Abstract
Understanding the true burden of tobacco smoking on adverse pregnancy outcomes is critical in generating appropriate interventions to improve outcomes. Self-reporting of human behaviour that is associated with stigma is associated with underreporting in general and may bias the impact of smoking in studies; however, self-reporting is frequently the most practical method of gleaning this information. The objective of this study was to evaluate concordance between self-reported smoking and concentrations of plasma cotinine, a biomarker of smoking, among participants enrolled in two related HIV cohorts. A total of 100 pregnant women (76 living with HIV [LWH] and 24 negative controls) in their third trimester, and 100 men and non-pregnant women (43 LWH and 57 negative controls) were included. Among all participants, 43 pregnant women (49% LWH and 25% negative controls) and 50 men and non-pregnant women (58% LWH and 44% negative controls) were self-reported smokers. The odds of discordance between self-reported smoking and cotinine levels were not significantly different between self-reported smokers and non-smokers, nor between pregnant women and others, but were significantly increased, regardless of self-reported status, among people LWH compared to negative controls. The overall concordance between plasma cotinine and self-reported data among all participants was 94% with a sensitivity and specificity of 90% and 96%, respectively. Taken together, these data demonstrate that participant surveying in a non-judgemental context can lead to accurate and robust self-report smoking data among both persons LWH and not, including in the context of pregnancy.
Similar content being viewed by others
Introduction
In 2016, over 1.1 billion people smoked tobacco (21.9% of the global population), varying widely between countries, from 4 to 47%1. Approximately one in ten Canadians reported smoking in 20192, which is similar to the United States with 12.5% of adult cigarette smoking in 20203. Rates of smoking are consistently higher among people living with HIV (LWH) than that of the general population. An analysis in the United States with data collected in 2016 noted that the rate of current smoking was twice as high among people LWH (47.0% vs 25.5%)4, with similar trends noted in a 2014 Canadian study (~ 30%)5. The adverse health effects of tobacco smoking and its association with cancers, cardiovascular disease and premature mortality are well-documented6, and are exacerbated in HIV populations in which smoking while LWH reduced life expectancy by > 6 years compared to LWH and not smoking7. Smoking is also an established risk factor for adverse pregnancy outcomes, including increasing the likelihood of preterm delivery, intrauterine growth restriction, low birth weight, and infant death8,9,10,11,12. The rate of smoking among pregnant women varies world-wide, from 10% in Japan13, to 17% in Australia14, ~ 20% in Canada15, and 30–35% in Spain16. Importantly, the effect of smoking on adverse pregnancy outcomes and pregnancy loss in North American women was reported to differ dramatically by HIV status, despite the use of effective antiretroviral therapies17.
Most cohort studies collect information on smoking using self-report data; however, depending on the context, the stigma associated with smoking may lead to underreporting and possible bias in studies relying on self-reported data18,19,20,21,22. In the non-pregnant population, misclassification is often higher among persons who self-report as non-smoking than the reverse, a phenomenon that appears increased in the context of pregnancy23. Indeed, previous studies reported higher non-disclosure rates of smoking among pregnant women than the general population24,25,26. For example, in a smoking cessation trial in the USA, pregnant women underreported their smoking by 14%24. Additionally, a study of pregnant women LWH in the United States identified only a weak agreement between self-reported and laboratory confirmed smoking, which the authors attribute to social desirability27. Canadian data are scarce and conflicted on the validity of self-reported smoking behaviors in pregnant and non-pregnant populations, and even less is known in the HIV population15,22,28,29,30,31,32. In the context of pregnancy, in order to provide optimal care for women, it is particularly important to foster a trusting, non-judgemental environment. This favors the obtention of accurate information by the prenatal care provider on tobacco smoking, as well as other risk behaviours that could adversely impact the health of the mother and the fetus. At the BC Women’s Hospital, and at partner institutions in Canada that provide prenatal care to women living with HIV, women centred non-judgemental trauma informed care is the model utilized, which facilitates disclosure of health behaviours and socio-structural issues for the benefit of the mother and her infant33,34.
Cotinine, a nicotine metabolite with a ~ 16 h half-life in plasma, urine, or saliva, is often used as a biomarker of smoking and can be used to determine concordance between levels of the marker and self-reported smoking status35. Cotinine has also been identified in cord blood and infant urine as an indicator of fetal exposure to tobacco36. In many studies, self-reported smoking is used as a key variable in analysis of clinical outcomes yet has been questioned as a valid reflection of actual smoking rates. Currently, there is a gap in knowledge regarding the validity of self-reported smoking status among pregnant women LWH. The objective of this study was to examine the concordance between self-reported smoking and plasma cotinine concentration among pregnant women LWH and negative controls enrolled in the Canadian CARMA (Children and women, Antiretrovirals and Markers of Aging)-PREG Cohort. Additionally, we examined the concordance between self-reported smoking and plasma cotinine concentration among people LWH and negative control women and men enrolled in the Canadian CARMA-CORE Cohort as a secondary cohort to validate our findings in a similar, non-pregnant, sample.
Results
In this study, at their study visit taking place between 28 and 38 weeks of gestation, 43 (43%) CARMA-PREG participants self-reported having smoked since last visit (Table 1). In this selected study sample, there was no significant difference in maternal age or HIV status between the self-reported smokers and non-smokers. However, there were fewer Indigenous women in the non-smokers group and fewer African Caribbean Black women in the smoker’s group (p < 0.001). Compared to non-smokers, self-reported smokers were more likely to have a low income (< $15,000/year, p < 0.001), and to use illicit drugs (p < 0.001) (Table 1).
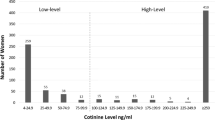
Among pregnant women who reported having smoked (n = 43) since their last visit, 3 (7%) reported smoking heavily (a pack a day or more), 28 (65%) reported smoking a moderate amount (2 to 19 cigarettes a day), and 3 (7%) reported light smoking (less than 2 cigarettes a day) (Figs. 1, S2). The frequency and/or quantity of tobacco use was unavailable for the remaining 9 (21%). Defining plasma cotinine ≥ 5 ng/mL as smoking-positive, we observed 95% concordance between self-reported smoking and smoking positivity according to plasma cotinine. For the women who self-reported as non-smokers, concordance with plasma cotinine < 5 ng/mL was 89% (Fig. 2). Two pregnant women who self-reported smoking showed plasma cotinine levels below 5 ng/mL (Fig. 2). Both women self-reported smoking fewer than two cigarettes per week on average during their pregnancy. The κ for women in CARMA-PREG was 0.839, indicating almost perfect agreement. The sensitivity and specificity of self-reporting in CARMA-PREG were 87% and 96%, respectively. Among pregnant women LWH (n = 76), the concordance between plasma cotinine and self-reported smoking (n = 37) and non-smoking (n = 39) was 95% and 85%, respectively (Fig. 2). Among negative control pregnant women (n = 24), 6 self-reported smoking and the remaining 18 reported no smoking, and the observed concordance was 100%. The κ for pregnant women LWH and negative control pregnant women, were 0.790 and 1.00, respectively. No significant difference in concordance between pregnant women LWH and negative control pregnant women was noted (p = 0.19).
To further examine the quality of self-reported smoking data in our cohorts we examined the concordance of self-reported smoking data and plasma cotinine in the CARMA-CORE cohort. The rate of smoking among participants in the entire cohort for whom this data was available (n = 623) was 40%. Random selection of sex-matched smokers (n = 50) and non-smokers (n = 50) resulted in age and HIV status balanced groups (Table 1), with ages ranging from 17 to 75 years, and both sexes were equally represented. The groups differed significantly by ethnicity, with more Indigenous people in the smoker group and more Asian or other ethnicities in the non-smoker group (p < 0.001). Similar to CARMA-PREG, self-reported smokers were more likely to have low income (p < 0.001) and use illicit drugs (p < 0.001). Additionally, smokers were more likely to use cannabis (p = 0.008).
Among the women and men who reported tobacco use at their first visit in the CARMA-CORE cohort, 11 (22%) reported smoking heavily, 29 (58%) reported smoking a moderate amount, and 7 (14%) reported light smoking (Figs. 1, S2). The intensity of tobacco use was unavailable for the remaining 3 (6%). We observed 96% concordance between self-reported smoking and plasma cotinine. For the women and men who self-reported as non-smokers, concordance with plasma cotinine < 5 ng/mL was 92%. The κ for participants in CARMA-CORE was 0.880. The sensitivity and specificity of self-reporting in CARMA-CORE were 92% and 96%, respectively. Among all CARMA-CORE participants LWH in this study (n = 43), the concordance between plasma cotinine and self-reported smoking (n = 25) and non-smoking (n = 18) was 96% and 78%, respectively (Fig. 2). Among the negative control women and men (n = 57), 25 self-reported smoking and the remaining 32 reported no smoking, and the observed concordance was 96% and 100%, respectively (Fig. 2). The κ for participants LWH and negative controls in CARMA-CORE were 0.755 and 0.964, respectively. No significant difference in concordance between participants LWH and negative controls in CARMA-CORE participants was noted (p = 0.08). Among all female CARMA-CORE participants (n = 51), the concordance between plasma cotinine and self-reported smoking (n = 25) and non-smoking (n = 26) was 100% (Fig. 2). Male CARMA-CORE participants (n = 49) had a concordance between plasma cotinine and self-reported smoking (n = 25) and non-smoking (n = 24) of 92% and 83%, respectively (Fig. 2). The κ for female and male CARMA-CORE participants were 1.00 and 0.755, respectively. There was a significant difference in concordance between male and female CARMA-CORE participants, in which men were more likely to have a discordant self-report (p = 0.01).
Taken together, if we group the two datasets, among 100 pregnant women, 51 non-pregnant women, and 49 men included in our analyses, 93 (47%) self-reported smoking. We observed an overall concordance of 94% between plasma cotinine and both self-reported smoking and non-smoking data with a κ of 0.860 and the sensitivity and specificity were 90% and 96%, respectively. Among all individuals LWH in both cohorts (n = 119), the concordance between plasma cotinine and self-reported smoking (n = 62) and non-smoking (n = 57) was 95% and 81%, respectively. Among all individuals who did not have an HIV diagnosis (n = 81), 31 self-reported smoking and the remaining 50 reported no smoking, with an observed concordance of 98% and 100% respectively. The κ coefficients for individuals LWH and negative controls were 0.780 and 0.974 respectively and although both high, these were significantly different (p = 0.009). It is notable that discordance was present for both self-reported smokers and non-smokers, indicating a bidirectional discordance among the HIV group. Among all women in both cohorts (n = 151), the concordance between plasma cotinine and self-reported smoking (n = 68) and non-smoking (n = 83) was 98% and 95%, respectively, with a κ of 0.894. There was no difference in concordance between people who used or did not use cannabis (p = 0.74).
For seven CARMA-PREG participants who self-reported smoking and had high plasma cotinine concentration (> 75 ng/mL), and seven participants for whom self-report and cotinine were discordant, we investigated the relationship between cotinine concentrations at third visit, at delivery, and in cord plasma. Participants with high cotinine levels at third visit also showed high levels of cotinine in maternal and cord plasma at delivery (Fig. S3). Two of the seven participants with discordant self-report and cotinine values reported light smoking but consistently had cotinine values below the 5 ng/mL limit (Fig. S4). Of the five remaining discordant participants who self-reported non-smoking but had cotinine values > 5 ng/mL at third visit, three had cotinine values < 5 ng/mL at delivery in maternal and cord plasma and two remained > 5 ng/mL throughout (Fig. S4).
For quality control purposes, we repeated the ELISA on 74 of the CARMA-PREG specimens. Qualitatively, we obtained identical results, indicating the assay is robust enough to assay specimens only once.
Discussion
The prevalence of maternal smoking in the entire CARMA-PREG cohort (28%) is higher than the estimated rate of smoking during pregnancy in Canada11,28,37, and is likely related to the demographic and socioeconomic makeup of our cohort. Of note, prevalence of smoking was similar (30%) in participants who were not included in the final analysis, indicating that bias was not introduced through the exclusion criteria. Although we purposefully selected similar numbers of smokers and non-smokers from the CARMA-CORE cohort for cotinine analysis, the rate of smoking in the entire cohort was 40%, four times as high as the Canadian average, but similar to reported rates among persons LWH in Canada2. Additionally, our results are consistent with previous Canadian studies showing that low income is associated with higher smoking rates38,39, including during pregnancy15,28,37.
As in many studies, the CARMA cohorts record smoking data via self-report, collected through interviews with research staff. We undertook the current study to examine the concordance between self-report and a biomarker of smoking, namely plasma cotinine, to ascertain whether self-reported smoking was a reliable variable for biomedical analyses in our studies. This was sparked, in part, by consistent observations of tobacco smoking exerting significant effects on the markers of cellular aging we are studying. Of note, people LWH worldwide are more likely to be smokers than people without HIV, implicating smoking as an important covariate in HIV studies40. We therefore felt a need to assess the robustness of our data. In participants from two separate cohorts with different research staff, we observed excellent concordance between self-report and cotinine, both among participants LWH and negative controls and in the pregnancy and non-pregnancy cohorts. Additionally, we observed a positive trend between self-reported smoking intensity and plasma cotinine concentration, indicating that the intensity of tobacco use was also accurately reported. Overall, only 9% of self-reported non-smokers were likely to be a smoker considering their cotinine result, and 4% of self-reported light smokers showed plasma cotinine levels ≤ 5 ng/mL. Given the half-life of cotinine, the timing of sampling could influence its detection. Hence, lower concordance between cotinine and self-reported smoking may be expected using this assay when the smoking frequency is very low, especially since its metabolism and clearance may be accelerated during pregnancy41.
The majority of the discordant results in this study were observed among people LWH. It is possible that this may in part be related to a reportedly faster nicotine metabolism in people LWH, which can further be affected by the antiretrovirals42,43,44,45. Given the high prevalence of smoking in some communities, this may also be related to a higher likelihood of second-hand smoke exposure. Additionally, men were more likely to be discordant, an interested finding that could warrant further research.
Our cohort participants were questioned on the use of cannabis and illicit drugs, topics that are sensitive to discuss, especially during pregnancy. Of note, the use of cannabis was not associated with self-reported smoking discordance, with only one discordant participant who self-reported as a non-smoker and reported cannabis use. This suggests that practices that mix tobacco with cannabis were either not performed, or were self-reported as a tobacco exposure.
Our results are similar to a Swedish study reporting that 6% of self-reported non-smoker pregnant women were more likely to be smokers and that 3% had cotinine concentrations suggestive of passive smoking46. However, the majority of studies report lower concordance. For example, in West Scotland, 25% of pregnant women who self-reported to be non-smokers had cotinine measurements above the threshold23. Similarly, in rural and small metropolitan areas in upstate New York, 35% of pregnant women had urinary cotinine levels suggesting inaccurate self-reported status19. In a systematic review conducted in 2009, 40 of the 54 included studies that compared prevalence estimates of self-reported smoking versus measured cotinine values in adults indicated that actual smoking status was underrepresented by self-reporting47. Such cases of underreporting may introduce bias to the associated analyses and may have implications not only in the research setting but also for clinicians and caregivers20. The integrity of self-reported data varies according to population and the social context in which the data are collected. Multiple factors could influence the interviewee’s responses to questions about smoking status, including participant characteristics, study method and setting, and the pressure mediated by social desirability. The present study was not designed to investigate how these factors may influence or predict the quality of self-reporting.
A strength of this study is that the participants were not aware that their self-reported smoking status would be confirmed via biochemical testing, thus a large majority of participants accurately reported their status without pressure to do so. Additionally, given that 98% of women living with HIV in Canada access ART (hence care) during pregnancy48, and that the vast majority of women invited to participate consented to do so, it is unlikely that recruitment bias exerts a large effect on our estimates. Another strength of this study is the high smoking rate within the cohorts, among both participants LWH and negative controls and the multi-ethnic makeup. As this study was conducted by research staff and participants were told the information they provided was confidential, the findings may not be generalizable to how women would share smoking information with their care providers. Although the cohort may not be fully generalizable to all pregnant and non-pregnant populations the goal of this study was to evaluate self-report concordance in the context of research, namely a prospective observational cohort study, and our results show high concordance among all participants. A limitation to this study is that data on exposure to second-hand smoke were not collected, an important factor that may help explain some of our discordant results among self-reported non-smokers.
Overall, our data suggest that it is possible to obtain robust data on smoking from pregnant and non-pregnant participants, living with HIV and not, through self-report in a research setting. Among CARMA cohort participants, our results indicate that self-reported smoking data are highly reliable as a surrogate for tobacco exposure. This suggests that study participants likely felt safe to speak candidly, and accurately self-reported their smoking habits to our non-judgemental research staff who explained during consent that all information is confidential, including from their care providers. This study will allow researchers utilizing this and similar datasets to have higher confidence when using self-reported smoking values in statistical modelling. The validity of this data is of particular importance as clinical studies, which rely on these types of self-reported data, help inform further clinical research.
Methods
Study sample
This is a cross-sectional study using data and specimens from two cohorts: CARMA-PREG and CARMA-CORE. CARMA-PREG is a prospective cohort which enrolled pregnant women LWH and negative controls between 2004 and 2020. Pregnant women LWH were recruited at the Oak Tree Clinic in British Columbia (BC) Women’s Hospital in Vancouver, BC, and the Sainte-Justine Hospital in Montreal, Quebec. The Oak Tree Clinic utilizes a model of women-centered HIV care to provide multiple services, including harm reduction and addition counselling by care providers, for women LWH33. Negative control women were recruited at BC Women’s Hospital. The enrolment of women in CARMA-PREG took place during their first prenatal visit and both women LWH and negative controls provided biological specimens at three visits during pregnancy, at delivery, and post-partum. Cord blood was also collected. Inclusion criteria for enrolment in the CARMA-PREG cohort were being pregnant with a known HIV status, and women LWH had to be receiving or be willing to receive antiretroviral therapy during pregnancy. Exclusion criteria for the cohorts included the inability to provide informed consent (language barriers) or to participate in research (health or social crisis). Additional exclusion criteria for the selection of the study sample for this sub-study of CARMA-PREG included the exclusion of participants missing third trimester plasma specimens, between 28 and 38 weeks of gestation, and the exclusion of those missing smoking information. The 167 participants excluded due to missing third trimester specimens included 109 from which the specimen was empty due to use in previous studies, 22 specimens were of too low volume for the assay, 15 were missing due to preterm birth (third trimester visit did not take place), and 21 were missing from the inventory. Despite having access to specimens from other trimesters, we chose to only include third trimester specimens in which the participants were all in the same period of pregnancy and equally visibly pregnant, a time in which stigma may be highest. For CARMA-PREG participants with repeat pregnancies, only the first was considered, and if a participant was enrolled in both cohorts, the CARMA-PREG data was prioritized. See Fig. S1 for details on the study participant selection.
Enrolment of participants LWH in CARMA-CORE took place during routine clinical visits at Oak Tree Clinic. Negative control participants were recruited through a variety of means, including word of mouth and advertisements posted in strategic areas in Vancouver to promote enrollment of negative control participants with similar sociodemographic characteristics to participants LWH, including smoking habits. Recruitment took place between 2008 and 2017 and participants had between one and seven study visits during that period. Inclusion criteria for enrolment in the CARMA-CORE cohort were a known HIV status, and the ability to provide informed consent. For the selection of the study sample for this sub-study, those younger than 14 years of age, missing plasma specimens from the first visit, or missing smoking information were excluded. Those younger than 14 years of age were not included as information on their smoking status was not collected. If a participant preferred not to answer, or their smoking status was unknown, then their smoking status was recorded as missing data.
Among all participants eligible for inclusion in this sub-study, 57 non-smoking CARMA-PREG participants were selected to match year of visit with the remaining n = 43 smokers, for a total of 100 participants. Smokers and non-smokers in CARMA-CORE were sex matched and 50 in each group were randomly selected from the whole cohort for a total of 100 participants.
For both cohorts, demographic data were collected upon entrance to the study, and clinical and substance use information, including tobacco exposure, were collected by self-report (any tobacco use since last visit) at each visit through participant interviews with trained research staff embedded in the clinical setting. For 81 of the 93 (87%) self-reported smokers, the intensity of tobacco smoking was collected and later categorized as heavy smoking (a pack a day or more), moderate smoking (2 to 19 cigarettes a day), or light smoking (less than 2 cigarettes a day). Smoking intensity definitions were adapted from the Government of Canada tobacco use statistics49. Research staff were instructed to not react or pass judgement during the survey, to ensure a safe research environment. In accordance with this, the research staff did not provide any recommendations for smoking cessation. Participants were also informed that their information would be confidential and would not be shared, even with their treating physician who was an investigator on the study. At the time of data collection, no self-report around tobacco exposure via vaping was sought as it was exceedingly rare, if occurring at all. For one participant, information around tobacco chewing (1 pinch 5 ×/day) was collected and converted to interpret one pinch as equivalent to 4 cigarettes50. No data were ever collected about second-hand smoking, nicotine patch, or nicotine gum use. Participants were not informed that cotinine would be measured, thus data were anonymized for these analyses.
Plasma cotinine measurement
Plasma cotinine levels were measured in specimens collected at their third trimester pregnancy visit from a total of 76 pregnant women LWH and 24 negative control CARMA-PREG participants. Additional plasma specimens at delivery and cord blood from select participants, 7 with high cotinine values and 7 with discordant self-report and cotinine values, were also assessed. In the CARMA-CORE cohort, plasma cotinine levels were measured in specimens collected during their first visit from a total of 43 people LWH and 57 negative control participants. Self-reported substance use information was collected on the same day as blood collection. Cotinine was measured by solid phase competitive enzyme-linked immunosorbent assay (ELISA) (Calbiotech, CA, USA). Any cotinine ≥ 5 ng/mL was defined as cotinine-positive, based on the limit of detection of the assay.
Statistical analyses
Comparisons between self-reported smoker and non-smoker groups were done using Fisher's exact test for categorical variables and Student t-test for continuous variables. The proportions of cotinine-negative among self-reported non-users and of cotinine-positive among self-reported users were used to express concordance. In addition, Cohen's kappa coefficient (κ), sensitivity, and specificity were used to determine the agreement between smoking as per self-report and cotinine among all participants and within the LWH and negative control groups. The association between the self-reported number of cigarettes per day and plasma cotinine levels were explored through Pearson’s correlation. All statistical analyses were performed using GraphPad Prism 8.4.3 and JMP Pro 15.
Ethics declarations
Ethical approval for this secondary use of data study was obtained from the Research Ethics Boards of the University of British Columbia and from the Hospital Research Review Committee of the Children’s and Women’s Health Centre of British Columbia (H03-70356, H04-70540, H07-03136, and H08-02018). All methods were performed in accordance with relevant guidelines and regulations. All participants provided written informed consent to have their cohort specimens biobanked.
Data availability
All study data are included in the article and/or supporting information. All requests for or questions about the data can be initiated by contacting helene.cote@ubc.ca.
References
World Health Organization. World Health Statistics Data Visualizations Dashboard. Tobacco Smoking. https://apps.who.int/gho/data/node.sdg.3-a-viz?lang=en.
East, K. A., Reid, J. L. & Hammond, D. Smoking and vaping among Canadian youth and adults in 2017 and 2019. Tob Control 32, 259–262 (2021).
Cornelius, M. E., Loretan, C. G., Wang, T. W., Jamal, A. & Homa, D. M. Tobacco Product Use Among Adults: United States, 2020. MMWR Morb. Mortal Wkly. Rep. 71, 397–405 (2022).
Asfar, T. et al. National estimates of prevalence, time-trend, and correlates of smoking in US people living with HIV (NHANES 1999–2016). Nicotine Tob. Res. 23(8), 1308–1317 (2021).
Bekele, T. et al. Trends and correlates of cigarette smoking and its impacts on health-related quality of life among people living with HIV: Findings from the Ontario HIV Treatment Network Cohort Study, 2008–2014. AIDS Patient Care ST 31(2), 49–59 (2017).
US Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General (Centers for Disease Control and Prevention US, 2014).
Helleberg, M. et al. Mortality attributable to smoking among HIV-1–infected individuals: A nationwide, population-based cohort study. Clin. Infect. Dis. 56(5), 727–734 (2013).
Cnattingius, S. The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob. Res. 6(Suppl 2), S125–S140 (2004).
US Department of Health and Human Services. The health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General (Centers for Disease Control and Prevention US, 2006).
Kvalvik, L. G., Skjaerven, R. & Haug, K. Smoking during pregnancy from 1999 to 2004: A study from the medical birth registry of Norway. Acta Obstet. Gynecol. Scand. 87(3), 280–285 (2008).
Al-Sahab, B., Saqib, M., Hauser, G. & Tamim, H. Prevalence of smoking during pregnancy and associated risk factors among Canadian women: A national survey. BMC Pregnancy Childbirth 10(1), 24 (2010).
Erickson, A. C. & Arbour, L. T. Heavy smoking during pregnancy as a marker for other risk factors of adverse birth outcomes: A population-based study in British Columbia, Canada. BMC Public Health 12(1), 102 (2012).
Kaneita, Y. et al. Prevalence of smoking and associated factors among pregnant women in Japan. Prev. Med. 45(1), 15–20 (2007).
Mohsin, M. & Bauman, A. E. Socio-demographic factors associated with smoking and smoking cessation among 426,344 pregnant women in New South Wales, Australia. BMC Public Health 5(1), 138 (2005).
Lange, S., Probst, C., Rehm, J. & Popova, S. National, regional, and global prevalence of smoking during pregnancy in the general population: A systematic review and meta-analysis. Lancet Glob. Health 6(7), e769–e776 (2018).
Ruiz, C. A. J. Nicotine replacement therapy during pregnancy. Arch. Bronconeumol. 42(8), 404–409 (2006).
Westreich, D. et al. Smoking, HIV, and risk of pregnancy loss. AIDS 31(4), 553–560 (2017).
Ford, R. P., Tappin, D. M., Schluter, P. J. & Wild, C. J. Smoking during pregnancy: How reliable are maternal self reports in New Zealand?. J. Epidemiol. Community Health 51(3), 246–251 (1997).
Britton, G. R. A., Brinthaupt, J., Stehle, J. M. & James, G. D. Comparison of self-reported smoking and urinary cotinine levels in a rural pregnant population. J. Obstet. Gynecol. Neonatal Nurs. 33(3), 306–311 (2004).
England, L. J. et al. Misclassification of maternal smoking status and its effects on an epidemiologic study of pregnancy outcomes. Nicotine Tob. Res. 9(10), 1005–1013 (2007).
Wong, S. L., Shields, M., Leatherdale, S., Malaison, E. & Hammond, D. Assessment of validity of self-reported smoking status. Health Rep. 23(1), D1 (2012).
Noonan, D., Jiang, Y. & Duffy, S. A. Utility of biochemical verification of tobacco cessation in the Department of Veterans Affairs. Addict. Behav. 38(3), 1792–1795 (2013).
Shipton, D. et al. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: A retrospective, cross sectional study. BMJ 339, b4347 (2009).
Boyd, N. R., Windsor, R. A., Perkins, L. L. & Lowe, J. B. Quality of measurement of smoking status by self-report and saliva cotinine among pregnant women. BMJ 2(2), 77–83 (1998).
Albrecht, S. A., Reynolds, M. D., Salamie, D. & Payne, L. A comparison of saliva cotinine, carbon monoxide levels, and self-report as indicators of smoking cessation in the pregnant adolescent. J. Addict. Nurs. 11(3), 93–101 (1999).
Markovic, N. et al. Substance use measures among women in early pregnancy. Am. J. Obstet. Gynecol. 183(3), 627–632 (2000).
Tassiopoulos, K. et al. Substance use in HIV-infected women during pregnancy: Self-report versus meconium analysis. AIDS Behav. 14(6), 1269–1278 (2010).
Millar, W. J. & Hill, G. Pregnancy and smoking. Health Rep. 15(4), 53 (2004).
Heaman, M. I. & Chalmers, K. Prevalence and correlates of smoking during pregnancy: A comparison of aboriginal and non-aboriginal women in Manitoba. Birth 32(4), 299–305 (2005).
Burstyn, I. et al. Evaluation of the accuracy of self-reported smoking in pregnancy when the biomarker level in an active smoker is uncertain. Nicotine Tob. Res. 11(6), 670–678 (2009).
Alberta Reproductive Health Report Working Group. Alberta Reproductive Health: Pregnancies and Births Table Update 2011. Alberta Health and Wellness 2011. https://open.alberta.ca/dataset/06456e95-348e-403f-8c04-5eb79391d252/resource/4601364f-a9f1-40e3-9f39-e8920da0f149/download/reproductive-health-2011.pdf.
Arbuckle, T. E., Liang, C. L., Fisher, M., Caron, N. J. & Fraser, W. D. Exposure to tobacco smoke and validation of smoking status during pregnancy in the MIREC study. J. Expo Sci. Environ. Epidemiol. 28(5), 461–469 (2018).
Kestler, M., Murray, M., Money, D., Sauve, L. & Pick, N. The Oak Tree Clinic: The envisioned model of care for women living with human immunodeficiency virus in Canada. Womens Health Issues 28(2), 197–198 (2018).
Skerritt, L. et al. Discussing reproductive goals with healthcare providers among women living with HIV in Canada: The role of provider gender and patient comfort. Sex Reprod. Health Matters 29(1), 1932702 (2021).
Jarvis, M. J., Russell, M. A., Benowitz, N. L. & Feyerabend, C. Elimination of cotinine from body fluids: Implications for noninvasive measurement of tobacco smoke exposure. Am. J. Public Health 78(6), 696–698 (1988).
Pichini, S. et al. Cord serum cotinine as a biomarker of fetal exposure to cigarette smoke at the end of pregnancy. Environ. Health Perspect. 108(11), 1079–1083 (2000).
Cui, Y., Shooshtari, S., Forget, E. L., Clara, I. & Cheung, K. F. Smoking during pregnancy: Findings from the 2009–2010 Canadian Community Health Survey. PLoS ONE 9(1), e84640 (2014).
Balfour, L. et al. An HIV-tailored quit-smoking counselling pilot intervention targeting depressive symptoms plus Nicotine Replacement Therapy. AIDS Care 29(1), 24–31 (2017).
Chaiton, M. & Callard, C. Mind the gap: Disparities in cigarette smoking in Canada. Tob. Use Insights 12, 058 (2019).
Johnston, P. I. et al. Worldwide relative smoking prevalence among people living with and without HIV: A systematic review and meta-analysis. AIDS 35, 957–970 (2021).
Taghavi, T., Arger, C. A., Heil, S. H., Higgins, S. T. & Tyndale, R. F. Cigarette consumption and biomarkers of nicotine exposure during pregnancy and postpartum. Addiction 113(11), 2087–2096 (2018).
Ashare, R. L. et al. Differences in the rate of nicotine metabolism among smokers with and without HIV. AIDS 33(6), 1083–1088 (2019).
Schnoll, R. A. et al. Brief report: Rate of nicotine metabolism and tobacco use among persons with HIV: Implications for treatment and research. J. Acquir. Immune Defic. Syndr. 80(2), e36–e40 (2019).
Earla, R., Ande, A., McArthur, C., Kumar, A. & Kumar, S. Enhanced nicotine metabolism in HIV-1–positive smokers compared with HIV-negative smokers: Simultaneous determination of nicotine and its four metabolites in their plasma using a simple and sensitive electrospray ionization liquid chromatography-tandem mass spectrometry technique. Drug Metab. Dispos. 42(2), 282–293 (2014).
Rezahosseini, O. et al. Plasma cotinine cutoff for distinguishing smokers from nonsmokers among persons living with HIV. J. Acquir. Immune Defic. Syndr. 82(5), e54 (2019).
Lindqvist, R., Lendahls, L., Tollbom, Ö., Åberg, H. & Håkansson, A. Smoking during pregnancy: Comparison of self-reports and cotinine levels in 496 women. Acta Obstet. Gynecol. Scand. 81(3), 240–244 (2002).
Gorber, S. C., Schofield-Hurwitz, S., Hardt, J., Levasseur, G. & Tremblay, M. The accuracy of self-reported smoking: A systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob. Res. 11(1), 12–24 (2009).
Haddad, N., Weeks, A., Robert, A. & Totten, S. HIV in Canada: Surveillance report, 2019. Can. Commun. Dis. Rep. 47(1), 77–86 (2021).
Canada Health. Terminology: Tobacco Use Statistics. https://www.canada.ca/en/health-canada/services/health-concerns/tobacco/research/tobacco-use-statistics/terminology.html.
Benowitz, N. L., Porchet, H., Sheiner, L. & Jacob, P. III. Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and nicotine gum. Clin. Pharmacol. Ther. 44(1), 23–28 (1988).
Acknowledgements
We thank all study participants and the dedicated Oak Tree Clinic and Sainte-Justine research staff and students including Rebecca Graham, Ashley Docherty, Jackson Chu, Daljeet Mahal, Laura Oliveira, Farnaz Changizi, Tessa Chaworth-Musters, Maninder Mahal, Annie Qiu, Clare Hall-Patch, Ariel Nesbitt, and Shanlea Gordon, and Silvie Valois.
Funding
This research was supported in part by two Canadian Foundation for AIDS Research grants (016012 to DMM & HCFC, and 0120 004 to HCFC & DMM) as well as two Canadian Institutes of Health Research (CIHR) team Grants (HET-85515 to HCFC, NP, & DMM, and TCO-125269 to HCFC, NP & DMM) and CIHR Canadian HIV Trials Network (CTN) funding to DM, IB and HCFC (CTN291). SS (2012–2013), AA (2016–2017), and MSRS (2019–2020) were supported by University of British Columbia (UBC) Centre for Blood Research Collaborative training awards. AA was supported in part by UBC Faculty of Medicine scholarships; and MSRS by a British Columbia Graduate Student Award. IB is supported by a salary award from the Fonds de Recherche en Santé du Québec (FRQS).
Author information
Authors and Affiliations
Contributions
M.S.R.S., S.S., A.A., D.M.M., and H.C.F.C. contributed to the study design. M.S.R.S., S.S., M.M.T.Z., I.G., and B.S. contributed to specimen preparation and data retrieval. M.S.R.S., S.S., and M.M.T.Z. and assayed (ELISA) specimens. M.S.R.S., S.S., and M.M.T.Z. performed the statistical analyses. E.J.M., J.V.S., C.E., N.P., M.C.M.M., and D.M.M. contributed to enrollment of participants and data collection. M.S.R.S., S.S., and A.A. contributed to writing the manuscript. All authors critically reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Smith, MS.R., Saberi, S., Ajaykumar, A. et al. Robust tobacco smoking self-report in two cohorts: pregnant women or men and women living with or without HIV. Sci Rep 13, 7711 (2023). https://doi.org/10.1038/s41598-023-34249-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34249-x
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.