Abstract
Objective
To report a more accurate prevalence estimate of late pregnancy nicotine exposures.
Study design
A cross-sectional study during a 2-month period in 2019. Participants were women delivering in any of the six county maternity hospitals who consented to universal drug testing at the time of delivery as part of routine hospital admission.
Results
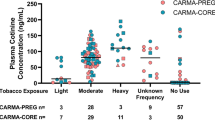
Of 2531 tested samples, 18.7% tested positive for high levels of cotinine indicating primary smoking or other primary use of tobacco products. Together, 33.0% of the study population tested positive for nicotine exposure during late pregnancy compared to vital records which reported 8.2% cigarette smoking during the third trimester of pregnancy and 10.5% cigarette smoking at any time during pregnancy through maternal self-report.
Conclusion
Captured vital birth smoking measures vastly underreport actual primary exposures to nicotine products. Vital birth data also fail to capture secondhand exposures which constitute a significant proportion of the population.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Pineles BL, Hsu S, Park E, Samet JM. Systematic review and meta-analyses of perinatal death and maternal exposure to tobacco smoke during pregnancy. Am J Epidemiol. 2016;184:87–97.
Hackshaw A, Rodeck C, Boniface S. Maternal smoking in pregnancy and birth defects: a systematic review based on 173 687 malformed cases and 11.7 million controls. Hum Reprod Update. 2011;17:589–604.
Rogers JM. Tobacco and pregnancy. Reprod Toxicol. 2009;28:152–60.
Gilliland FD, Li YF, Peters JM. Effects of maternal smoking during pregnancy and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med. 2001;163:429–36.
Drake P, Driscoll AK, Mathews TJ. Cigarette smoking during pregnancy: United States, 2016. NCHS Data Brief. 2018:1–8.
United States Department of Health and Human Services (US DHHS) CfDCaPC, National Center for Health Statistics (NCHS), Division of Vital Statistics. Natality public-use data 2007–2018, on CDC WONDER Online Database. http://wonder.cdc.gov/natality-current.html.
Curtin SC, Matthews TJ. Smoking prevalence and cessation before and during pregnancy: data from the birth certificate, 2014. Natl Vital Stat Rep. 2016;65:1–14.
Dietz PM, Homa D, England LJ, Burley K, Tong VT, Dube SR, et al. Estimates of nondisclosure of cigarette smoking among pregnant and nonpregnant women of reproductive age in the United States. Am J Epidemiol. 2011;173:355–9.
Dukic VM, Niessner M, Pickett KE, Benowitz NL, Wakschlag LS. Calibrating self-reported measures of maternal smoking in pregnancy via bioassays using a Monte Carlo approach. Int J Environ Res Public Health. 2009;6:1744–59.
Pickett KE, Rathouz PJ, Kasza K, Wakschlag LS, Wright R. Self-reported smoking, cotinine levels, and patterns of smoking in pregnancy. Paediatr Perinat Epidemiol. 2005;19:368–76.
Tong VT, Althabe F, Aleman A, Johnson CC, Dietz PM, Berrueta M, et al. Accuracy of self-reported smoking cessation during pregnancy. Acta Obstet Gynecol Scand. 2015;94:106–11.
Howland RE, Mulready-Ward C, Madsen AM, Sackoff J, Nyland-Funke M, Bombard JM, et al. Reliability of reported maternal smoking: comparing the birth certificate to maternal worksheets and prenatal and hospital medical records, New York City and Vermont, 2009. Matern Child Health J. 2015;19:1916–24.
Hall ES, Wexelblatt SL, Greenberg JM. Self-reported and laboratory evaluation of late pregnancy nicotine exposure and drugs of abuse. J Perinatol. 2016;36:814–8.
Marynak KL, Gammon DG, King BA, Loomis BR, Fulmer EB, Wang TW, et al. National and state trends in sales of cigarettes and e-cigarettes, U.S., 2011-2015. Am J Prev Med. 2017;53:96–101.
Whittington JR, Simmons PM, Phillips AM, Gammill SK, Cen R, Magann EF, et al. The use of electronic cigarettes in pregnancy: a review of the literature. Obstet Gynecol Surv. 2018;73:544–9.
McGraw D. Current and future trends in electronic cigarette use. Int J Psychiatry Med. 2014;48:325–32.
Cantrell J, Kreslake JM, Ganz O, Pearson JL, Vallone D, Anesetti-Rothermel A, et al. Marketing little cigars and cigarillos: advertising, price, and associations with neighborhood demographics. Am J Public Health. 2013;103:1902–9.
Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18:188–204.
Benowitz NL, Hukkanen J, Jacob P, 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009;192:29–60.
Etzel RA. A review of the use of saliva cotinine as a marker of tobacco smoke exposure. Prev Med. 1990;19:190–7.
Dempsey D, Jacob P 3rd, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharm Exp Ther. 2002;301:594–8.
Shin HS, Kim JG, Shin YJ, Jee SH. Sensitive and simple method for the determination of nicotine and cotinine in human urine, plasma and saliva by gas chromatography-mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2002;769:177–83.
Tuomi T, Johnsson T, Reijula K. Analysis of nicotine, 3-hydroxycotinine, cotinine, and caffeine in urine of passive smokers by HPLC-tandem mass spectrometry. Clin Chem. 1999;45:2164–72.
Wexelblatt SL, Ward LP, Torok K, Tisdale E, Meinzen-Derr JK, Greenberg JM. Universal maternal drug testing in a high-prevalence region of prescription opiate abuse. J Pediatr. 2015;166:582–6.
Balhara YP, Jain R. A receiver operated curve-based evaluation of change in sensitivity and specificity of cotinine urinalysis for detecting active tobacco use. J Cancer Res Ther. 2013;9:84–9.
Haufroid V, Lison D. Urinary cotinine as a tobacco-smoke exposure index: a minireview. Int Arch Occup Environ Health. 1998;71:162–8.
Farber HJ, Groner J, Walley S, Nelson K, Section On Tobacco Control. Protecting children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:e1439–67.
Kurti AN, Redner R, Lopez AA, Keith DR, Villanti AC, Stanton CA, et al. Tobacco and nicotine delivery product use in a national sample of pregnant women. Prev Med. 2017;104:50–6.
Wang TW, Asman K, Gentzke AS, Cullen KA, Holder-Hayes E, Reyes-Guzman C, et al. Tobacco product use among adults—United States, 2017. Morb Mortal Wkly Rep. 2018;67:1225–32.
Siu AL, U.S. Preventive Services Task Force. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: U.S. preventive services task force recommendation statement. Ann Intern Med. 2015;163:622–34.
Acknowledgements
This study includes data provided by the Ohio Department of Health, which should not be considered an endorsement of the study or its conclusions. This work was supported by a grant from Chiesi Farmaceutici and by a gift from Amgis Foundation Inc. Although both organizations provided financial support, neither participated in study design, data collection, data analysis and interpretation, or manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SLW and ESH have been consultants for Braeburn Pharmaceuticals. SLW is on the Abbott Nutrition speaker’s bureau. All other authors have no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hall, E.S., McAllister, J.M., Kelly, E.A. et al. Regional comparison of self-reported late pregnancy cigarette smoking to mass spectrometry analysis. J Perinatol 41, 2417–2423 (2021). https://doi.org/10.1038/s41372-021-01045-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-021-01045-2
This article is cited by
-
Rates of substance and polysubstance use through universal maternal testing at the time of delivery
Journal of Perinatology (2022)