computed the relativistic vibrational energy spectra for the \({3^3\Sigma _g^+}\) state of the Cs\({}_{2}\) molecule.
Based on the IRMP, the predictions of molar enthalpy, entropy and Gibbs free energy for the P\({}_{2}\) dimer were calculated24,25,26. Udoh et al.27 utilized the NU method to find the solutions of the Schrödinger equation (SE) in D-dimensions for the IRMP and estimated the ro-vibrational energies of H\({}_{2}\)(\({X^1\Sigma _g^+}\)) and NO(\({a^4\Pi _i}\)) diatomic molecules. Horchani and Jelassi28 used the IRMP to explore the impact of quantum correction on the thermodynamic characteristics of the Cs\({}_{2}\) (\({3^3\Sigma _g^+}\)) molecule. The vibrational energies for nitrogen molecule and sodium dimer were found29 by studying the solutions of the SE with the IRMP. Al-Raeei30 derived an expression of the bond equilibrium length of the IRMP and used it to analyze six dimers and molecules.Yanar31 computed the vibrational energies of the SiF\({^+}\)(\({X^1\Sigma ^+}\)) molecule utilizing particular cases of the general molecular potential, such as the Morse potential, IRMP, and others.
Fractional derivatives calculus has been an appealing area of research in recent decades because of its application in different scientific fields such as physics, chemistry, biology, engineering, medicine, and economics. In the literature, various fractional derivative definitions have been introduced, such as Riemann-Liouville32, Caputo33, Jumarie34, and others35.
According to Khalil36, an alternative fractional derivative definition that preserves classical features is the conformable fractional derivative (CFD). In the context of the CFD, the characteristics of heavy mesons were discussed using the N-dimensional radial SE for the trigonometric RMP37, hot-magnetized inter-action potential38, dependent temperature potential39, and generalized Cornell potential40. Abu-Shady41 used the concept of the CFD to present the mathematical model for describing the Coronavirus disease (COVID-19).
The generalized fractional derivative (GFD) is a novel concept for the fractional derivative that produces results consistent with those of classical definitions, was recently proposed by Abu-Shady and Kaabar42. The extended NU method was employed in conjunction with the GFD to solve the SE and determine the masses of heavy mesons43 and also the mass spectra of heavy tetraquarks and diquark44. The masses of heavy flavor baryons with and without hyperfine interactions were calculated using the generalized fractional iteration approach in Ref.45. In the scope of the GFD, the analytical exact iteration method was used to analyze the thermodynamic properties of heavy mesons in strongly coupled quark-gluon plasma46. In Ref.47, the fractional forms of various special functions were derived using the GFD. By using the generalized fractional Nikiforov‑Uvarov (GFNU) method48, the solutions of the SE with the generalized Woods-Saxon potential were derived. More recently, the D-dimensional SE was studied via the GFNU technique using the Deng-Fan potential49 and the improved Tietz potential (ITP)50. Furthermore the vibrational and ro-vibrational energies of several DMs were predicted.
It is vital to note that no previous research into SE solutions for the IRMP has been disclosed within the framework of the GFD. To this end, the purpose of this work is to explore solutions to the D-dimensional SE for the IRMP in the scope of the GFD. The structure of this work is as follows: The basics of the GFNU approach are explained in Section “The basics of the GFNU method”. The solutions of the D-dimensional SE for the IRMP are found within the scope of the GFD in Section “Solution of the SE with the IRMP in D-dimensions”. The numerical results of the vibrational energy levels of different DMs are provided and analyzed in Section “Discussion”. Finally, Section “Conclusion” provides a succinct conclusion of the work.
The basics of the GFNU method
The basics of the GFNU method are introduced in this part for solving the generalized fractional differential equation, which takes the following form49, 50.
where \({\tilde{\sigma }(z)}\) and \({\sigma (z)}\) are polynomials of maximum \({2\gamma }\)-th degree and \({\tilde{\tau }(z)}\) is a function at most \({\gamma }\)-th degree. Utilizing the primary characteristics of the GFD42
where
with
and inserting Eqs. (5) and (6) into Eq. (4) gives
Eq. (4) can be changed into the hypergeometric equation shown below:
where
where the generalized fractional is denoted by the subscript GF. Now taking
and putting Eq. (12) into Eq. (10) leads to
where X(z) is given by:
and
The function \({Y(z)}=Y_\nu (z)\) is a hypergeometric-type function with polynomial solutions provided by the Rodrigues formula
where \({C_\nu }\) is a constant of the normalization, and \({\rho (z)}\) is the weight function given by:
The polynomial \({\pi _{GF}(z)}\) is determined by:
The function h(z) can be obtained if the function under the square root is the square of a polynomial. Hence, the eigenvalue expression is:
where
Finally, by putting Eqs. (14) and (16) into Eq. (12), the eigenfunctions \({\mathcal {W}(z)}\) can be determined.
Solution of the SE with the IRMP in D-dimensions
The radial SE for a DM in the D-dimensional space with the potential V(r) is given by50.
where E, D, J and are the energy eigenvalue, the dimensionality number, and the vibrational quantum number respectively, and \({\hbar }\) is the reduced Planck’s constant. By putting,
Eq. (21) turns to
with
Inserting the IRMP (2) into Eq. (23) gives:
To determine the approximate analytical solutions of Eq. (25), the Pekeris approximation recipe is applied to the centrifugal term \({(\delta ^2-\frac{1}{4})\big /r^2}\) as19,20,21
where the coefficients \({b_0, b_1}\) and \({b_2}\) are defined as follows19,20,21
Inserting Eq. (26) into Eq. (25) yields
By employing the variable \({z=-e^{-\alpha r}}\), Eq. (30) turns into
where
with
By changing the integer orders in Eq. (31) to fractional orders, the generalized fractional version of the SE for the IRMP is being represented as follows:
Inserting Eqs. (5) and (6) into Eq. (36) yields
By comparing Eq. (37) with Eq. (10) yields the following functions:
By putting Eq. (38) into Eq. (18), the function \({\pi _{GF}(z)}\) is found as follows:
Eq. (39) can be reduced to the following:
where
with
By applying the restriction that the discriminant of the function under the square root of Eq. (40) should be zero, the function h(z) can be found as follow
By inserting Eq. (43) into Eq. (40) yields
The negative sign in Eq. (44) is selected to get a physically acceptable solution, the \({\pi _{GF}(z)}\) then changes to
and
Therefore, the functions \({g(z), \tau _{GF}(z)}\) and \({g_\nu (z)}\) are written as follows:
By integrating Eqs. (47) and (49), the fractional form of the energy eigenvalue of a DM in D dimensions can be expressed as:
where
In the absence of the influence of the fractional parameters, the following ordinary expression for the energy eigenvalues can be produced by putting \({\gamma =\beta =1}\):
By utilizing Eq. (14), the function X(z) becomes
Using Eq. (17), the function \({\rho (z)}\) can be stated as follows
With the help of Eq. (16), the function \({Y_\nu (z)}\) is written as
The complete solution of Eq. (31) is obtained by applying Eq. (12) as follows
Discussion
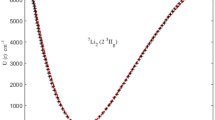
In this part, the obtained results are applied to a selection of DMs with widespread uses in optical and molecular physics. First, the potential function curves for the chosen DMs are initially generated using the IRMP. The molecular parameters used in this study are presented in Table 1, which are collected from the literature51,52,53,54,55,56,57,58,59,60,61. In Figs. (1, 2, 3), potential function curves generated by the IRMP are displayed alongside the experimental RKR points for the considered DMs. These Figs. show that the generated IRMP curves closely correspond to the observed RKR data points51,52,53,54,55,56,57,58,59,60,61. We evaluate the average absolute deviations (AAD) from the RKR experimental data in order to demonstrate the effectiveness of the IRMP.
A prominent goodness-of-fit metric for evaluating the reliability of an empirical potential energy model is the AAD from the dissociation energy, which is defined as62.
where \({V_{RKR}(r)}\) is the RKR potential and N is the number of experimental data points. Our AAD values for the chosen DMs are shown in Table 2. According to the Lippincott criterion,62 the AAD of the potential model must be less than 1\({\%}\) of the dissociation energy in order to fit the RKR potential curve. Thus, a better model is indicated by the smaller value of the AAD.
As revealed by Table 2, the IRMP is a perfect model for simulating the RKR potential since the computed AAD outcomes for all of the considered DMs are less than 1\({\%}\) of the dissociation energies. Further potential models for the K\({}_{2}\)(\({X^1\Sigma _g^+}\)) molecule that have AAD results are the Morse, Modified Morse, and Hulbert-Hirschfelder potentials52. Our AAD value is 0.6999\({\%}\), whereas the AAD results for the Morse and Hulbert-Hirschfelder potentials are 2.395\({\%}\), and 0.681\({\%}\) respectively. Consequently, both the IRMP and Hulbert-Hirschfelder potential are superior to the Morse potential for simulating the RKR data of the K\({}_{2}\)(\({X^1\Sigma _g^+}\)) molecule.
In order to verify the reliability of the expressions generated for the IRMP using the GFNU technique, the pure vibrational energy levels of different DMs are computed in three-dimensional space (\({D=3}\)). Comparisons between the calculated energies and the experimental RKR data as well as earlier investigations are provided in Tables 3, 4, 5, 6, 7, 8. To further support the veracity of our findings, we also examine the mean absolute percentage deviation (MAPD) of the IRMP from the RKR experimental points. The MAPD is expressed as50:
where \({E_{RKR}}\) are the experimental RKR energies and \({E_{nJ}}\) are the computed energies using the IRMP. The vibrational energies of the selected DMs are calculated using Eqs. (50) and (53) in both the fractional and ordinary instances respectively. The results in Tables 3, 4, 5, 6, 7, 8 clearly show that the vibrational energies estimated using the IRMP are in close agreement with the RKR experimental data. Also for all of the chosen DMs, the calculated MAPD demonstrates that are within 1% of the allowed error from the experimental RKR values.
The vibrational energies of the ScI (\({B^1\prod }\)) molecule are displayed in Table 3, along with comparisons to the findings of Refs.63,64,65. Diaf et al. employed the path integrals formalism to compute the vibrational energies of the ScI (\({B^1\prod }\)) molecule with the q-deformed Scarf potential in Ref.63. While the modified forms of the generalised Mobius square and hyperbolical-type potentials were used in Refs.64,65. The findings of these comparisons show that they coincide with the other potential models63,64,65. The vibrational energies for the N\({}_{2}\)(\({X^1\Sigma _g^+}\)) molecule are listed in Table 4 compared to the observed RKR data and the outcomes of Refs.52, 66. The authors in Ref.66 employed the deformed hyperbolic barrier potential to calculate the energy levels of the N\({}_{2}\)(\({X^1\Sigma _g^+}\)) molecule. Whereas the authors of Ref.52 used the Morse and deformed modified Rosen-Morse (DMRM) potentials.
Table 4 illustrates that our findings agree better with the RKR data than those computed using the other potential models52, 66. Furthermore, our MAPD values are the smallest in both the ordinary and fractional cases. As a result, our IRMP estimates for modelling the N\({}_{2}\)(\({X^1\Sigma _g^+}\)) molecule are more accurate than the other works52, 66. The vibrational energies for the K\({}_{2}\)(\({X^1\Sigma _g^+}\)) molecule are reported in Table 5. When comparing our results with those of Eyube et al.67 for the K\({}_{2}\)(\({X^1\Sigma _g^+}\)) molecule, it becomes clear that our results from the IRMP are more precise for fitting the RKR data for the K\({}_{2}\)(\({X^1\Sigma _g^+}\)) molecule than those from the improved q-deformed Scarf oscillator (IQSO) and the ITP. The vibrational energies of the CS(\({X^1\Sigma ^+}\)), AsS(\({X^2\prod }\)) and AsP(\({X^1\Sigma ^+}\)) molecules are listed in Table 6. As illustrated in Table 6, our outcomes coincide with the RKR data. In Table 7, the computed values for the SrO(\({X^1\Sigma ^+}\)), YO(\({X^2\Sigma ^+}\)) and ScO(\({X^2\Sigma ^+}\)) molecules with the observed RKR values are presented. As can be seen in Table 7, the calculated and observed outcomes are in close agreement. The vibrational energies of the SiP(\({X^2\prod }\)) and SiN(\({X^2\Sigma ^+}\)) are listed in Table 8 molecules with the RKR experimental values. It appears that the estimated results and the RKR data agree well. In Table 8, we also provide a comparison of the computed vibrational energies for the SiF\({^+}\)(\({X^1\Sigma ^+}\)) molecule with the outcomes of Ref.31 and observed values. Yanar31 calculated the vibrational energies for the SiF\({^+}\)(\({X^1\Sigma ^+}\)) molecule using the IRMP as well as the improved generalized Pöschl-Teller (IGPT) potential . It is clear that the current findings for the SiF\({^+}\)(\({X^1\Sigma ^+}\)) molecule are in good accord with those of Ref.31. As illustrated in Tables 3, 4, 5, 6, 7, 8, the influence of incorporating fractional parameters on the vibrational energies for the molecules studied in this work is crucial for modelling the experimental RKR data. Consequently, our results can be investigated to examine various molecules in future studies.
Conclusion
In this paper, the GFD is utilized for the first time to investigate the bound state solutions of the D-dimensional SE using the IRMP. Based on the GFNU, the analytical forms for the energy eigenvalues and wave functions of the IRMP are derived as a function of the fractional parameters in the D-dimensional space by employing the Pekeris-type approximation to the centrifugal term. The present results are applied to a number of DMs that have extensive applications in different physical domains. With the help of the molecular parameters, the potential energy curves are generated in terms of IRMP for the selected DMs. For the chosen DMs, the AAD of the IRMP from the observed RKR data is presented. According to our estimated AAD, the IRMP can successfully fit the experimental RKR data of several DMs. To validate the mechanism used in this research, the pure vibrational energies for different DMs are calculated in both ordinary (\({\gamma =\beta =1}\)) and fractional (\({\gamma \ne 1, \beta \ne 1}\)) cases in three-dimensional space (\({D=3}\)). It is found that the current computed pure vibrational energy values are preferable to those from earlier works and are in full harmony with the experimental data. It is further shown that the pure vibrational energies of different DMs computed in the existence of fractional parameters fit the observed RKR data better than those computed in the ordinary case. This leads one to the conclusion that fractional order significantly affects the vibrational energy levels of DMs. The MAPD from the observed RKR data points is assessed to further substantiate the accuracy of our findings. According to the assessed MAPD, our values are accurate to within a 1% error margin of the experimental RKR values. Therefore, the current findings indicate that the IRMP is a precise model for estimating the observed RKR data for all of the DMs considered in this investigation.
Data availability
All data generated or analysed during this study are available upon reasonable request from the corresponding author.
References
Ahmadov, A. I., Demirci, M., Mustamin, M. F. & Orujova, M. Sh. Bound state solutions of the Klein-Gordon equation under a non-central potential: The Eckart plus a ring-shaped potential. Eur. Phys. J. Plus 138, 1–13 (2023).
Inggil, A. S., Suparmi, A. & Faniandari, S. Solution of Klein-Gordon equation screened Hartmann ring-shaped plus Kratzer potential using hypergeometry method. AIP Conf. Proc. 2540, 100011 (2023).
Abu-Shady, M., Abdel-Karim, T. A. & Khokha, E. M. Binding energies and dissociation temperatures of heavy quarkonia at finite temperature and chemical potential in the-dimensional space. Adv. High Energy Phys. 2018, 7356843 (2018).
Abu-Shady, M., Abdel-Karim, T. A. & Khokha, E. M. Heavy-light mesons in the non-relativistic quark model using Laplace transformation method. Adv. High Energy Phys. 2018, 7032041 (2018).
Abu-Shady, M. & Khokha, E. M. Bound state solutions of the dirac equation for the generalized cornell potential model. Int. J. Mod. Phys. A 36, 2150195 (2021).
Khokha, E. M., Abu-Shady, M. & Abdel-Karim, T. A. The influence of magnetic and Aharanov-Bohm fields on energy spectra of diatomic molecules in the framework of the Dirac equation with the generalized interaction potential. Int. J. Quant. Chem. 2022, e27031 (2022).
Morales, D. A. Supersymmetric improvement of the Pekeris approximation for the rotating Morse potential. Chem. Phys. Lett. 394, 68–75 (2004).
Bayrak, O., Boztosun, I. & Ciftci, H. Exact analytical solutions to the Kratzer potential by the asymptotic iteration method. Int. J. Quant. Chem. 107, 540–544 (2007).
Dong, S. H. Relativistic treatment of spinless particles subject to a rotating Deng-Fan oscillator. Commun. Theor. Phys. 55, 969–971 (2011).
Gu, X. Y. & Sun, J. Q. Any l-state solutions of the Hulthén potential in arbitrary dimensions. J. Math. Phys. 51, 022106 (2010).
Roy, A. K. Ro-vibrational spectroscopy of molecules represented by a Tietz-Hua oscillator potential. J. Math. Chem. 52, 1405–1413 (2014).
Rosen, N. & Morse, P. M. On the vibrations of polyatomic molecules. Phys. Rev. 42, 210 (1932).
Royappa, A. T., Suri, V. & McDonough, J. R. Comparison of empirical closed-form functions for fitting diatomic interaction potentials of ground state first-and second-row diatomics. J. Mol. Struct. 787, 209 (2006).
Jia, C. S. et al. Equivalence of the Wei potential model and Tietz potential model for diatomic molecules. J. Chem. Phys. 137, 014101 (2012).
Jia, C. S. & Jia, Y. Relativistic rotation-vibrational energies for the \(\text{ Cs}_2\) molecule Eur. Phys. J. D 71, 1–7 (2017).
Jia, C. S. et al. Prediction of entropy and Gibbs free energy for nitrogen. Chem. Eng. Sci. 202, 70–74 (2019).
Wang, P. Q. et al. Improved expressions for the Schiöberg potential energy models for diatomic molecules. J. Mol. Spec. 278, 23–26 (2012).
Chen, T., Lin, S. R. & Jia, C. S. Solutions of the Klein-Gordon equation with the improved Rosen-Morse potential energy model. Eur. Phys. J. Plus 128, 69 (2013).
Hu, X. T., Zhang, L. H. & Jia, C. S. D-dimensional energies for cesium and sodium dimers. Can. J. Chem. 92, 386–391 (2014).
Tan, M. S., He, S. & Jia, C. S. Molecular spinless energies of the improved Rosen-Morse potential energy model in D dimensions. Eur. Phys. J. Plus 129, 264 (2014).
Liu, J. Y., Hu, X. T. & Jia, C. S. Molecular energies of the improved Rosen-Morse potential energy model. Can. J. Chem. 92, 40–44 (2014).
Akanni, Y. W. & Kazeem, I. Approximate analytical solutions of the improved Tietz and improved Rosen-Morse potential models. Chin. J. Phys. 53, 060401 (2015).
Song, X. Q., Wang, C. W. & Jia, C. S. Thermodynamic properties for the sodium dimer. Chem. Phys. Lett. 673, 50–55 (2017).
Jia, C. S. et al. Enthalpy of gaseous phosphorus dimer. Chem. Eng. Sci. 183, 26–29 (2018).
Jia, C. S., Zeng, R., Peng, X. L., Zhang, L. H. & Zhao, Y. L. Entropy of gaseous phosphorus dimer. Chem. Eng. Sci. 190, 1–4 (2018).
Peng, X. L., Jiang, R., Jia, C. S., Zhang, L. H. & Zhao, Y. L. Gibbs free energy of gaseous phosphorus dimer. Chem. Eng. Sci. 190, 122–125 (2018).
Udoh, M. E., Okorie, U. S., Ngwueke, M. I., Ituen, E. E. & Ikot, A. N. Rotation-vibrational energies for some diatomic molecules with improved Rosen-Morse potential in D-dimensions. J. Mol. Mod. 25, 170 (2019).
Horchani, R. & Jelassi, H. Effect of quantum corrections on thermodynamic properties for dimers. Chem. Phys. 532, 110692 (2020).
Onate, C. A. & Akanbi, T. A. Solutions of the Schrödinger equation with improved Rosen Morse potential for nitrogen molecule and sodium dimer. Results Phys. 22, 103961 (2021).
Al-Raeei, M. The bond length of the improved Rosen Morse potential, applying for: Cesium, hydrogen, hydrogen fluoride, hydrogen chloride, lithium, and nitrogen molecules. Results Chem. 4, 100560 (2022).
Yanar, H. More accurate ro-vibrational energies for \(\text{ SiF}^+\)(\(X^1\Sigma ^+\)) molecule. Phys. Scr. 97, 045404 (2022).
Miller, K. S. & Ross, B. An Introduction to the Fractional Calculus and Fractional Differential Equations (Wiley, New York, 1993).
Caputo, M. Linear models of dissipation whose Q is almost frequency independent-II Geophysical. J. Int. 13, 529–539 (1967).
Jumarie, G. Modified Riemann-Liouville derivative and fractional Taylor series of nondifferentiable functions further results. Comp. Math. Appl. 51, 1367–1376 (2006).
Oliveira, E. & Machado, J. A review of definitions for fractional derivatives and integral. Math. Prob. Eng. 2014, 238459 (2014).
Karayer, H., Demirhan, D. & Büyükkılıç, F. Conformable fractional Nikiforov-Uvarov method. Commun. Theor. Phys. 66, 12–18 (2016).
Abu-Shady, M. & Ezz-Alarab, S. Y. Conformable fractional of the analytical exact iteration method for heavy quarkonium masses spectra. Few-Body Syst. 62, 1–8 (2021).
Abu-Shady, M., Ahmadov, A. I., Fath-Allah, H. M. & Badalov, V. H. Spectra of heavy quarkonia in a magnetized-hot medium in the framework of fractional non-relativistic Quark model. J. Theor. Appl. Phys. 16, 162225 (2022).
Abu-Shady, M. Quarkonium masses in a hot QCD medium using conformable fractional of the Nikiforov-Uvarov method. Int. J. Mod. Phys. A 34, 1950201 (2019).
Al-Jamel, A. The search for fractional order in heavy quarkonia spectra. Int. J. Mod. Phys. A 34, 1950054 (2019).
Abu-Shady, M. The conformable fractional of mathematical model for the Coronavirus Disease 2019 (COVID-19 Epidemic). Fur. App. Math. 1, 34–42 (2021).
Abu-Shady, M. & Kaabar, M. K. A. A generalized definition of the fractional derivative with applications. Math. Prob. Eng. 2021, 9444803 (2021).
Abu-Shady, M. & Inyang, E. P. Heavy-meson masses in the framework of trigonometric Rosen-Morse potential using the generalized fractional Derivative. East Eur. J. Phys. 4, 80–86 (2022).
Abu-Shady, M., Ahmed, M. M. A. & Gerish, N. H. Generalized fractional of the extended Nikiforov-Uvarov method for heavy tetraquark masses spectra. Mod. Phys. Lett. A 38, 2350028 (2023).
Abu-Shady, M. & Fath-Allah, H. M. Masses of single, double, and triple heavy baryons in the hyper-central quark model by using GF-AEIM. Adv. High Energy Phys. 2022, 4539308 (2022).
Abu-Shady, M. & Ezz-Alarab, S. Y. Thermodynamic properties of heavy mesons in strongly coupled quark gluon plasma using the fractional of non-relativistic quark model. Ind. J. Phys.https://doi.org/10.1007/s12648-023-02695-y (2023).
Abu-Shady, M. & Kaabar, M. K. A novel computational tool for the fractional-order special functions arising from modeling scientific phenomena via Abu-Shady-Kaabar fractional derivative. Comp. Math. Meth. Med. 2022, 2138775 (2022).
Abu-Shady, M. & Inyang, E. P. The fractional Schrodinger equation with the generalized Woods-Saxon potential. East Eur. J. Phys. 41, 63–68 (2023).
Abu-Shady, M., Khokha, E. M. & Abdel-Karim, T. A. The generalized fractional NU method for the diatomic molecules in the Deng-Fan model. Eur. Phys. J. D 76, 159 (2022).
Abu-Shady, M. & Khokha, E. M. On prediction of the fractional vibrational energies for diatomic molecules with the improved Tietz potential. Mol. Phys. 120, e2140720 (2022).
Reddy, R. R. et al. Potential energy curves, dissociation energies and Franck-Condon factors of NI and ScI molecules. J. Quant. Spectrosc. Radiat. Transf. 74, 125 (2002).
Desai, A. M., Mesquita, N. & Fernandes, V. A new modified Morse potential energy function for diatomic molecules. Phys. Scr. 95, 085401 (2020).
Ross, A. J., Crozet, P., d’Incan, J. & Effantin, C. The ground state, \(X^1\Sigma _g^+\), of the potassium dimer. J. Phys. B Atom. Mol. Phys. 19, L145 (1986).
Reddy, R. R., Rao, T. V. R. & Viswanath, R. Potential energy curves and dissociation energies of NbO, SiC, CP, \(\text{ PH}^+\), \(\text{ SiF}^+\), and \(\text{ NH}^+\). Astrophys. Space Sci. 189, 29 (1992).
Reddy, R. R., Ahammed, Y. N., Gopal, K. R., Azeem, P. A. & Rao, T. V. R. Dissociation energies of astrophysically important MgO, SO, SiN and TiO from spectroscopic data. J. Quant. Spect. Rad. Trans. 66, 501–508 (2000).
Jakubek, Z. J., Nakhate, S. G. & Simard, B. The SiP molecule: The first observation and spectroscopic characterization. J. Chem. Phys. 116, 6513–6520 (2002).
Murthy, N. S., Manisekaran, T. & Bapat, N. S. Dissociation energy of the ground states of SrO, SnCl, NaH, and RbH from the true potential energy curves. J. Quant. Spect. Rad. Trans. 29, 183–187 (1983).
Reddy, R. R., Nazeer Ahammed, Y., Rama Gopal, K., Abdul Azeem, P. & Anjaneyulu, S. Rkrv potential energy curves, dissociation energies, \(\gamma\)-centroids and franck-condon factors of YO, CrO, BN, ScO, SiO and AlO molecules. Astrophys. Space Sci. 262, 223–240 (1998).
Rajamanickam, N., Prahllad, U. D. & Narasimhamurthy, B. On the dissociation energy of AsP molecule. Spect. Lett. 15, 557–564 (1982).
Reddy, R. R., Reddy, A. S. R. & Rao, T. V. R. Estimation of dissociation energy of the AsS molecule. Pramana 25, 187–190 (1985).
Reddy, R. R., Nazeer Ahammed, Y., Rama Gopal, K. & Baba Basha, D. Estimation of potential energy curves, dissociation energies, franck-condon factors and r-centroids of comet interesting molecules. Astrophys. Space Sci. 286, 419–436 (2003).
Lippincott, E. R. A new relation between potential energy and internuclear distance. J. Chem. Phys. 21, 2070 (1953).
Diaf, A., Hachama, M. & Ezzine, M. M. H. l-states solutions for the q-deformed Scarf potential with path integrals formulation. Phys. Scr. 96, 105212 (2021).
Okorie, U. S., Ikot, A. N., Ibezim-Ezeani, M. U. & Abdullah, H. Y. Diatomic molecules energy spectra for the generalized Mobius square potential model. Int. J. Mod. Phys. B 34, 2050209 (2020).
Eyube, E. S., Notani, P. P. & Dikko, A. B. Modeling of diatomic molecules with modified hyperbolical-type potential. Eur. Phys. J. Plus 137, 329 (2022).
Ezzine, M. M. H., Hachama, M. & Diaf, A. Feynman kernel analytical solutions for the deformed hyperbolic barrier potential with application to some diatomic molecules. Phys. Scr. 96, 125260 (2021).
Eyube, E. S., Nyam, G. G. & Notani, P. P. Improved q-deformed Scarf II oscillator. Phys. Scr. 96, 125017 (2021).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contributed to the work's conception and design. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abu-Shady, M., Khokha, E.M. A precise estimation for vibrational energies of diatomic molecules using the improved Rosen–Morse potential. Sci Rep 13, 11578 (2023). https://doi.org/10.1038/s41598-023-37888-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-37888-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.