Abstract
Cornea topography maps allow ophthalmologists to screen and diagnose cornea pathologies. We aim to automatically identify any cornea abnormalities based on such cornea topography maps, with focus on diagnosing keratoconus. To do so, we represent the OCT scans as images and apply Convolutional Neural Networks (CNNs) for the automatic analysis. The model is based on a state-of-the-art ConvNeXt CNN architecture with weights fine-tuned for the given specific application using the cornea scans dataset. A set of 1940 consecutive screening scans from the Saarland University Hospital Clinic for Ophthalmology was annotated and used for model training and validation. All scans were recorded with a CASIA2 anterior segment Optical Coherence Tomography (OCT) scanner. The proposed model achieves a sensitivity of 98.46% and a specificity of 91.96% when distinguishing between healthy and pathological corneas. Our approach enables the screening of cornea pathologies and the classification of common pathologies like keratoconus. Furthermore, the approach is independent of the topography scanner and enables the visualization of those scan regions which drive the model’s decisions.
Similar content being viewed by others
Introduction
Vision is arguably the most important way we humans navigate and interact with our environment. However, we tend to only notice this once the visual performance is deteriorated due to some pathology. Keratoconus, for example, is a progressive eye disease that affects the cornea of the eye, and is one of the most common cornea pathologies1. The cornea reduces in thickness, and thus starts to bulge out due to the intraocular pressure, an effect that typically results in visual deterioration2,3. In advanced stages, a cornea transplant is the only feasible treatment option. If diagnosed early, however, less invasive treatments like Corneal Cross Linking (CXL) or Intracorneal Ring Segments (ICRS) can be used to reduce disease progression4.
When visiting an ophthalmologist, often the corneal topography is inspected using an OCT scan. OCT scans are non-invasive and are often part of standard diagnostic procedures in ophthalmology. However, especially in early stages, keratoconus and other corneal pathologies are hard to detect, making a diagnosis based on these scans extremely difficult. According to a study from the Department of Opthalmology at the Cantonal Hospital of Lucerne5, the difficulty of diagnosis may also be compounded by weaker than expected levels of keratoconus expertise among general ophthalmologists. Some corneal topography devices already calculate an index like the CASIA2 Ectasia Severity Index (ESI)6 or the Pentacam Belin-Ambrósio enhanced ectasia display (BAD)7, on how deformed a cornea is. Some assumptions underlying those indexes, like the separation of the cornea in < 3 mm and > 3 mm zones in the BAD or the focus on asymmetry in the ESI (thus neglecting central keratoconus cases), seem arbitrary to the authors. Those algorithms are also unable to distinguish between different corneal pathologies8.
Data and image classification techniques have become increasingly relevant and useful in everyday life situations and also in the medical field9,10,11. Such techniques are successfully used in oncology12, dermatology13 and many other domains14 to help diagnose diseases.
Major advancements in the field of computer vision and image classification helped to improve the accuracy and reliability in such classification tasks15. Among such advancements, Convolutional Neural Networks (CNN) are a class of deep neural networks which take inspiration from the way mammal brains interpret vision signals from the eye16. CNN are especially powerful in analyzing unstructured data such as images. Using a deep learning approach, pathologies can be detected and classified automatically, without the need for experts to interpret indexes that are different for every device.
The main aim of this study was to develop a method of using preprocessed corneal OCT screening data to automatically assess corneal health, and potentially to discriminate between different diseases and conditions such as keratoconus. This could be of significant benefit in a screening setting as part of a standard OCT diagnosis, where identifying corneal pathologies and abnormalities at an early stage would allow appropriate treatments to be applied promptly, improving outcomes.
Using preprocessed OCT data from a CASIA2 device, we demonstrate a model initially developed for determining whether a cornea is healthy or abnormal, and evaluate the potential of further training the model to distinguish between individual disorders and abnormalities using more detailed labelled data.
Methods
Our proposed process first represents the numerical results of OCT scans as images, and then use a CNN to diagnose corneal abnormalities, especially keratoconus. The visual representation of the OCT as basis for diagnosis has several advantages. Firstly it corresponds to the way humans identify abnormalities in the cornea; secondly, as the parts of the image that are most influential for a particular outcome of a neural network can be easily identified and visualized, results can be examined and checked (explainable AI, or XAI17); and finally, a visual representation is relatively independent of the device and the setting used.
As a first step, classical Machine Learning (ML) methods such as k-means and random forest have been trained and evaluated on the raw scan data as baseline. The use of raw scan data means that in the future other cornea diagnostics devices like corneal tomography or Scheimpflug devices18 may not be supported by these methods. All the devices however can produce topography maps as those maps are used for diagnosis by ophthalmologists. Also, these classical ML methods lag in performance compared to modern CNN architectures, and they were not considered further.
In terms of technology, the entire processing was done in the programming language Python, version 3.10, running on a computer with an NVIDIA RTX A5000 GPU.
Cornea scans dataset
All anterior OCT scans were performed with a CASIA2 anterior OCT device by the Saarland University Hospital Clinic for Ophthalmology as part of a standard patient screening procedure. The device calculates various features from the measured cornea data. For this study the anterior axial refraction, posterior axial refraction, anterior elevation, posterior elevation and pachymetry were used. The data is provided as a 2D matrix of (32 \(\times \) 400) data points in a polar format. Some faulty scans by the device with missing or invalid data points were filtered out as part of a data quality assessment19. The CASIA2 masks data points where no valid data is available, for example when the eyelid obstructs the cornea. Scans with any masked value in the central 2mm area were discarded. This resulted in approximately 10% of the scans being filtered. Some of these scans for example had a pachymetry of several centimeters and more. Supplementary Figure S1 shows an example of a faulty scan.
The raw measurement data were represented as images using the function contourf in the Python package matplotlib20; these images were annotated by a cornea expert to obtain the labels for the supervised learning task. To support the annotation process, the keratoconus ABCD score21 and the asymmetry and astigmatism in the inner 1.5 mm of the cornea were also provided.
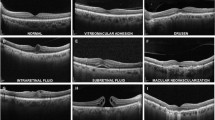
As each cornea scan could potentially contain any cornea pathology, the most common cornea pathologies and abnormalities were annotated. All technically well-recorded scans were used to get a realistic real-world data sample. Besides keratoconus (the most frequent pathologic condition), several other clearly defined conditions have been used as labels despite insufficient training data for a few of them. Specifically, the following labels were given: healthy, keratoconus, post laser, keratoglobus, pellucid marginal corneal degeneration (PMD), other (less common diseases/scars/irregularities in the cornea) and not appreciable (N/A) (faulty, invalid or unidentifiable scans by the OCT device). Supplementary Figures S2 to S8 show examples of the different labels.
Traditional statistical approaches are not able to filter out all invalid scans. Some scans might look valid from a data distribution perspective but do not allow an ophthalmologist to assess the health condition. Those scans were labeled as N/A and then also used during model training, as in a clinical setting such invalid scans might be present.
The annotated 1940 cornea scans from 899 patients were randomly split into a training (containing 80% of the available data) and validation dataset. Hold-out validation was performed to evaluate the models’ performance while training. Table 1 presents the split of the cornea scans dataset and the number of scans used for every set.
To evaluate the final model, a completely new, disjoint and more recent test dataset was collected, consisting of 242 scans from 188 patients. The scans were performed with the same CASIA2 device in the same clinic as the training and validation scans. The test dataset only contains scans from patients not present in the training or validation datasets. The labeling was done in the same way as the labeling of the training and validation dataset, but this time by two different cornea experts. For 15 (6.20%) scans the two experts disagreed on the label. These cases were discussed between the experts in order to agree on a final label.
Deep neural network architecture
Recent trends in computer vision using deep learning have shown that the ConvNeXt convolutional neural network architecture22 is well suited for classification of image data. Compared to traditional machine learning approaches, these architectures do not require domain knowledge to do feature engineering, as the relevant features are extracted automatically by the hidden layers of the neural network based on the training data. For this study, we have started with the existing ConvNeXt architecture provided by the Python package torchvision23 within the pytorch framework24.
In order to use ConvNeXt for cornea disease classification, some adjustments to its architecture were necessary. For each cornea, the axial refractive power and the elevation of both the anterior and posterior surface of the cornea, as well as its thickness (pachymetry), are obtained from the scan. This results in five partially dependent maps, each of which can be represented as gray-scale image. Cornea abnormalities are then visible at the same location in several of these gray-scale images. As CNN architectures are powerful extractors of relevant features from spacial data, all gray-scale images of a cornea were stacked together to form a five-channel pseudo-image. The original ConvNeXt architecture takes three channels as input, one channel each for red, green and blue. For each of the three color channels, the information can be represented as gray-scale map. To make all available information from a cornea scan available to the model, the three channels from ConvNeXt are expanded to five channels, copying the pre-trained weights from torchvision. Figure 1 illustrates how the model receives the cornea maps as input, and how the predicted output is visualized. We call this new architecture CorNeXt.
List of all the feature maps and how they are used as input for the CorNeXt model architecture. The topography maps are generated using matplotlib20 based on the exported raw data from the CASIA2 device. (a) The raw data extracted from the CASIA2 device from a single cornea scan. (b) The features anterior and posterior axial refractive and elevation and pachymetry stacked as the five input channels for the CorNeXt model. (c) The softmax probability distribution of the models’ prediction over all classes.
The CorNeXt model was trained in multiple iterations to evaluate several model configurations. The final model hyper-parameter configuration is shown in Table 2. We found that training all weights of the CNN model resulted in better performance than just training the classification head. As a loss function negative log likelihood was used.
All weights of the model are transfer learned from the pre-trained ConvNeXt weights provided by torchvision. As optimizer AdamW is used, inspired by the original ConvNeXt implementation together with the ReduceLROnPlateau learning rate scheduler. While training, the model is augmented using RandomVerticalFlip. Augmentation is limited to RandomVerticalFlip because the position of the eye is fixed when creating an OCT scan.
To evaluate the model performance, the accuracy, F1 score, sensitivity, specificity and the Area Under the Receiver Operating Characteristic (AUROC) are calculated using the torchmetrics library25. The best model was selected based on the F1 score with a weighted average considering each class’s support. The F1 score is a method to measure a statistical test’s accuracy and allows a meaningful evaluation even when the dataset has an uneven class distribution.
Determining whether a prediction was made for the correct reasons is the core topic of model explainability17. To do so, integrated gradient26 from the captum27 library was used to visualize which input features are most relevant for a model to make a certain prediction.
A Python based web application was developed to enable easy interaction with the CorNeXt model. In addition to that, the application allows researchers and ophthalmologists to visualize the data used for the prediction to verify whether the predictions are plausible.
Results
In a screening setting, the focus is on reliably identifying patients with any kind of cornea abnormality. For this evaluation, all metrics are evaluated as a binary classification consisting of the two classes healthy and abnormal. A scan is considered abnormal when it has been labeled as either keratoconus, post laser, PMD, keratoglobus or other. Note that all data points annotated as n/a were removed for this evaluation as it is not clear if the cornea is healthy or not in those cases. Post laser was treated as abnormal for two reasons: (1) namely before laser or cataract surgeries, it is essential to identify any cornea abnormality (including previous laser treatment), and (2) more often than not, post laser is misinterpreted as some other cornea pathology, since laser treatment can alter the shape of the cornea by quite some extent depending on the desired correction of the refractive error28. The evaluation result on the test cornea scans dataset is shown in Table 3. Figure 2 shows the confusion matrix and the confidence of the model when classifying a cornea as healthy or abnormal with respect to the correct label. A threshold of 0.5 on the softmax output was used to determine the predicted class. The CorNeXt model is able to distinguish between healthy and abnormal with very good performance.
The performance of the CorNeXt model on the test cornea scans dataset in distinguishing between healthy and abnormal cornea scans. (a) The confusion matrix of the model. (b) How confident the model is when classifying a cornea scan as healthy or abnormal. The threshold for probabilistic model output was set to 0.5 (marked by the vertical line).
In a further experiment, we investigate whether the model is able to yield a more granular interpretation of the scans and distinguish between different conditions. In general, the model identifies the different classes with varying performance. For all classes except healthy and keratoconus, there are too few samples in the test dataset to make reliable statements on model performance.
The performance of the model on the test dataset is shown in Table 4 and the confusion matrix is displayed in Fig. 3.
The performance of the model on the validation cornea scans dataset is reported in the supplementary material (Supplementary Tables S1, S2; Supplementary Figure S9). Comparing the performance on the different datasets can reveal potential overfitting: the comparison between validation and test data shows that the conclusions on the model architecture that were based on the performance of different variants on the validation set, are confirmed on new data.
When interpreting the results, it should be kept in mind that all the data is consecutive screening data. Hence, there might be a potential drift in the characteristics of the scanned eyes.
As the correct interpretation of an OCT scan is critial, we emphasize the importance of explainable artifical intelligence17. For image inputs, integrated gradients26 show which areas were most relevant to yield the predicted class label, and thus allow an expert to compare the key regions for the model with the most relevant area for a human-made diagnosis.
Figure 4 shows the integrated gradients on a correctly identified sample input scan with keratoconus. For each of the five input channels, a corresponding gray-scale heatmap depicts the importance of a given pixel in the input channel for the final label. The obtained class label is mainly based on the ectatic area in the cornea; which corresponds to the area deemed most important for a keratoconus by human experts.
We also present a modern web application as shown in Fig. 5 to analyze anterior segment OCT scans and predict cornea pathologies. The application shows the probability for each of the most common cornea pathologies, predicted using the developed model. In addition to that, axial refraction, elevation and pachymetry maps and state-of-the art metrics like keratoconus ABCD score21 as well as asymmetry and astigmatism calculations are displayed to cross-check the prediction results.
The scan details page of the developed Eye Analyzer application. At the very top the predictions of the CorNeXt model are visualized. This includes a probability distribution over all classes using a softmax output. Furthermore, the keratoconus ABCD score and asymmetry/astigmatism in the inner 1.5 mm region are displayed. The topography maps are also shown as a way to cross-check the models’ prediction results. Those maps are generated using the same methods as the input images for the model.
Discussion
The model accurately distinguishes healthy corneas from corneas with abnormalities. The classification of different types of abnormality heavily depends on the available training data and variance inside a class.
The performance between classes differs significantly. While healthy and keratoconus are well recognized, post laser, keratoglobus, PMD, other and N/A seem to be very challenging for the model. Unfortunately the test dataset does not contain enough samples of these classes to draw any meaningful conclusions about the performance of the model.
The confusion matrix in Fig. 3 shows that the model has difficulties distinguishing between the post laser, keratoconus and healthy classes. Looking at some samples of misclassification, we see that (1) the class post laser has quite a high variance and (2) some post laser treatments look very similar to healthy eyes. Hyperopic post laser treatments can also be misclassified as keratoconus, as the topography looks very similar to a keratoconus eye.
A comparison of the performance of the proposed CorNeXt with methods and results published elsewhere is challenging, mostly because there is no reference dataset. Similar works30 show large variations in terms of the types of cornea scanners used, sample size, validation methods and performance measurements. As far as we know, this study is the first to use a completely random sample set of consecutive screening data. Consequently, we believe that the results presented here come close to the performance that could be expected in a clinical setting.
An intuitive application called Eye-Analyzer was developed to enable ophthalmologists and researchers to easily analyze cornea OCT scans and interact with the AI model. The application can help to diagnose ectatic cornea diseases like keratoconus even in an early stage, where minimal invasive treatment options such as CXL or ICRS implementations are available. This might delay or even prevent corneal grafting or living with sometimes significantly decreased vision. The Eye-Analyzer application could also be used to analyze corneas before a cataract or laser surgery, looking for any abnormalities.
Currently, the Eye-Analyzer application can only be used for research purposes, as validation or certification as a medical product would be required to be used by ophthalmologists to diagnose patients corneas. Furthermore, some classes have barely satisfactory performance and more training data would need to be used to have a more stable performance predicting keratoglobus, PMD, post laser and other.
This study demonstrates that it’s possible to analyze anterior OCT cornea screening scans using CNNs. The proposed CorNeXt model is able to automatically assess corneal health with state-of-the-art performance compared to publicly available work30. Predicting the most common corneal diseases in screening data seems to be a much more challenging task. The performance of different classes varies depending on their variance and the number of samples available for training and validation. For some classes like keratoglobus, PMD, post laser and other there was insufficient validation data to draw any conclusions on their performance. Nevertheless, we still think it is important to not exclude this data, as it would artificially alter the dataset and could not be considered screening data anymore.
It is highly recommended to continue development of the model. The model predictions should also be compared to that of senior ophthalmologists specialized in cornea diseases. In addition to that, the dataset should be independently labeled by another ophthalmologist to better understand labeling differences between human experts. Using more training data would certainly also help to increase the model’s performance. This could be done either by labeling more data or adding an application feature for users to give feedback on the prediction results. Furthermore, a gold standard challenge to diagnose cornea abnormalities could be created, making comparison of such methods more reliable in the future.
Data availability
The data that supports the findings of this study was provided by Saarland University Hospital Clinic for Ophthalmology under license. The data can be made available upon request to the authors with permission of the Saarland University Hospital Clinic for Ophthalmology.
References
Espandar, L. & Meyer, J. Keratoconus: Overview and update on treatment. Middle East Afr. J. Ophthalmol. 17, 15. https://doi.org/10.4103/0974-9233.61212 (2010).
Kenney, M. C. & Gaster, R. N. Keratoconus. In Ocular Disease 42–48 (W.B. Saunders, 2010). https://doi.org/10.1016/B978-0-7020-2983-7.00006-1.
Kurna, S. A., Altun, A., Gencaga, T., Akkaya, S. & Sengor, T. Vision related quality of life in patients with keratoconus. J. Ophthalmol.https://doi.org/10.1155/2014/694542 (2022).
Oyeniran, E. & Tauqeer, Z. Update in the management of keratoconus. Ad. Ophthalmol. Optometry 6, 307–324. https://doi.org/10.1016/j.yaoo.2021.04.019 (2021).
Baenninger, P. B. et al. Mismatch of corneal specialists’ expectations and keratoconus knowledge in general ophthalmologists—a prospective observational study in Switzerland. BMC Med. Educ. 21, 297. https://doi.org/10.1186/s12909-021-02738-0 (2021).
Dembski, M., Nowińska, A., Ulfik-Dembska, K. & Wylęgała, E. Swept source optical coherence tomography analysis of the selected eye’s anterior segment parameters. J. Clin. Med.https://doi.org/10.3390/jcm10051094 (2021).
Doctor, K. et al. Simplifying and understanding various topographic indices for keratoconus using Scheimpflug based topographers. Indian J. Ophthalmol. 68, 2732. https://doi.org/10.4103/ijo.IJO_2111_20 (2020).
Spira, C. et al. Comparison of the specificity and sensitivity of various instrument-guided keratoconus indices and classifiers. Ophthalmologe 112, 353–358. https://doi.org/10.1007/s00347-014-3135-8 (2015).
An, F., Li, X. & Ma, X. Medical image classification algorithm based on visual attention mechanism-MCNN. Oxid. Med. Cell. Longev. 2021, 6280690. https://doi.org/10.1155/2021/6280690 (2021).
Hirano, H., Minagi, A. & Takemoto, K. Universal adversarial attacks on deep neural networks for medical image classification. BMC Med. Imaging 21, 9. https://doi.org/10.1186/s12880-020-00530-y (2021).
Litjens, G. et al. A survey on deep learning in medical image analysis. Med. Image Anal. 42, 60–88. https://doi.org/10.1016/j.media.2017.07.005 (2017).
Debelee, T. G., Kebede, S. R., Schwenker, F. & Shewarega, Z. M. Deep learning in selected cancers’ image analysis—a survey. J. Imaginghttps://doi.org/10.3390/jimaging6110121 (2020).
Caffery, L. J. et al. Transforming dermatologic imaging for the digital era: Metadata and standards. J. Digit. Imaging 31, 568. https://doi.org/10.1007/s10278-017-0045-8 (2018).
Basu, K., Sinha, R., Ong, A. & Basu, T. Artificial intelligence: How is it changing medical sciences and its future?. Indian J. Dermatol. 65, 365. https://doi.org/10.4103/ijd.IJD_421_20 (2020).
Qu, H., Rahmani, H., Xu, L., Williams, B. & Liu, J. Recent advances of continual learning in computer vision: An overview. arXivhttps://doi.org/10.48550/arXiv.2109.11369 (2021).
Lindsay, G. W. Convolutional neural networks as a model of the visual system: Past, present, and future. J. Cogn. Neurosci. 33, 2017–2031. https://doi.org/10.1162/jocn_a_01544 (2021).
Tjoa, E. & Guan, C. A survey on explainable artificial intelligence (XAI): Toward medical XAI. IEEE Trans. Neural Netw. Learn. Syst. 32, 4793–4813. https://doi.org/10.1109/tnnls.2020.3027314 (2021).
Kanellopoulos, A. J. Scheimpflug vs Scanning-slit corneal tomography: Comparison of corneal and anterior chamber tomography indices for repeatability and agreement in healthy eyes. Clin. Ophthalmol. (Auckland, N.Z.) 14, 2583. https://doi.org/10.2147/OPTH.S251998 (2020).
Pipino, L., Lee, Y. & Wang, R. Data quality assessment. Commun. ACMhttps://doi.org/10.1145/505248.506010 (2003).
Hunter, J. D. Matplotlib: A 2d graphics environment. Comput. Sci. Eng. 9, 90–95. https://doi.org/10.5281/zenodo.7697899 (2007).
Duncan, J. K., Belin, M. W. & Borgstrom, M. Assessing progression of keratoconus: Novel tomographic determinants. Eye Vis.https://doi.org/10.1186/s40662-016-0038-6 (2016).
Liu, Z. et al. A convnet for the 2020s. https://doi.org/10.48550/ARXIV.2201.03545 (2022).
Maintainers, T. & contributors. TorchVision: PyTorch’s Computer Vision library (2016).
Paszke, A. et al. Pytorch: An imperative style, high-performance deep learning library. In Advances in Neural Information Processing Systems Vol. 32 (eds Wallach, H. et al.) 8024–8035 (Curran Associates Inc., 2019).
Nicki Skafte Detlefsen et al. TorchMetrics—measuring reproducibility in PyTorch. https://doi.org/10.21105/joss.04101 (2022).
Sundararajan, M., Taly, A. & Yan, Q. Axiomatic attribution for deep networks. https://doi.org/10.48550/ARXIV.1703.01365 (2017).
Kokhlikyan, N. et al. Captum: A unified and generic model interpretability library for pytorch (2020). 2009.07896.
Ong, H. S. et al. Corneal ectasia risk and percentage tissue altered in myopic patients presenting for refractive surgery. Clin. Ophthalmol. (Auckland, N.Z.) 13, 2003. https://doi.org/10.2147/OPTH.S215144 (2019).
Waskom, M. L. Seaborn: Statistical data visualization. J. Open Source Softw. 6, 3021. https://doi.org/10.21105/joss.03021 (2021).
Maile, H. et al. Machine learning algorithms to detect subclinical keratoconus: Systematic review. JMIR Med. Inform. 9, e27363. https://doi.org/10.2196/27363 (2021).
Acknowledgements
We would like to express our gratitude to Dr. Kitti Kormányos and Dr. Nóra Szentmáry for sharing their expertise in the field of ophthalmology and providing useful feedback on the created application and algorithms. We would also like to thank Dr. Kitti Kormányos for labeling the data. Without their help, this study would not have been possible.
Author information
Authors and Affiliations
Contributions
All authors contributed to the design of the study. A.L. provided the required dataset, and B.F. conceived the experiments, designed and implemented all algorithms and the application. All authors analyzed the results and worked on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fassbind, B., Langenbucher, A. & Streich, A. Automated cornea diagnosis using deep convolutional neural networks based on cornea topography maps. Sci Rep 13, 6566 (2023). https://doi.org/10.1038/s41598-023-33793-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-33793-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.