Abstract
To study the clinical significance of autoantibodies in Chinese patients with new-onset systemic lupus erythematosus (SLE), we enrolled 526 new-onset patients who met the 1997 Updated American College of Rheumatology SLE Classification Criteria for a retrospective cohort study. Chi-square test and Wilcoxon rank-sum test were used to detect the relationship of autoantibodies with clinical manifestations and serological results respectively. Our results demonstrated that the positive rate of anti-ribosomal P protein (anti-P) antibody in female patients was higher than that in male patients (41.2% vs. 22%, P = 0.008). Patients with anti-SSB (43.95 ± 73.12 vs. 40.92 ± 75.75, P = 0.004; 63.93 ± 103.56 vs. 55.06 ± 120.84, P = 0.008 respectively) antibodies had higher levels of alanine aminotransferase (ALT) and aspartate transaminase (AST), whereas those with anti-P antibody (28.90 ± 25.70 vs. 50.08 ± 93.00, P = 0.014; 38.51 ± 48.19 vs. 69.95 ± 142.67, P = 0.047, respectively) had lower levels of them. Anti-dsDNA antibody (P = 0.021) was associated with pulmonary arterial hypertension (PAH). The patients with anti-Ro60 (P = 0.044), anti-P (P = 0.012) and anti-dsDNA (P = 0.013) antibodies were less likely to develop Interstitial lung disease. Anti-SmRNP antibody was correlated to lower prevalence of neuropsychiatric symptoms (P = 0.037), and patients with anti-centromere antibody (ACA) were more likely to develop serositis (P = 0.016).We identified five clusters of SLE-related autoantibodies, confirmed previously reported associations of autoantibodies, and discovered new associations.
Similar content being viewed by others
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease related to genetic as well as environmental factors like viral infection and drugs, which induce production of specific autoantibodies. The immunocomplexes formed by these autoantibodies with autoantigens are deposited in capillaries, leading to systemic injuries1,2. Therefore, SLE has a broad spectrum of clinical manifestations3. However, the exact pathological basis of SLE remains unclear.
Some autoantibodies show high diagnostic sensitivity and specificity for SLE. For instance, anti-nuclear antibody (ANA), anti-double stranded DNA (anti-dsDNA) antibody and anti-Sm antibody have been included in the American College of Rheumatology (ACR) as diagnostic markers of SLE4. Furthermore, some of these autoantibodies are directly related to clinical manifestation. For e.g., the anti-dsDNA antibodies have been linked with nephritis5, and anti-RNP antibodies with Reynold’s phenomenon6. Therefore, a greater understanding of the relationship between autoantibodies and clinical manifestations can help predict organ injury and identify the SLE patients with high risk of developing complication for timely intervention. There are much more researches on anti-dsDNA, anti-Sm, anti-nucleosome antibodies. However, only a few studies have been conducted on other autoantibodies, and the results are inconsistent. Furthermore, little is known regarding the diagnostic role of these autoantibodies in patients with new-onset SLE.
This research aims at exploring the relationship between SLE-related autoantibodies, including anti-dsDNA, anti-Sm, anti-ribosomal P protein (anti-P), anti-chromatin, anti-SSA/Ro60 (anti-Ro60), anti-SSA/Ro52 (anti-Ro52), anti-SSB, anti-centromere and anti-SmRNP antibodies, and clinicopathological features such as sex, age, disease activity, serological results and clinical manifestations in Chinese patients with new-onset SLE by retrospective cohort study.
Methods
Study population
According to Rao’s study7, to achieve clinically significant difference of clinical features and laboratory data between groups , a minimum sample size of 120 gives adequate study power to detect differences between groups (α = 0.05, power = 80%, two tailed test). A total of 526 new-onset SLE patients who met the 1997 updated ACR SLE classification criteria were enrolled between 2012 and 2021 from the First Affiliated Hospital of Bengbu Medical College after giving verbal informed consent. The basic information, clinical manifestations and serological results were collected during hospitalization. Disease activity was measured by the SLEDAI-2000 criteria. Ninety-one patients (17.3%) had late-onset SLE with age of diagnosis ≥ 50 years8.
The study had received ethical approval by the Ethics Committee of the First Affiliated Hospital of Bengbu Medical College [No. 2022 (149)].
Clinical symptoms and complications
According to 1997 update ACR criteria4, we collected the information of the following symptoms. Facial rash includes malar rash and discoid rash. The clinical feature of arthritis is tenderness, swelling, or effusion of 2 or more peripheral joints. The diagnosis of these symptoms and oral ulcerations (oral or nasal ulcerations) mainly depends on physical exam and case history. The symptoms of neuropsychiatric systemic lupus erythematosus (NPSLE) are seizures or psychosis (exclude drug or known metabolic derangements). Diagnosis can be aided by a combination of clinical manifestations, as well as and CT and MRI. Those with persistent proteinuria > 0.5 g or 3 + or cellular casts are diagnosed with renal disorder. The main diagnostic method of serositis (including pleuritis and percarditis) is imaging examination including ultrasonic examination and CT.
Furthermore, we registered other common or special symptoms and complications9. Patients with fever (excluding infection), alopecia, Reynold’s phenomenon and appendicular rash can be diagnosed by physical exam and case history. The diagnosis of interstitial lung disease (ILD) and pulmonary arterial hypertension (PAH) depends on HRCT and echocardiography, respectively.
Immunological tests
ANA levels in 475 patients were tested by immunofluorescence assay (EUROIMMUN, China), and in 22 patients by ELISA (KHB, Shanghai, China). Other autoantibodies were detected by line immunoassay (BioPlex 2200, Bio-Rad).
Statistical analysis
Statistical analysis was performed using SPSS 16.0. Continuous variables were described as mean ± standard deviation. Using the Kolmogorov–Smirnov test to verify normal distribution of variables. The links between sex, age at diagnosis, clinical manifestations and autoantibodies were determined by the Chi-square test. The Wilcoxon rank-sum test was used to compare the laboratory measurements and disease activity between patients positive and negative for the autoantibodies. The correlation between the different autoantibodies were detected using Cluster analysis with Ward’s method. A value of P < 0.05 was considered significant for the above-mentioned tests.
Ethical approval and consent to participate
Informed consent was obtained from all subjects and their legal guardians. The study had received ethical approval by the Ethics Committee of the First Affiliated Hospital of Bengbu Medical College. All methods were carried out in accordance with relevant guidelines and regulations.
Results
Characteristics of study subjects
The demographic characteristics, disease activity and clinical manifestations of 526 new-onset SLE patients are summarized in Table 1. The median age of patients at diagnosis was 36.52 ± 14.31 years, and 90.5% of the patients were women. The majority of patients presented medium to high disease activity (> 70%), and more than half of the patients had fever (50.2%). In addition, the common initial symptom were arthritis (48.7%), facial rash (34.0%), renal disorder (33.5%), serositis (19.2%), appendicular rash (18.1%), Reynold’s phenomenon (17.7%), alopecia (16.0%), and ILD (11.2%). In contrast, PAH, oral ulcerations and NPSLE were less than 8% frequent.
Prevalence of autoantibodies
As shown in Table 2, 97.4% of 497 patients tested positive for the anti-ANA antibody. In addition, the anti-chromatin antibody also had a high positive rate of 72.6%, followed by the anti-dsDNA (69%), anti-Ro60 (66%), anti-SmRNP (62.4%), anti-Sm (56.3%), anti-Ro52 (51.5%), anti-P (39.4%) and anti-SSB (28.7%) antibodies. In contrast, less than 5% of the patients were positive for the anti-centromere antibody.
The association of autoantibodies with sex and age
The relationship between sex, age at onset and autoantibodies is shown in Table 3. The prevalence of anti-P antibody was significantly higher in female patients compared to that in male patients (41.2% vs 22.0%, P = 0.008). Furthermore, the positive rate of anti-Sm antibody in early-onset patients was 58.9% compared to 44% in the late-onset patients (P = 0.009).
The association of autoantibodies with disease activity and laboratory data
The relationship between disease activity, laboratory data and autoantibodies is shown in Table 4. The SLEDAI score was significantly higher in patients with the anti-dsDNA (P < 0.001), anti-P (P = 0.042) and anti-chromatin (P = 0.001) antibodies compared to patients lacking these autoantibodies. In addition, the prevalence of anti-dsDNA (P < 0.001), anti-P (P < 0.001), anti-Ro60 (P = 0.009), anti-Ro52 (P = 0.014) and anti-SSB (P = 0.004) antibodies could result in a significantly rapider erythrocyte sedimentation rate (ESR). The presence of anti-Sm (P = 0.001), anti-Ro60 (P < 0.001), anti-Ro52 (P < 0.001), anti-SSB (P < 0.001) and anti-SmRNP (P = 0.001) antibodies correlated with higher levels of Immunoglobulin G (IgG), whereas patients with anti-SSB antibodies (P = 0.006) had lower concentrations of Immunoglobulin A (IgA). Complement C3 and C4 were significantly lower in patients with anti-dsDNA (P < 0.001), anti-P (P = 0.003; P = 0.001 respectively), anti-SSB (P < 0.001; P = 0.001, respectively) and anti-chromatin (P = 0.001; P = 0.023, respectively) antibodies, and patients with anti-centromere antibodies (P = 0.041) had lower levels of complement C4. Furthermore, subjects with anti-dsDNA (P < 0.001), anti-Ro60 (P = 0.002), anti-Ro52 (P = 0.003) and anti-SSB (P < 0.001; P = 0.013, respectively) antibodies had lower levels of hemoglobin (Hb) and lower white blood cell (WBC) counts. The anti-SmRNP antibodies (P = 0.021) correlated with higher Hb levels, and the anti-centromere antibodies (P = 0.039) with lower WBC counts. While anti-dsDNA (P = 0.038) and anti-SSB (P = 0.031) antibodies correlated significantly with lower platelet (Plt) count, patients with anti-SmRNP antibodies (P = 0.011) had a higher Plt count. Patients with anti-SSB (P = 0.004; P = 0.008, respectively) antibodies had higher levels of alanine aminotransferase (ALT) and aspartate transaminase (AST), but patients with anti-P (P = 0.014; P = 0.047, respectively) had a lower levels of ALT and AST. Moreover, patients with anti-Sm antibody (P = 0.022) had a higher levels of AST, whereas the presence of anti-SmRNP antibody (P = 0.032) was associated with lower AST levels. The prevalence of anti-dsDNA (P < 0.001), anti-Ro60 (P = 0.020) and anti-SSB (P = 0.021) antibodies were related to lower albumin (Alb) concentrations. Finally, anti-dsDNA (P = 0.049; P = 0.037 respectively) correlated significantly with higher levels of serum creatinine (sCr) and blood urea nitrogen (BUN), whereas the anti-P antibody (P = 0.035; P = 0.008, respectively) correlated with lower levels of these indicators.
The association of autoantibodies with clinical manifestations
The correlation between clinical manifestations and the different autoantibodies are summarized in Table 5. Fever was significantly associated with the presence of anti-dsDNA (P = 0.009), anti-P (P = 0.031) and anti-SmRNP (P = 0.041) antibodies. Facial rash was more frequent in patients positive for anti-dsDNA (P = 0.013), anti-P (P < 0.001) and anti-SmRNP (P = 0.031) antibodies, whereas patients with anti-R52 antibody (P = 0.039) were less likely to develop facial rash. Alopecia was associated with anti-dsDNA (P = 0.020), anti-Sm (P = 0.020) and anti-SmRNP (P = 0.034) antibodies, Reynold’s phenomenon with anti-Sm (P = 0.007) and anti-SmRNP (P < 0.001) antibodies, and serositis with anti-dsDNA (P = 0.014), anti-Ro52 (P = 0.047) and anti-centromere (P = 0.016) antibodies. While the presence of anti-P (P = 0.003) and anti-SmRNP (P = 0.001) antibodies correlated with a higher risk of appendicular rash, patients with anti-SSB antibody (P = 0.038) were less likely to develop the same. PAH was associated significantly with the presence of anti-dsDNA antibody (P = 0.021), whereas the anti-P antibody (P = 0.009; P = 0.012, respectively) was correlated with a lower risk of PAH and ILD. In addition, the patients with anti-dsDNA (P = 0.013) and anti-R60 (P = 0.044) antibodies were less likely to develop ILD. Renal disorder was associated with the presence of anti-dsDNA (P < 0.001) and anti-SSB (P = 0.006) antibodies. Anti-SmRNP antibody was also correlated with oral ulcerations (P = 0.026), whereas patients with anti-SmRNP antibody (P = 0.037) were less likely to develop NPSLE. There was no relationship between the different autoantibodies and arthritis.
The relationship between autoantibodies
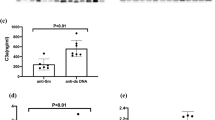
The relationship between autoantibodies were detected by Cluster analysis. As shown in Fig. 1, the autoantibodies were classified into 5 clusters. Cluster 1 included the anti-Sm and anti-SmRNP antibodies, and Cluster 2 was composed of anti-R60 and anti-R52 antibodies. Both clusters were formed early in the disease course. Cluster 3 comprised of anti-dsDNA and anti-chromatin antibodies, Cluster 4 included the anti-SSB and anti-centromere antibodies, and Cluster 5 included the anti-P antibody.
The relationship between the autoantibodies were detected by Cluster analysis. Chrom: chromatin; cen: centromere. Cluster 1 was composed of anti-Sm antibody and anti-SmRNP antibody. Cluster 2 was composed of anti-R60 antibody and anti-R52 antibody. Cluster 3 was composed of anti-dsDNA antibody and anti-chromatin antibody. Cluster 4 was composed of anti-SSB and anti-Centromere antibodies. Cluster 5 was composed of anti-P antibody.
Discussion
SLE is characterized by the presence of specific autoantibodies1. The therapy which focus on autoantibodies has been cared much. For example, blocking anti-dsDNA antibodies in a mouse model of SLE alleviated organ injury5. In this study, we retrospectively analyzed the correlation of several autoantibodies with sex, age at onset, disease activity, laboratory data and clinical manifestations in Chinese patients with new-onset SLE, in order to identify novel therapy.
In this study, fever and arthritis are common initial symptoms, which was also observed in other study9,10. Arthritis were more prevalent in some studies11,12, which may be attributed to different definition of it. The prevalence of facial rash from our study was similar to the study by Sebastiani et al.11, but lower than that in some previous studies12,13. Meanwhile, the ocurring rate of appendicular rash which is part of skin manifestation, alopecia and Reynold’s phenomenon lower than that in other studies10,12,13. Skin manifestation is influenced by sun-exposing, and discoid rash can induce alopecia9,11. Cold stimulation is a cause of Reynold’s phenomenon. The difference may be due to geological location, climate, as well as genetic factors. NPSLE with more nonspecific symptoms like headache and mood disorder is difficult to diagnose. Therefore, the prevalence of NPSLE was different in previous cohorts, but still a rare initial presentation11,13. The incidence of serositis in our cohort was consistent with the finding of Leuchten et al.13, but lower than that in study by Sebastiani et al.11. The first incidence rate of serositis which diagnosis is dependent on the imaging examination may be underestimated. The frequency of renal disorder was essentially comparable with that from other researches11,13. We did not find the first incidence rate of PAH and ILD in previous studies, which are rare symptoms, but the outcomes were coincide with the incidence of them in the course of SLE14.
The prevalence of anti-P (39.4%), anti-Ro60 (66.0%) and anti-Ro52 (51.5%) antibodies were higher in our cohort compared to that reported in previous studies15,16,17,18,19,20, which may be attributed to different ethnicities21.
The anti-dsDNA antibody is a reliable diagnostic biomarker for SLE16, and its presence is related to tissue damage in the kidneys, skin and brain5. Our results just conformed to the first two. The difference may be due to different course of disease and sample size. Another possible explanation is the difficulty of diagnosis for NPSLE and the rarity of it as initial symptom. We found that the anti-dsDNA antibody was associated with disease activity, leukopenia, anemia, serositis, thrombocytopenia, ESR, complement C4 and Alb, which is consistent with previous findings22,23,24. Higher ESR is the likely result of kidney damage, which in turn lowers Alb levels25. Furthermore, presence of the anti-dsDNA antibody was related to more serious kidney injury, lower levels of complement C3, fever, alopecia, PAH and less prevalence of ILD. According to the research by Li et al.26, the frequency of lupus nephritis, serositis and hypocomplementemia in patients with SLE and PAH were significant higher. Meanwhile, the disease activity of them were higher, and our results of association of anti-dsDNA were coincide with above symptoms. PAH and ILD were rare initial sign. We will collect more data of new-onset SLE paitents with them, and conduct follow-up studies in risk factors of these complications in the future.
The anti-Sm antibody is highly specific for SLE, and we detected significantly higher positive rate of this autoantibody in patients with early-onset SLE compared to those with late-onset disease. Previous studies have shown that SLE patients with anti-Sm antibody tend to be younger compared to those lacking the antibody17,27. In addition, the anti-Sm antibody was associated with Reynold’s phenomenon and elevated liver enzymes in our study, as demonstrated by other groups as well24,28. Alopecia and higher levels of IgG also correlated with the presence of anti-Sm antibody in our cohort. There is evidence that anti-Sm antibody is associated with disease activity17,29, renal disorder28,30,31 and lower levels of complement proteins31,32. The differences in the testing methods for anti-Sm antibody may explain the variations in results33.
The anti-P antibody has been previously associated with disease activity18,34, lower levels of complement34, fever18,21 and malar rash18,35,36 in SLE patients, which was also observed in our study. However, our findings contradict the previously reported correlation between anti-P antibody and renal disorder21,36,37. In fact, we found that the extent of kidney damage of patients with anti-P antibody was slighter than that without it. Furthermore, the liver function of patients with anti-P antibody was better compared to those lacking the antibody, which is also inconsistent with previous studies21,38. In a previous study39, the levels of anti- P antibody increased during the active phase of nephritis and resumed to normal in remission stage. In addition, the patient with anti-P antibody was not diagnosed with liver damage at early stage of SLE. Therefore, these discrepancies can be attributed to differences in course of disease, meanwhile, it also may be associated with ethnicity and study design. More follow-up is warranted to observe the long-term complications. Moreover, the presence of anti-P antibody was correlated to ESR, appendicular rash, and lower prevalence of PAH and ILD in our study. However, we did not observe any correlation between anti-P antibody and NPSLE which is difficult to diagnose. One noteworthy finding was that the prevalence of anti-P antibody was higher in female patients than in male patients.
The prevalence of anti-SSA and anti-SSB antibodies was higher in SLE patients with secondary Sjogren’s syndrome. Earlier studies have demonstrated that anti-SSA (Ro) antibody is related to hemocytopenia40 and ILD41. Our findings regarding the former were similar, whereas that regarding ILD were contradictory, which can be attributed to differences in sample size and ethnic groups. Moreover, ILD mostly occurred in long-course patients14, different course of disease may explain the discrepancy. We also found that anti-SSA antibody was correlated to ESR and higher levels of IgG. In addition, we observed a correlation between anti-Ro60 antibody and lower Alb concentrations. The presence of the anti-Ro52 antibody was related to lower prevalence of malar rash, which contradicts the findings of Harley et al.,40 which can be explained by different definition. Some studies have reported an association between anti-SSA antibody and PAH26,42, and the risk factors of PAH are pericarditis and pleurisy26, which were not observed in our study. However, we detected a correlation between the anti-Ro52 antibody and serositis, which has been reported previously24. There may be a potential link between anti-SSA and PAH, and further studies are needed to verify this hypothesis. Previous studies have shown that anti-SSB antibody is associated with higher levels of IgG7, lower levels of Complement C323 and hematological symptoms7,42, which was confirmed in our study as well. In addition, we found that anti-SSB antibody correlated to ESR, lower levels of IgA and Complement C4, lower Alb concentrations, lower prevalence of appendicular rash, higher prevalence of renal disorder and more serious hepatic damage. We observed that the patients with anti-SSB antibody got lower levels of Hb, WBC and Plt which is initial hematological symptoms. It may be caused by disease activity, renal disorder or liver damage, and this new result need further follow-up observation.
The anti-centromere antibody (ACA) has been detected in subjects with CREST syndrome. The positivity rate of ACA in SLE patients in our study was 3.8%. In addition, ACA was correlated to lower levels of Complement C4, lower WBC count and serositis. However, we did not detect any association between ACA and Reynold’s phenomenon, most likely due to the few samples positive for ACA. The prevalence of anti-chromatin antibody (also called anti-nucleosome antibody) is high in SLE patients20, and is associated with disease activity and renal disorder20,43,44. We also detected an association between this antibody and disease activity but without renal disorder, which may be due to genetic, course of disease and ethnic influences. Furthermore, the anti-chromatin antibody was correlated to hypo-complementemia in our study. The anti-SmRNP antibody is derived from anti-RNP antibody45, and is associated with higher levels of IgG and Hb, higher Plt count, lower levels of AST, fever, skin manifestations, oral ulcerations, Reynold’s phenomenon, alopecia and lower prevalence of NPSLE. There is some overlap between the clinical manifestations of anti-SmRNP and anti-RNP antibodies, such as Reynold’s phenomenon46. Using cluster analysis, the autoantibodies were classified into 5 clusters. Only cluster 1 and cluster 5 fit well with previous studies42,47, which could be explained by the different autoantibodies we detected. In addition, anti-dsDNA antibody was highly relevant with anti-chromatin antibody in previous study, which was observed in our study (cluster 3)44.
Our study has certain salient features, such as a large cohort and all patients with new-onset SLE. Thus, our findings are more relevant in terms of identifying targets for delaying the progression of SLE, since because the prevalence of autoantibodies can change during disease course48. Nevertheless, we could not examine the changes in the spectrum and levels autoantibodies during the course of SLE due to the cross-sectional design of our study. Second, our cohort consisted of only Chinese patients, and the results may not be applicable to other ethnic populations. Third, samples with rare autoantibodies and clinical manifestations were few, and the findings will have to be validated with further studies.
In conclusion, detection of specific autoantibodies in SLE patients can predict organ injury and other complications, and aid in timely intervention. We recommend that patients with anti-dsDNA antibodies should undergo echocardiography to detect PAH in a timely manner, and liver function tests should be conducted for those with anti-SSB antibody. The blood routine examination need to be tested regularly for patients with anti-SSA, anti-SSB and anti-centromere antibodies. Patients with anti- dsDNA and anti-SSB antibody should pay attention to tests of renal function. Imaging examination of such as echocardiography and CT need to be performed to detect serositis for patients with anti-dsDNA, anti-Ro52 and anti-centromere antibodies.
Data availability
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.
References
Olsen, N. J. & Karp, D. R. Autoantibodies and SLE: The threshold for disease. Nat. Rev. Rheumatol. 10(3), 181–186. https://doi.org/10.1038/nrrheum.2013.184 (2014).
Xiao, Z. X., Miller, J. S. & Zheng, S. G. An updated advance of autoantibodies in autoimmune diseases. Autoimmun Rev. 20(2), 102743. https://doi.org/10.1016/j.autrev.2020.102743 (2021).
Fava, A. & Petri, M. Systemic lupus erythematosus: Diagnosis and clinical management. J. Autoimmun. 96, 1–13. https://doi.org/10.1016/j.jaut.2018.11.001 (2019).
Hochberg, M. C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 40(9), 1725. https://doi.org/10.1002/art.1780400928 (1997).
Wang, X. & Xia, Y. Anti-double stranded DNA antibodies: Origin, pathogenicity, and targeted therapies. Front. Immunol. 10, 1667. https://doi.org/10.3389/fimmu.2019.01667 (2019).
Sulcebe, G. & Morcka, K. Diagnostic and prognostic significance of different antinuclear antibodies in more than 1000 consecutive Albanian patients with rheumatic diseases. Clin. Exp. Rheumatol. 10(3), 255–261 (1992).
Rao, L. et al. Specificity of anti-SSB as a diagnostic marker for the classification of systemic lupus erythematosus. Exp. Ther. Med. 5(6), 1710–1714. https://doi.org/10.3892/etm.2013.1051 (2013).
Arnaud, L., Mathian, A., Boddaert, J. & Amoura, Z. Late-onset systemic lupus erythematosus: Epidemiology, diagnosis and treatment. Drugs Aging 29(3), 181–189. https://doi.org/10.2165/11598550-000000000-00000 (2012).
Kuhn, A. et al. The diagnosis and treatment of systemic lupus erythematosus. Deutsches Arzteblatt Int. 112(25), 423–432. https://doi.org/10.3238/arztebl.2015.0423 (2015).
Metry, A. M. et al. Systemic lupus erythematosus: Symptoms and signs at initial presentations. AntiInflamm. Antiallergy Agents Med. Chem. 18(2), 142–150. https://doi.org/10.2174/1871523018666181128161828 (2019).
Sebastiani, G. D., Prevete, I., Iuliano, A. & Minisola, G. The importance of an early diagnosis in systemic lupus erythematosus. Isr. Med. Assoc. J. IMAJ 18(3–4), 212–215 (2016).
Nossent, J. et al. Disease activity and damage accrual during the early disease course in a multinational inception cohort of patients with systemic lupus erythematosus. Lupus 19(8), 949–956. https://doi.org/10.1177/0961203310366572 (2010).
Leuchten, N. et al. Early symptoms of systemic lupus erythematosus (SLE) recalled by 339 SLE patients. Lupus 27(9), 1431–1436. https://doi.org/10.1177/0961203318776093 (2018).
Hannah, J. R. & D’Cruz, D. P. Pulmonary complications of systemic lupus erythematosus. Sem. Respir. Crit. Care Med. 40(2), 227–234. https://doi.org/10.1055/s-0039-1685537 (2019).
Pisetsky, D. S., Bossuyt, X. & Meroni, P. L. ANA as an entry criterion for the classification of SLE. Autoimmun. Rev. 18(12), 102400. https://doi.org/10.1016/j.autrev.2019.102400 (2019).
Dema, B. & Charles, N. Autoantibodies in SLE: Specificities, isotypes and receptors. Antibodies (Basel Switz.) 5(1), 2. https://doi.org/10.3390/antib5010002 (2016).
Flechsig, A. et al. What is the clinical significance of anti-Sm antibodies in systemic lupus erythematosus? A comparison with anti-dsDNA antibodies and C3. Clin. Exp. Rheumatol. 35(4), 598–606 (2017).
Abraham, M. & Derk, C. T. Anti-ribosomal-P antibodies in lupus nephritis, neuropsychiatric lupus, lupus hepatitis, and Chagas’ disease: Promising yet limited in clinical utility. Rheumatol. Int. 35(1), 27–33. https://doi.org/10.1007/s00296-014-3058-3 (2015).
Didier, K. et al. Autoantibodies associated with connective tissue diseases: What meaning for clinicians?. Front. Immunol. 9, 541. https://doi.org/10.3389/fimmu.2018.00541 (2018).
Burlingame, R. W. & Cervera, R. Anti-chromatin (anti-nucleosome) autoantibodies. Autoimmun. Rev. 1(6), 321–328. https://doi.org/10.1016/s1568-9972(02)00083-6 (2002).
Pasoto, S. G., Viana, V. S. & Bonfa, E. The clinical utility of anti-ribosomal P autoantibodies in systemic lupus erythematosus. Expert Rev. Clin. Immunol. 10(11), 1493–1503. https://doi.org/10.1586/1744666X.2014.966692 (2014).
Conti, F. et al. Systemic Lupus Erythematosus with and without Anti-dsDNA antibodies: Analysis from a large monocentric cohort. Med. Inflamm. 2015, 328078. https://doi.org/10.1155/2015/328078 (2015).
Correa-Rodríguez, M. et al. Clinical and serological associations of autoantibodies in patients with systemic lupus erythematosus. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 69(8), 1417–1425. https://doi.org/10.1136/jim-2021-001887 (2021).
Vilá, L. M. et al. Clinical and prognostic value of autoantibodies in puerto Ricans with systemic lupus erythematosus. Lupus 15(12), 892–898. https://doi.org/10.1177/0961203306069352 (2006).
Aringer, M. Inflammatory markers in systemic lupus erythematosus. J. Autoimmun. 110, 102374. https://doi.org/10.1016/j.jaut.2019.102374 (2020).
Li, M. et al. Chinese SLE treatment and research group (CSTAR) registry: II. Prevalence and risk factors of pulmonary arterial hypertension in Chinese patients with systemic lupus erythematosus. Lupus 23(10), 1085–1091. https://doi.org/10.1177/0961203314527366 (2014).
López, P., Mozo, L., Gutiérrez, C. & Suárez, A. Epidemiology of systemic lupus erythematosus in a northern Spanish population: Gender and age influence on immunological features. Lupus 12(11), 860–865. https://doi.org/10.1191/0961203303lu469xx (2003).
Arroyo-Ávila, M. et al. Clinical associations of anti-Smith antibodies in PROFILE: A multi-ethnic lupus cohort. Clin. Rheumatol. 34(7), 1217–1223. https://doi.org/10.1007/s10067-015-2941-y (2015).
Ahn, S. S. et al. Anti-Smith antibody is associated with disease activity in patients with new-onset systemic lupus erythematosus. Rheumatol. Int. 39(11), 1937–1944. https://doi.org/10.1007/s00296-019-04445-y (2019).
Alba, P. et al. Anti-dsDNA, anti-Sm antibodies, and the lupus anticoagulant: Significant factors associated with lupus nephritis. Ann. Rheum. Dis. 62(6), 556–560. https://doi.org/10.1136/ard.62.6.556 (2003).
Ni, J. D. et al. Clinical and serological correlates of anti-Sm autoantibodies in Chinese patients with systemic lupus erythematosus: 1,584 cases. Rheumatol. Int. 29(11), 1323–1326. https://doi.org/10.1007/s00296-009-0855-1 (2009).
Singh, R. R., Malaviya, A. N., Kailash, S. & Varghese, T. Clinical significance of anti-Sm antibody in systemic lupus erythematosus & related disorders. Indian J. Med. Res. 94, 206–210 (1991).
López-Longo, F. J. et al. Expresión clínica del lupus eritematoso sistémico con anticuerpos anti-U1-RNP y anti-Sm [Clinical expression of systemic lupus erythematosus with anti-U1-RNP and anti-Sm antibodies]. Rev. Clin. Esp. 197(5), 329–335 (1997).
Mahler, M. et al. Multi-center evaluation of autoantibodies to the major ribosomal P C22 epitope. Rheumatol. Int. 32(3), 691–698. https://doi.org/10.1007/s00296-010-1685-x (2012).
Briani, C. et al. Neurolupus is associated with anti-ribosomal P protein antibodies: An inception cohort study. J. Autoimmun. 32(2), 79–84. https://doi.org/10.1016/j.jaut.2008.12.002 (2009).
Mahler, M. et al. International multicenter evaluation of autoantibodies to ribosomal P proteins. Clin. Vaccine Immunol. CVI 13(1), 77–83. https://doi.org/10.1128/CVI.13.1.77-83.2006 (2006).
Gerli, R. & Caponi, L. Anti-ribosomal P protein antibodies. Autoimmunity 38(1), 85–92. https://doi.org/10.1080/08916930400022699 (2005).
Kiss, E. & Shoenfeld, Y. Are anti-ribosomal P protein antibodies relevant in systemic lupus erythematosus?. Clin. Rev. Allergy Immunol. 32(1), 37–46. https://doi.org/10.1007/BF02686080 (2007).
Reichlin, M. Autoantibodies to the ribosomal P proteins in systemic lupus erythematosus. Clin. Exp. Med. 6(2), 49–52. https://doi.org/10.1007/s10238-006-0094-7 (2006).
Harley, J. B., Scofield, R. H., & Reichlin, M. Anti-Ro in Sjögren’s syndrome and systemic lupus erythematosus. Rheumatic diseases clinics of North America, 18(2), 337–358 (1992).
Boulware, D. W. & Hedgpeth, M. T. Lupus pneumonitis and anti-SSA (Ro) antibodies. J. Rheumatol. 16(4), 479–481 (1989).
Li, J. et al. Chinese SLE treatment and research group registry: III. Association of autoantibodies with clinical manifestations in Chinese patients with systemic lupus erythematosus. J. Immunol. Res. 2014, 809389. https://doi.org/10.1155/2014/809389 (2014).
Gómez-Puerta, J. A., Burlingame, R. W. & Cervera, R. Anti-chromatin (anti-nucleosome) antibodies: Diagnostic and clinical value. Autoimmun. Rev. 7(8), 606–611. https://doi.org/10.1016/j.autrev.2008.06.005 (2008).
Muller, S., Dieker, J., Tincani, A. & Meroni, P. L. Pathogenic anti-nucleosome antibodies. Lupus 17(5), 431–436. https://doi.org/10.1177/0961203308090030 (2008).
Migliorini, P., Ardman, B., Kaburaki, J. & Schwartz, R. S. Parallel sets of autoantibodies in MRL-lpr/lpr mice. An anti-DNA, anti-SmRNP, anti-gp70 network. J. Exp. Med. 165(2), 483–499. https://doi.org/10.1084/jem.165.2.483 (1987).
Migliorini, P., Baldini, C., Rocchi, V. & Bombardieri, S. Anti-Sm and anti-RNP antibodies. Autoimmunity 38(1), 47–54. https://doi.org/10.1080/08916930400022715 (2005).
Hoffman, I. E. et al. Specific antinuclear antibodies are associated with clinical features in systemic lupus erythematosus. Ann. Rheum. Dis. 63(9), 1155–1158. https://doi.org/10.1136/ard.2003.013417 (2004).
Choi, M. Y. et al. Longitudinal analysis of ANA in the systemic lupus international collaborating clinics (SLICC) inception cohort. Ann. Rheum. Dis. 81(8), 1143–1150. https://doi.org/10.1136/annrheumdis-2022-222168 (2022).
Acknowledgements
We thank Xiaoyue Xu and Gang Liu for their excellent statistical advice.
Funding
This study was supported by the Natural Science Foundation of Anhui Provincial (2108085MH258).
Author information
Authors and Affiliations
Contributions
C.X. designed this work. C.X., X.F. performed patient clinical assessment. Z.X., L.D., D.H., N.L. collected the data of patients. M.G., Z.X. analyzed the data. C.X., M.G. wrote the manuscript. C.X., X.F. reviewed the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gong, M., Dai, L., Xie, Z. et al. Serological and clinical associations of autoantibodies in Chinese patients with new-onset systemic lupus erythematosus. Sci Rep 13, 10101 (2023). https://doi.org/10.1038/s41598-023-37100-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-37100-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.