Abstract
The profile of secondary metabolites in ten members of tribe Mentheae (Nepetoideae, Lamiaceae) from Peru by liquid chromatography associated with high resolution mass spectrometry, is presented. Salvianolic acids and their precursors were found, particularly rosmarinic acid, caffeic acid ester derivatives, as well as a diversity of free and glycosylated flavonoids as main substances. At all, 111 structures were tentatively identified.
Similar content being viewed by others
Introduction
The tropical Andes are considered one of the most diverse areas on the planet in terms of vascular plants. The flora of Perú is extremely rich, and its territory is home to some 25,000 species, almost 10% of all plants in the world. However, the percentage of them scientifically studied is quite low1. Phytochemical research on Peruvian biodiversity proved to be fundamental in the development of modern medicine, e.g. the isolation of cocaine from Erythroxylum coca was a milestone in the development of local anesthetics2, similarly the isolation of the first antimalarial agent, quinine from Cinchona ledgeriana cortex initiated “the alkaloids golden age”3. Most of those phytochemical investigations were conducted overseas, a fact that reflects the absence or the restricted access of resources and infrastructure for developing classical phytochemical research in Peru. Today, modern platforms maybe applied for the metabolic characterization of Peruvian flora, a task that can be achieved by a liquid chromatography associated with high resolution mass spectrometry (LC-HRMS) method since it is less time consuming compared to classic methods of isolation and structure identification. Some recent investigations that exemplify the use of LC-HRMS for describing the phytochemical profile of Peruvian flora include the metabolic profile on medicinal plants of the genus Chuquiraga (Asteraceae)4 and that related to Capsicum (Solanaceae) fruits5.
Perú has several traditional medicine systems, that of the northern Andes6,7, that of the southern Andes8 and that of the Amazonian forest9, each one of them with its main and minor plants and particular practices. With the passage of time, those traditional medicines are getting combined a fact that is especially noticeable in Lima city, the capital of Peru10. One aspect that is worth to highlight is that, especially in Andean medicines, but not in Amazonian ones, there is an important contribution of plants belonging to the Lamiaceae family to the traditional medicine systems.
The large family Lamiaceae has twelve subfamilies. The Nepetoideae subfamily, with 3400 species and 105 genera, has three tribes11: Elsholtzieae, Ocimeae and Mentheae, the latter with 65 genera. The Mentheae tribe is chemically characterized by having volatile terpenoids and a phenolic acid called rosmarinic acid that makes these plants aromatic and with medicinal properties12,13 Mentheae can also be classified into 3 subtribes: Menthinae (43 genera), Salviinae (10 genera) and Nepetiinae (12 genera)14,15. In Peru (Herbario Nacional Universidad de San Marcos-Perú, October 2017), the main genera of Mentheae were Clinopodium (29 species), Hedeoma (1 specie), Lepechinia (11 species), Minthostachys (7 species) and Salvia (60 species). Clinopodium, Hedeoma, and Minthostachys belong to the Menthinae subtribe, while Lepechinia and Salvia belong to the Salviinae subtribe. Investigations on the non-volatile components in Peruvian Mentheae are relatively scarce compared to the works related to essential oils16 . In a previous work17 the contents of rosmarinic acid, triterpenic acids, oleanolic and ursolic were quantified in thirteen Peruvian Mentheae. The highest content of rosmarinic acid was observed in Lepechina meyenii (Walp.) Epling and the highest content of triterpenic acids in Clinopodium revolutum (Ruiz & Pavón) Govaerts. Subsequently18, the non-volatile compounds were unambiguously or reasonably identified in two Lepechinia species: L. meyenii and L. floribunda (Benth.) Epling, by LC-HRMS, where the presence of salvianolic acids and diterpenoids were notable.
LC-HRMS methods have been used to comprehensively analyze the phenolic components of plants, this implies procedures for the systematic manually identification of mass spectra19,20 and also the use of suitable software21,22, in both cases the procedure involves recording of diagnostic ions for classification and then the identification of characteristic ionic products and neutral losses for confirmation. In the present communication, the profile of secondary metabolites by LC-HRMS is reported for ten Peruvian Mentheae: Clinopodium (4 species), Salvia (4 species), Hedeoma (1 species) and Minthostachys (1 species).
Results
Phytochemical profile
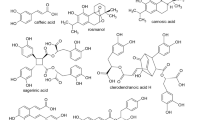
The LC-HRMS metabolite profile of the ethanolic extracts of the ten peruvian Mentheae was obtained in the negative mode (ESI (−)) and the detected compounds appear in Table 1. Assignments were made based on the literature21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37. Isomers of quinic acid (m/z 191.0556), danshensu (m/z 197.0450), protocatechuic aldehyde (m/z 137.0239), and caffeic acid (m/z 179.0350) occur in most plants. Equally abundant are the monocaffeoylquinic acids present in seven species. Minthostachys mollis contains four different monocaffeoylquinic acids. Several derivatives of ferulic acid and p-coumaric acid could also be identified. The 4 (para) substitution or the 3,4 substitution with respect to C3 cannot be determined by MS, however this is the substitution reported in Mentheae19,20,23,38,39,40,41,42,43,44,45,46,47,48,49. Caffeic acid, protocatechuic aldehyde and protocatechuic acid share the same substitution pattern. Furthermore, a diversity of flavonoids (flavonols, flavones, flavanones, flavanonols) was found in all the samples, both free and glycosylated. Minthostachys mollis, Clinopodium sericeum and Clinopodium pulchellum are the most diverse with respect to their flavonoids. The most frequent flavonoid aglycones were luteolin (m/z 285.0404), quercetin (m/z 301.0354), kaempferol (m/z 285.0404) and apigenin (m/z 269.0455). Eupatorin is present in five of the species studied50. In Clinopodium revolutum, apigenin and luteolin C- hexosides were detected. In all the samples the presence of rosmarinic acid (m/z 359.0772) was detected. In Clinopodium revolutum, salvianic acid C (m/z 377.0881), which is the result of hydrating the double bond of rosmarinic acid, has been detected, and, in Salvia sagitatta, teucrol (m/z 315.0880)51, a decarboxylated rosmarinic acid was observed. Isorinic acid (m/z 343.0827) a rosmarinic acid molecule without the 3-OH was present in Clinopodium brevicalyx, Salvia sagitatta, Salvia cuspidata and Hedeoma mandoniana. Methyl (m/z 373.0931) and ethyl (m/z 387.1088) esters of rosmarinic acid were present in Salvia cuspidata and Clinopodium brevicalyx. In Salvia cuspidata and Clinopodium revolutum, the dimer of rosmarinic acid, sagerinic acid (m/z 719.1598), which is a molecule with a stabilized cyclobutane ring, was found. Clinopodium pulchellum displayed the presence of salvianolic acid A (m/z 493.1143) and salvianolic acid F (m/z 313.0722). In Clinopodium brevicalyx, Clinopodium sericeum and Hedeoma mandoniana, the presence of salvianolic acid B (m/z 717.1443) was observed, a particularly important substance due to its effect on neurodegenerative diseases52. However, the plant with the greatest diversity of salvianolic acids was Clinopodium sericeum, "romero de jalca", in addition to salvianolic acid B, lithospermic acid (m/z 537.1038), two isomers of salvianolic acid A and two isomers of salvianolic acid F. This type of substances is very important due to its effect on cell fibrosis (scar formation) in direct relation to cancer53. Among the other substances found, it should be noted that the Rosmarinus type diterpenoids, common in Lepechinia18,54 are scarce in this work; only Salvia sagitatta and Salvia cuspidata show the presence of carnosol (m/z 329.1761) and the phenolic diterpenoid, rosmadial (m/z 343.1552) in the last one28. Salvia haenkei contains the ent-(5R,9R)-15,16-epoxy-10S-hydroxycleroda-3,7,13(16),14-tetraene-17,12S; 18,19 diolide (m/z 355.1190)26, while Salvia cuspidata had a lignan, isolariciresinol (m/z 359.1502) previously reported in Linum seeds31,55, and 5-epi-icetexone (m/z 341.1396) described as an anti- Trypanosoma cruzii molecule56. Oleanolic and ursolic triterpenic acids, quantified in a previous report by Serrano et al.17, do not appear in this analysis due to the elution program used, which does not reach 100% acetonitrile57. Figure 1 shows the typical ESI (−) chromatogram of Salvia sagitatta and Fig. 2 shows the chromatogram of Clinopodium sericeum. The chemical structures of the main metabolites detected are displayed in Fig. 3.
Discussion
This is the first time that the phytochemical profile has been obtained for the ten Peruvian Mentheae (Lamiaceae) here reported. The botanical genera studied were Salvia (Salviinae), Clinopodium, Hedeoma and Minthostachys (Menthinae). While Salvia and Clinopodium are genera of worldwide distribution, Hedeoma and Minthostachys are American and South American genera, respectively. All Salvia species in this work belong to the Salvia subgenus Calosphace Benth. (Epling)63. Assignments were based on the search for diagnostic ions, characteristic product ions and neutral losses19,20,25,40,41. The fragmentation patterns shown in said references are particularly useful for this work since they are specifically directed to Lamiaceae/Mentheae. The phytochemical profiles of those Mentheae here surveyed are quite similar to their European and Asian relatives. All the species analyzed show the presence of rosmarinic acid, while, quinic acid, 3,4-dihydroxyphenyl-lactic acid “danshensu”, protocatechuic aldehyde and caffeic acid are present in most of the samples. Monocaffeoylquinic acids, also called chlorogenic acids, are also frequent but better expressed in Minthostachys. Dicaffeoylquinic acid was detected only in Clinopodium revolutum. All samples contained flavonoids with more diversity in Minthostachys and Clinopodium. Flavonoid-free aglycones predominate in several plants: In Salvia sagitatta, cirsimaritin is abundant64, while eupatorin predominates in Clinopodium revolutum50, genkwanin in Salvia haenkei36 and hesperetin in Clinopodium pulchellum27. In several plants, rosmarinic acid is the main peak: Clinopodium brevicalyx, Salvia oppositiflora, Clinopodium sericeum and Hedeoma mandoniana. Some type of salvianolic acid is present in all the samples, although in some cases, they are very small modifications of the rosmarinic acid molecule. Dimers and trimers of rosmarinic acid are present in Clinopodium brevicalyx, Salvia oppositiflora, Salvia cuspidata, Clinopodium sericeum, Hedeoma mandoniana and Clinopodium pulchellum. In Clinopodium sericeum, not only is the diversity of salvianolic acids important but also their abundance in salvianolic acid A, which would allow the preparation of the said substance from it71.
Conclusion
Peruvian Mentheae are a rich source of flavonoids, phenolic acids and terpenoids. The present study involved LC-HRMS analysis of ten species. A total of 111 compounds were detected. Most of these were identified by key ion filtering strategy and comparison with literature data. This methodology can be used to the authentication and differentiation of larger numbers of Mentheae species: The San Marcos Herbarium, Lima-Perú, in 2017 had 108 Mentheae.
Methods
Plant material
The plants used in this study are as follows: Clinopodium brevicalyx Epling (Harley & Granda) (Menthinae) (HUT 59506), Salvia oppositiflora (R. and P.) (Salviinae) (HUT 59502), Minthostachys mollis Griseb. (Menthinae) (HUT 59766), Salvia sagittata R. and P. (Salviinae) (HUT59499), Salvia cuspidata subsp. cuspidata (R. and P.) (Salviinae) (HUT 59505), Clinopodium revolutum (R. and P.) (Menthinae) (HUT 58329), Clinopodium sericeum (Briq. et Benth) Govaerts (Menthinae) (HUT 58,332), Salvia haenkei Benth. (Salviinae) (HUT 59500), Hedeoma mandoniana Wedd. (Menthinae) (HUT 59763), Clinopodium pulchellum Kunth (Govaerts) (Salviinae) (HUT 59765). All of them were collected in Peru (2014–2018) by the author (C.S.) according to the procedures of the Universidad San Antonio Abad and following the guidelines of the Herbarium Truxillense of the Universidad Nacional de Trujillo (HUT)—Perú https://facbio.unitru.edu.pe. Specimens were identified and deposited by the botanist Eric Frank Rodríguez (Herbarium Truxillense).
Sample preparation for metabolite profiling
Fifty milligrams of pulverized aerial parts were subjected to an ultrasonic bath for five minutes with 1 mL of ethanol for three times. The filtrates were evaporated in vacuo and stored at 4 °C until use.
LC-HRMS
Chromatographic separation was performed on a Thermo Scientific Dionex Ultimate 3000 UHPLC system with an Acclaim RP C18 150 × 4.6 mm × 1.8 µm chromatographic column at 25 °C and a gradient of (a) 0.1% H2CO2 in water and (b) acetonitrile: [time, % (b)]: (0.5); (5,5); (10.30); (15.30); (20,70); (25.70); (35.5) and 12 min of equilibration before each injection. The flow rate was 1 mL min−1, and the injection volume was 10 μL. The extracts were dissolved in 1.5 mL of methanol and filtered through 0.22 µm PTFE. For high resolution mass spectrometry, a Q-Exactive MS (Thermo Fisher Germany) equipped with electrospray ionization (ESI) in negative mode was used. The MS collection parameters were as follows: spray voltage 2500 V; capillary temperature, 400 °C. Sheath gas flowed at a rate of 75 units. Auxiliary gas flowed at 20 units. Scanning range of 100–1500 m/z. Resolution of 35,000. The mass tolerance threshold was 5 ppm. Data acquisition and processing were performed with XCalibur 2.3 (Thermo Fisher Scientific).
Diagnostic ions for classification
Quinic acids derivatives: 337.0929 p-coumaroylquinic acid, 367.1035 feruloylquinic acid, 353.0878 caffeoylquinic acid, 515.1195 dicaffeoylquinic acid.
Phenylpropionic acids: 163.0401 p-coumaric acid, 179.0350 caffeic acid, 359.07772 rosmarinic acid.
Flavonoids: 253.0506 chrysin, 269.0455 apigenin, 285.0404 luteonin and kaemferol, 301.0354 quercetin.
Data availability
The datasets used and/or analyses during the current study are available from the corresponding author on reasonable request.
References
Lock, O., Pérez, E., Villar, M., Flores, D. & Rojas, R. Bioactive compounds from plants used in Peruvian traditional medicine. Nat. Prod. Commun. 11, 315–337 (2016).
Paulet, P. La cocaína. Boletín del Ministerio de Fomento del Perú 1, 23–42 (1903).
Roersch, A. Colonial agroindustrialism: Science, industry and the state in the dutch golden alkaloid age, 1850–1950. Doctoral Thesis University of Utrecht 2015.
Ccana-Ccapatinta, G. et al. High resolution liquid chromatography–mass spectrometry based metabolomics for the classification of Chuquiraga (Barnadesioideae, Asteraceae): New phenylpropanoid derivatives as chemical markers for Chuquiraga spinosa. J. Nat. Prod. 86, 683–693 (2023).
Espichán, F., Rojas, R., Quispe, F., Cabanac, G. & Marti, G. Metabolomic characterization of 5 native Peruvian chili peppers (Capsicum spp.) as a tool for species discrimination. Food Chem. 386, 132704 (2022).
Vásquez, L., Escurra, J., Aguirre, R., Vásquez, G., Vásquez, L. Plantas Medicinales del Norte del Perú. FINCyT. Lambayeque 2010.
Bussmann R., Sharon, D. Plantas de los Cuatro Vientos. Flora Mágica y Medicinal del Perú. Trujillo 2007.
Roersch, C. Plantas Medicinales del Sur Andino del Perú (Koeltz Scientific Books, 1994).
Rutter, R. Catálogo de Plantas Útiles de la Amazonía del Perú (Instituto Lingüístico de Verano, 1990).
Bussmann, R., Paniagua, N., Castañeda, R., Prado, Y. & Mandujano, J. Health in a pot. The ethnobotany of emolientes and emolienteros in Perú. Econ. Bot. 69, 83–88 (2015).
Zhao, F. et al. An updated tribal classification of Lamiaceae based on plastome phylogenomics. BMC Biology 19, 2 (2021)
Wink, M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 64(1) 3–19 (2003).
Amoah, S., Sandjo, L., Kratz, J. & Biavatti, M. Rosmarinic acid-pharmaceutical and clinical aspects. Planta Med. 82, 388–406 (2016).
Bräuchler, C., Heubl, G. & Meimberg, H. Molecular phylogeny of Menthinae (Lamiaceae, Nepetoideae, Mentheae)-taxonomy, biogeography and conflicts. Mol. Phylogenet. Evol. 55, 501–523 (2010).
Drew, B. & Sytsma, K. Phylogenetics, biogeography, and staminal evolution in the tribe Mentheae (Lamiaceae). Am. J. Bot. 99, 933–953 (2012).
Senatore, F. & de Feo, V. Flavonoid glycosides from Minthostachys spicata (Lamiaceae). Biochem. Syst. Ecol. 23, 573–574 (1995).
Serrano, C., Villena, G. & Rodríguez, E. Algunos componentes fitoquímicos y actividad antioxidante en representantes de la tribu Mentheae (Lamiaceae) del Perú. Arnaldoa 27, e101–e107 (2020).
Serrano, C., Villena, G. & Rodríguez, E. Phytochemical profile and rosmarinic acid purification from two peruvian Lepechinia Willd. Species (Salviinae, Mentheae, Lamiaceae). Sci. Rep. 11, 7260 (2021).
Qiao, X. et al. A targeted strategy to analyze untargeted mass spectral data: Rapid chemical profiling of Scutellaria baicalensis using ultra high performance liquid chromatography coupled with hybrid quadrupole orbitrap mass spectrometry and key ion filtering. J. Chromatogr. A 1441, 83–95 (2016).
Li, J. et al. Characterization of the multiple chemical components of Glechomae herba using ultra high performance liquid chromatography coupled to quadrupole time of flight tandem mass spectrometry with diagnostic ion filtering strategy. J. Sep. Sci. 42, 1312–1322 (2019).
Shan, Q., Cao, G., Cai, H., Cong, X. & Cai, B. Novel software based method to classify structurally similar compounds combined with high performance liquid chromatography-quadrupole time of flight mass spectrometry to identify complex components of herbal medicines. J. Chromatogr. A 1264, 13–21 (2012).
Cerrato, A. et al. A new software-assisted analytical workflow based on high-resolution mass spectrometry for the systematic study of phenolic compounds in complex matrices. Talanta 209, 120573 (2020).
Taamalli, A. et al. LC–MS-based metabolite profiling of methanolic extracts from the medicinal and aromatic species Mentha pulegium and Origanum majorana. Phytochem. Anal. 26, 320–330 (2015).
Li, C. et al. Precursor ion scan enhanced rapid identification of the chemical constituents of Danhong injection by liquid chromatography–tandem mass spectrometry: An integrated strategy. J. Chromatogr. A 1602, 378–385 (2019).
Luo, Y., Wen, Q., Jian Sheng, C., Feng, Y. & Tan, T. Characterization of the polymeric phenolic acids and flavonoids in Clerodendranthi spicati herba using ultra high performance liquid chromatography coupled to quadrupole time of flight tandem mass spectrometry with target and nontarget data mining strategy. Rapid Commun. Mass Spectrom. 33, 1884–1893 (2019).
Almanza, G. et al. Clerodane diterpenoids and an ursane triterpenoid from Salvia haenkei. Computer- assisted structural elucidation. Tetrahedron 53, 14719–14728 (1997).
Wianowska, D. & Gil, M. Recent advances in extraction and analysis procedures of natural chlorogenic acids. Phytochem. Rev. 18, 273–302 (2018).
Wang, L. et al. Determination and pharmacokinetic study of three diterpenes in rat plasma by UHPLC-ESI-MS/MS after oral administration of Rosmarinus officinalis L. Extract. Mol. 22, 934 (2017).
Fabre, N., Rustan, I., Hoffmann, E. & Quetin, J. Determination of flavone, flavonol, flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectroscopy. J. Am. Soc. Mass Spectrosc. 12, 707–715 (2001).
Yang, W. et al. Collision-induced dissociation of 40 flavonoid aglycones and differentiation of the common flavonoids subtypes using electrospray ionization ion-trap tandem mass spectrometry and quadrupole time of flight mass spectrometry. Eur. J. Mass Spectrom. 18, 493–503 (2012).
Meagher, L., Beecher, G., Flanagan, V. & Li, B. Isolation and characterization of the lignans, isolariciresinol and pinoresinol, in flaxseed meal. J. Agric. Food Chem. 47, 3173–3180 (1999).
Troalen, L., Phillips, A., Peggie, D., Barran, P. & Hulme, A. Historical textile dyeing with Genista tinctoria L.: A comprehensive study by UPLC-MS/MS analysis. Anal. Methods 6(22), 8915–8923 (2014).
Choi, S., Lee, S. & Lee, K. A comparative study of hesperetin, hesperidin and hesperidin glucoside: Antioxidant, anti-inflammatory, and antibacterial activities in vitro. Antioxidants 11, 1618 (2022).
Ciric, A., Prosen, H., Jelikic, M. & Durdevic, P. Evaluation of matrix effect in determination of some bioflavonoids in food samples by LC–MS/MS method. Talanta 99, 780–790 (2012).
Sohuila, T., Zohra, B. & Tahar, H. Identification and quantification of phenolic compounds of Artemisia herba-alba at three harvest time by HPLC–ESI–Q-TOF–MS. Int. J. Food Prop. 22, 843–852 (2019).
Song, Y., Zhang, S., Liu, H. & Jin, X. Determination of genkwanin in rat plasma by liquid chromatography-tandem mass spectrometry: Application to a bioavailability study. J. Pharm. Biomed. Anal. 84, 129–134 (2013).
Castañeta, G. et al. Untargeted metabolomics by using UHPLC-ESI-MS/MS of an extract obtained with ethyl lactate Green solvent from Salvia rosmarinus. Separations 9, 327 (2022).
Sharma, Y., Velamuri, R., Fagan, J. & Schaefer, J. Full-spectrum analysis of bioactive compounds in rosemary (Rosmarinus officinalis L.) as influenced by different extraction methods. Molecules 25, 4599 (2020).
Velamuri, R., Sharma, Y., Fagan, J. & Schaefer, J. Application of UHPLC-ESI-QTOF-MS in phytochemical profiling of sage (Salvia officinalis) and rosemary (Rosmarinus officinalis). Planta medica Int. Open 7, e133–e144 (2020).
Li, Q. et al. Chemical constituents and quality control of two Dracocephalum species based on high-performance liquid chromatographic fingerprints coupled with tandem mass spectrometry and chemometrics. J. Sep. Sci. 21, 4071–4085 (2016).
Shen, Y. et al. Rapid profiling of polymeric phenolic acids in Salvia miltiorrhiza by hybrid data-dependent/targeted multistage mass spectrometry acquisition based on expected compounds prediction and fragment ion searching. J. Sep. Sci. 41, 1888–1895 (2018).
Boudair, T., Lozano, J., Boulaem, H., del Mar, M. & Segura, A. Phytochemical characterization of bioactive compounds composition of Rosmarinus eriocalyx by RP-HPLC-ESI-QTOF-MS. Nat. Prod. Res. 33, 2208–2214 (2019).
Mena, P. et al. Phytochemical profiling of flavonoids, phenolic acids, terpenoids, and volatile fraction of a rosemary (Rosmarinus officinalis L.) extract. Molecules 21, 1576 (2016).
Sulniuté, V., Pukalskas, A. & Venskutonis, P. Phytochemical composition of fractions isolated from ten Salvia species by supercritical carbon dioxide and pressurized liquid extraction methods. Food Chem. 224, 37–47 (2017).
Barros, L. et al. Phenolic profiles of cultivated, in vitro cultured and comercial samples of Melissa officinalis L. infusions. Food Chem. 136, 1–8 (2013).
Fialová, S., Slobodnikova, L., Veizerova, L. & GranCai, D. Lycopus europaeus: Phenolic fingerprint, antioxidant activity and antimicrobial effect on clinical Staphylococcus aureus strains. Nat. Prod. Res. 29, 2271–2274 (2015).
Miron, T., Herrero, M. & Ibáñez, E. Enrichment of antioxidant compounds from lemon balm (Melissa officinalis) by pressurized liquid extraction and enzyme-assisted extraction. J. Chromatogr. A 1288, 1–9 (2013).
Guo, Z., Liang, X. & Xie, Y. Qualitative and quantitative analysis on the chemical constituents in Orthosiphon stamineus Benth. using ultra high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 164, 135–147 (2019).
Liu, A., Guo, H., Ye, M., Lin, Y. Detection, characterization and identification of phenolic acids in Danshen using high-performance. liquid chromatography with diode array detection and electrospray ionization mass spectrometry. J. Chromatogr. A 1161, 170–82 (2007).
Li, L. et al. Identification of metabolites of eupatorin in vivo and in vitro based on UHPLC-Q-TOF-MS/MS. Molecules 24, 2658 (2019).
Amani, M. et al. Teucrol, a decarboxyrosmarinic acid and its 4′-O-triglycoside, teucroside from Teucrium pilosum. Phytochemistry 55, 927–931 (2000).
Zhao, R., Liu, X., Zhang, L., Yang, H. & Zhang, Q. Current progress of research on neurodegenerative diseases of salvianolic acid B. Oxid. Med. Cell. Longev. 2019, 3281260 (2019).
Ma, L., Tang, L. & Yi, Q. Salvianolic acids: Potential source of natural drugs for the treatment of fibrosis and cancer. Front. Pharmacol. 10, 1–13 (2019).
Chabán, M. et al. Antibacterial effects of extracts obtained from plants of Argentina: Bioguided isolation of compounds from the anti-infectious medicinal plant Lepechinia meyenii. J. Ethnopharmacol. 239, 111930 (2019).
Fischer, U., Jacksh, A., Carle, R. & Kammerer, D. Determination of lignans in edible and nonedible parts of pomegranate (Punica granatum L.) and products derived therefrom, particularly focusing on the quantitation of isolariciresinol using HPLC-DAD-ESI/MSn. J. Agric. Food Chem. 60, 283–292 (2012).
Nieto, M., García, E., Giordano, O. & Tonn, C. Icetexane and abietane diterpenoids from Salvia gilliesi. Phytochemistry 53, 911–915 (2000).
Avula, B. et al. Comparative analysis of five Salvia species using LC-DAD-QToF. J. Pharm. Biomed. Anal. 209, 114520 (2021).
Gil, M. & Wianowska, D. Chlorogenic acids-their properties, occurrence and analysis. Annales Universitatis Mariae Curie-Sklodowska Lublin Polonia LXXII, 61–104 (2017).
Schutz, K., Kammerer, D., Carle, R. & Schieber, A. Identification and quantification of caffeoylquinic acids and flavonoids in artichoke (Cynara scolymus L.) heads, juice and pomace by HPLC-DAD-ESI/MSn. J. Agric. Food Chem. 52, 4090–4096 (2004).
Abu Reidah, I. et al. HPLC–ESI-Q-TOF-MS for a comprehensive characterization of bioactive phenolic compounds in cucumber whole fruit extract. Food Res. Int. 46, 108–117 (2012).
Krzyzanowska, J., Pecio, L., Moldoch, J., Ludwiczuk, A. & Kowalczyk, M. Novel phenolic constituents of Pulmonaria officinalis L. LC-MS/MS comparison of spring and autumn metabolites profiles. Molecules 23, 2227 (2018).
Kim, S. et al. PubChem 2023 update. Nucleic Acids Res. 51, 1373–1380 (2023).
Gonzáles Gallegos, J. et al. Richness and distribution of Salvia subg. Calosphace (Lamiaceae). Int. J. Plant Sci. 181, 831–856 (2020).
Benali, T. et al. The current state of knowledge in biological properties of cirsimaritin. Antioxidants 11, 1842 (2022).
Satake, T. et al. Studies on the constituents of fruits of Helicteres isora L. Chem. Pharm. Bull. 47, 1444–1447 (1999).
Misic, D. et al. Simultaneous UHPLC/DAD/(+/−)HESI-MS/MS analysis of phenolic acids and nepetalactones in methanol extracts of Nepeta species: A possible application in chemotaxonomic studies. Phytochem. Anal. 26, 72–85 (2015).
Keckes, S. et al. The determination of phenolic profiles of Serbian unifloral honeys using ultra-high-performance liquid chromatography/high resolution accurate mass spectrometry. Food Chem. 138, 32–40 (2013).
Ertas, A. et al. A detailed study on the chemical and biological profiles of essential oil and methanol extract of Thymus nummularius (Anzer tea): Rosmarinic acid. Ind. Crops Prod. 67, 336–345 (2015).
Brito, A., Ramírez, J., Areche, C., Sepúlveda, B. & Simirgiotis, M. HPLC-UV-MS profiles of phenolic compounds and antioxidant activity of fruits from three Citrus species consumed in Northern Chile. Molecules 19, 17400–17421 (2014).
Peter, S., Peru, K., Fahlman, B., McMartin, M. & Headley, J. The application of HPLC ESI MS in the investigation of the flavonoids and flavonoid glycosides of a Caribbean Lamiaceae plant with potential for bioaccumulation. J. Environ. Sci. Health B 50, 819–826 (2015).
Lu, L., Zhang, H., Qian, Y. & Yuan, Y. Isolation of salvianolic acid A, a minor phenolic carboxylic acid of Salvia miltiorrhiza. Nat. Prod. Commun. 5, 805–808 (2010).
Acknowledgements
CS thanks to Proyecto “Cuatro Moléculas” and Vicerrectorado de Investigación de la Universidad Nacional de San Antonio Abad del Cusco-Perú (CIPCU 003-2021-UNSAAC). Special thanks to Dr. Carlos Areche, Chemistry Department, Universidad de Chile, For LC-HRMS experiments.
Author information
Authors and Affiliations
Contributions
C.S., G.V., G.C., M.L. conception, design of the work. C.S., G.V., G.C. wrote the main manuscript. E.R., B.C. plant material collection and taxonomical identification. C.S., G.V. and M.L. phytochemical analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Serrano, C.A., Villena, G.K., Rodríguez, E.F. et al. Phytochemical analysis for ten Peruvian Mentheae (Lamiaceae) by liquid chromatography associated with high resolution mass spectrometry. Sci Rep 13, 10714 (2023). https://doi.org/10.1038/s41598-023-37830-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-37830-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.