Abstract
A diplogastrid nematode was isolated from a dung beetle, Onthophagus sp., collected from a rotten mushroom in Kyoto, Japan. The species is characterised by its cheilostomatal shape, separated into 12 narrow plates (rugae), deep stegostom, large ellipsoidal amphids, conical female tail and characteristic receptaculum seminis in the female. Based on its phylogenetic status and stomatal composition, the species is typologically similar to two other diplogastrid genera, Neodiplogaster and Mononchoides. The species can be distinguished from these two genera by the size and shape of the amphid (small pore in Neodiplogaster), female tail shape (long and filiform in Mononchoides) and presence of receptaculum seminis (absence in the two nominal genera), and is described as a monotypic member of a new genus, Onthodiplogaster japonica n. gen., n. sp. Observation of feeding behaviour suggested that O. japonica n. gen., n. sp. does not show clear stomatal dimorphism or polymorphism, which is found in its close relatives, but the species can feed on nematodes (predation), fungi and bacteria. This monomorphic omnivory possibly represents its habitat of dung and other rotten materials, where the environment is biologically divergent, and its condition changes rapidly.
Similar content being viewed by others
Introduction
The nematode family Diplogastridae Micoletzky is a highly divergent group with respect to feeding and stomatal morphology (feeding structure). The family is derived from bacteria feeders, and diverged to more than 30 genera, including several insect parasites, fungal feeders and predators1,2. Some diplogastrid genera have monomorphic short or long tube-like stomas without a conspicuous tooth, and feed on bacteria; others have various, short tube or barrel-like stomas with various-shaped teeth, ridges and denticles1,2. Interestingly, the general stomatal morphology is not clearly correlated with phylogeny, i.e., each form appears in separate lineages, suggesting genetic plasticity in stomatal morphology. In addition, many lineages of the family show stomatal dimorphism or polymorphism3,4. For example, the genus Pristionchus Kreis, a model system for developmental (phenotypic) plasticity, is a bacteria feeder with narrow short tube-like stoma (stenostomatous form), and its predatory form with wide barrel-shaped stoma (eurystomatous form) occurs according to environmental cues, e.g., shortage of bacteria and high population density5. The other diplogastrid genus, Neodiplogaster Cobb, is a group of fungal feeders, and its eurystomatous form is induced by environmental factors and the presence of potential prey species4,6.
Pristionchus and related genera have been investigated extensively in terms of the evolutionary biology of phenotypic plasticity and several other fields5,7,8,9,10. These omnivorous (dimorphic or polymorphic) species feed on bacteria or fungi and prey nematodes with two (or multiple) types of mouth forms.
In this study, during a field survey of insect-associated nematodes, a species of diplogastrid nematode was isolated from a dung beetle, Onthophagus sp. The nematode species showed omnivorous feeding, i.e., fungi, bacteria and prey nematodes, without clear stomatal dimorphism. The nematode is taxonomically described and illustrated as Onthodiplogaster japonica n. gen., n. sp. In addition, its feeding range was experimentally evaluated using cultured materials.
Materials and methods
Several adult dung beetles, Onthophagus sp., were collected from a rotten mushroom, Boletus violaceofuscus W. F. Chiu (Agaricomycetes: Boletaceae) in the experimental Quercus myrsinifolia Blume stand in the Kansai Research Center, Forestry and Forest Products Research Institute, Kyoto, Japan (GPS: 34.94166666 N, 135.77361111 E, 77 m a.s.l.) in July 2021. The beetles were dissected individually on 2.0% water agar (2.0% agar without nutrients) and stored in the laboratory at room temperature (20–25 °C). The plates were observed for nematode propagation on the dissected beetle body for 2 weeks. Propagated nematodes were observed under a dissecting microscope (S8 Apo; Leica, Wetzlar, Germany) to determine their feeding habit, i.e., bacterial feeder, fungal feeder, or predator, and transferred to appropriate media using a stainless-steel insect pin (Insect Pin No. 00; Shiga Kontyu, Tokyo, Japan). The nematodes were considered bacteria feeders and were transferred to nematode growth medium (NGM) seeded with Escherichia coli strain OP50, a standard food for bacteriophagous nematodes. The successfully cultured nematode strain was stored as a laboratory culture with the code NKZ390.
Morphological observation
Adult nematodes were recovered from 2-week-old cultures on E. coli OP50. Approximately one-third of the nematodes were used for morphological observation, according to Kanzaki11. There, about 100 males and females were casually observed to confirm the consistency of their morphology, and several individuals were examined closely. In addition, several individuals found feeding on fungi or preying on nematodes (Acrobeloides sp.) were examined for their stomatal morphology. Micrographs were obtained using a digital camera system (MC170 HD; Leica), and morphological drawings were made using a drawing tube connected to the microscope (Eclipse Ni; Nikon, Tokyo, Japan). Drawings and micrographs were used to construct figures with Photoshop Elements 2020 (Adobe, San Jose, CA, USA).
The remaining individuals were heat-killed at 55 °C for 30 s., and fixed in TAF (2.0% triethanolamine, 7.0% formalin) for 1 week. Nematodes were then processed to glycerine using the modified Seinhorst method12 and mounted in glycerine according to Maeseneer and d’Herde13. Mounted materials were used for morphometric analysis and deposited as type specimens.
Scanning electron microscopy
The surface structures of the new species were observed by scanning electron microscopy (SEM). Adult females and males were collected from cultures on an NGM plate seeded with E. coli OP50 and transferred to M9 buffer. Nematodes were killed by heating on a hot plate (60 °C for 1 min.), and pre-fixed in 2% paraformaldehyde plus 2.5% glutaraldehyde in 0.1 M phosphate-buffered saline (PBS) for 15 h at 4 °C. Nematodes were post-fixed with 1% osmium tetroxide in 0.1 M PBS for 1.5 h and dehydrated in an ethanol series (25, 50, 70, 80, 90, and twice in 99.5% ethanol, 5 min each). Next, samples were immersed three times (5 min each) in 100% tert-butyl alcohol and lyophilised (JFD-310; JEOL, Tokyo, Japan). Samples were coated with osmium using a sputter coater (HPC-1SW; Vacuum Device, Ibaraki, Japan), and observed by SEM (JSM-6700F; JEOL).
Feeding preference examination
The nematode was initially cultured on E. coli OP50. However, because of its typological similarity to the fungal feeding/predator Neodiplogaster, the feeding preference of O. japonica n. gen., n. sp. was examined using the prey species Acrobeloides sp., the standard food for Seinura spp. in our laboratory14,15, and two species of fungi, Botrytis cinerea Pers., standard food for Neodiplogaster acaloleptae Kanzaki6,16, and an unidentified Mucorales fungus isolated from a carrier Onthophagus sp.
Potential foods were inoculated on appropriate media, i.e., NGM was seeded with E. coli OP50 for Acrobeloides sp., and 2.0% malt extract agar (2.0% malt extract, 2.0% agar) was used for the two fungal species. Nematodes reared on E. coli OP50 were isolated by pouring deionised water on the media, rinsed three times with deionised water, and approximately 100 individuals of various stages were inoculated onto the potential foods. Therefore, E. coli OP50 was retained in the fungal culture. Three culture plates were used for each potential food. Thereafter, the feeding behaviour of the nematode was observed under a dissecting microscope and a light microscope, and its propagation was confirmed under a dissecting microscope.
Molecular profiling and phylogeny
Several adult individuals were selected from cultured material (as described above), and transferred to nematode lysis solution17,18 individually for DNA extraction. These nematodes were digested at 55 °C for 20 min, and the lysates served as the templates for PCR. First, the materials were individually amplified and the D2-D3 expansion segments of the large subunit of ribosomal RNA (D2-D3 LSU) were sequenced according to Ye et al.19 to confirm the species identity. Thereafter, approximately 4 kb of ribosomal RNA including near-full-length small subunit (SSU; ~ 1.7 kb), internal transcribed spacer region (~ 0.9 kb) and D1-D4 expansion segments of the large subunit (D1-D4 LSU; ~ 1.4 kb) were determined following the methodology by Ekino et al.20 and Kanzaki et al.21. The partial sequence of mitochondrial cytochrome oxidase subunit I (mtCOI; 660 bp) was determined following the methodology of Kanzaki & Futai22. The sequences of the new species were deposited in the GenBank database under accession numbers LC721118 (rDNA) and LC721119 (mtCOI).
The SSU and D2-D3 LSU were subjected to molecular phylogenetic analysis. First, both sequences were compared with those in the database by BLAST homology searching (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome). According to the BLAST results and prior reports23,24, sequences for phylogenetic analysis were retrieved from the database (Table S1). Although the partial sequence of mtCOI gene was determined, the sequence was used only as a characteristic of the species, because the number of diplogastrid mtCOI sequences deposited in the GenBank database is not sufficient for phylogenetic analysis.
The sequences were aligned using MAFFT25,26 (http://align.bmr.kyushu-u.ac.jp/mafft/software/). The substitution model and parameters were determined by MEGA 727 for each locus, and Bayesian phylogenetic analysis was conducted in MrBayes 3.228,29; four chains were run for 4 × 106 generations. Markov chains were sampled at intervals of 100 generations30. Two independent runs were performed, and, after confirming the convergence of runs and discarding the first 2 × 106 generations as burn-in, the remaining topologies were used to generate a 50% majority-rule consensus tree.
Results
Recovered nematodes
In addition to O. japonica n. gen., n. sp. Tokorhabditis atripennis Ragsdale, Kanzaki, Yamashita & Shinya was isolated, and will be the subject of a future study.
Phylogenetic status of Onthodiplogaster japonica n. gen., n. sp
The phylogenetic status of O. japonica n. gen., n. sp. is shown in Fig. 1 and Suppl. Fig. S1. Onthodiplogaster japonica n. gen., n. sp. is included in the clade that harbours Tylopharynx de Man, Mononchoides Rahm, Neodiplogaster, Sachsia Meyl, Paroigolaimella Paramonov, Eudiplogaserium Meyl, Cutidiplogaster Fürst von Lieven, Uni, Ueda, Barbuto & Bain and the undescribed genus “ST” (short tail) Diplogastridae sp. isolated from manatee skin24 with maximal support (100% posterior probability). Several important typological characteristics, e.g., presence of receptaculum seminis, are indicated by asterisks in the phylogenetic tree (Fig. 1).
Molecular phylogenetic relationships among diplogastrid nematodes. The Bayesian tree inferred from near full length of SSU and D2-D3 LSU of ribosomal RNA genes. The GTR + G + I model was applied to both loci, and the parameters are as follows: AIC = 50,457.865; lnL = − 24,991.621; freqA = 0.25, freqC = 0.21, freqG = 0.27, freqT = 0.27; R(a) = 0.93, R(b) = 2.55, R(c) = 2.11, R(d) = 0.91, R(e) = 3.75, R(f) = 1.00; Pinva = 0.39; Shape = 0.58 for SSU, and AIC = 54,594.498; lnL = -27,059.552; freqA = 0.21, freqC = 0.22, freqG = 0.32, freqT = 0.25; R(a) = 0.46, R(b) = 1.69, R(c) = 0.90, R(d) = 0.43, R(e) = 3.42, R(f) = 1.00; Pinva = 0.21; Shape = 1.00 for D2–D3 LSU. Posterior probability (PP) values exceeding 50% are given on appropriate clades. Several clades far from the one new genus belong were compressed, and full phylogenetic tree is provided as Suppl. Fig. S1.
Feeding preference of Onthodiplogaster japonica n. gen., n. sp
Nematodes showed active feeding and propagation on Acrobeloides sp. (Suppl. Movies S1, S2) and B. cinerea (Suppl. Movie S3). Although feeding behaviour was observed on the Mucorales fungus (Suppl. Movie S4), the nematode did not propagate on the fungus, and could not be subcultured. In addition, although O. japonica n. gen., n. sp. sometimes ate dead individuals, it was unclear whether the behaviour was cannibalism or scavenging. The behaviour of feeding on dead conspecific nematodes was not observed when co-cultured with Acrobeloides sp. on OP50.
The feeding behaviour, i.e., movements of stoma and pharynx and their coordination, was similar to that of Neodiplogaster and Mononchoides species6,31 regardless of food source.
Taxonomic description of Onthodiplogaster n. gen
The generic epithet Onthodiplogaster, ‘ontho’ (= dung in Latin) + Diplogaster is derived from its carrier insect, Onthophagus spp., and its primary habitat. Although the type strain was isolated from a beetle on a rotten mushroom, the primary habitat of this phoretic nematode is usually determined by the carrier insect, and thus, the primary habitat of the nematode is hypothesized to be dung, carrion and other rotten materials which the dung beetle inhabits.
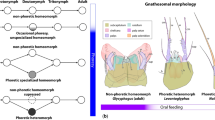
Diplogastridae. Body cylindrical, moderate to stout; typical for the family. Cuticle thick, with fine annulation and clear longitudinal striations. Lateral field weakly developed, sometimes difficult to distinguish from striations, but can be distinguished by lack of annulation, seemingly composed of two bands, i.e., three lines were observed. Six equal-sized lip sectors not clearly separated from each other, forming a dome shape, without clear constriction, continuous with body contour. Six short and papilliform labial sensilla present in male and female, and four small, papilliform cephalic papillae present in male, as typical for diplogastrid nematodes. Amphid large, ellipsoidal, located at the level of the posterior end of cheilostom. Stomatal dimorphism possibly present, i.e., the individuals feeding on other nematodes (predation) have somewhat wider stoma than microbe-feeding individuals, but no structural difference was observed. In addition, male has generally narrower stoma than female. Stoma separated into three sections: cheilo-, gymno- and stegostom. Cheilostom short, twice as wide as its depth, separated into 12 narrow plates (rugae). Gymnostom forming dorsally opened (notched) short cuticular tube with serrated anterior edge, separated into two (anterior and posterior) subsections, each derived from different arcade syncytia, but the margin is not clearly observed, and anterior end internally overlaps with the posterior end of cheilostom. Stegostom separated into three subsections: pro-meso-, meta- and telostegostom. Pro-mesostegostom not clearly cuticularised, internally overlapping with gymnostom. Metastegostom with a square- or diamond-shaped movable dorsal tooth, a square- or diamond-shaped right subventral tooth with several anterior spinous processes, and several left subventral ridges; anterior end of both teeth varies a little among individuals, from relatively simple triangular to claw-like shape; both teeth and ridges were connected to the stegostomatal cylinder (= telostegostom). Stegostom forming a cuticular cylinder, with a dorsal apodeme which appears as a narrow extension of dorsal telostegostomatal wall in lateral view, and wing or trapezoidal shape in ventral view. The posterior ends of right and left subventral sectors of telostegostom slightly expand, forming weakly developed apodeme. Dorsal pharyngeal gland orifice on the middle of dorsal tooth, i.e., the gland duct penetrates the dorsal tooth. Male gonad single, anteriorly reflexed. Male with nine pairs of papilliform genital papillae. Male tail with a spike of moderate length and sharply pointed tip. Female gonad paired, with oval or kidney-shaped receptaculum seminis at the dorsal side of the junction among vagina and anterior and posterior gonads. Female tail elongate conoid with pointed tip, i.e., without filiform tip.
-
Phylum Nematoda Potts, 1932
-
Class Chromadorea Inglis, 1983
-
Order Rhabditida Chitwood, 1933
-
Family Diplogastridae Micoletzky, 1922
Onthodiplogaster Kanzaki, Ikeda & Shinya, n. gen.
https://zoobank.org/NomenclaturalActs/60A942AC-1064-4F93-BAC1-71DA6341C30C.
Type species: Onthodiplogaster japonica n. gen., n. sp.
Relationships
Onthodiplogaster n. gen. is characterised by its stomatal morphology, possessing 12 rugae in cheilostom and a cuticular stegostomatal cylinder, large ellipsoidal amphid and the presence of oval or kidney-shaped receptaculum seminis in the female.
These characteristics have been reported for other genera of the family. The cheilostomatal rugae are present in Fictor Paramonov, Neodiplogaster, Mononchoides and two species of Allodiplogaster Paramonov & Sobolev1,32,33. In addition, Oigolaimella Paramonov has cheilostom separated into many plates with a pointed anterior end referred to as a “corona”34. Large amphid is reported in Demaniella Steiner, Goffartia Hirschmann, Sachsia and several species of Mononchoides1,35,36,37. Receptaculum seminis is present in Diplogasteroides de Man sensu lato, Pseudodiplogasteroides Körner and Acrostichus Rahm1,4. Phylogenetically, Onthodiplogaster n. gen. is included in a clade with Tylopharynx, Mononchoides, Neodiplogaster, Sachsia, Paroigolaimella, Eudiplogasterium, and Cutidiplogaster (Fig. 1, Suppl. Fig. S1).
Although several genera have stomatal dimorphism (polymorphism), the diplogastrid genera are primarily separable from each other by their stomatal morphology1, and the new genus can be readily distinguished from Tylopharynx, Sachsia, Paroigolaimella, Eudiplogasterium, and Cutidiplogaster by the character.
Onthodiplogaster n. gen. is distinguished from Tylopharynx by its cheilostom, plated versus forming a ring (or short tube), gymnostom, forming ring or short tube with versus without anterior serratae, metastegostom, with square- to diamond-shaped dorsal and right subventral teeth and left subventral ridges versus triangular dorsal and right subventral teeth with claw-like tip and undeveloped left subventral sector, and stegostomatous apodeme weakly developed versus ball-shaped (rounded)31; from Sachsia by its cheilostom, plated versus forming a ring (or short tube), gymnostom, forming ring or short tube with versus without anterior serratae, metastegostom, with square- to diamond-shaped dorsal and right subventral teeth and left subventral ridges versus possessing only thorn-like dorsal tooth, and telostegostom forming deep cylinder versus short funnel shape1,38; from Paroigolaimella by cheilostom composed by 12 versus six plates, gymnostom forming ring (short tube) with anterior serratae versus simple tube, metastegostom with square- to diamond-shaped dorsal and right subventral teeth and left subventral ridges versus small triangular dorsal tooth and subventral warts (spiny plates), and telostegostom forming deep cylinder versus short funnel shape1,4; from Eudiplogasterium by its gymnostom forming ring (short tube) with anterior serratae versus simple tube without anterior serratae, metastegostom with square- to diamond-shaped dorsal and right subventral teeth and left subventral ridges versus small triangular dorsal tooth and small subventral ridges, and telostegostom forming deep cylinder versus short funnel shape4; and Cutidiplogaster by its cheilostom composed by 12 plates versus double-layered short tube, gymnostom forming ring with versus without anterior serratae, metastegostom with square- to diamond-shaped dorsal and right subventral teeth and left subventral ridges versus only small flap-like dorsal tooth, and telostegostom forming deep cylinder versus long annulated tube39.
Among these genera, Onthodiplogaster n. gen. is typologically close to Mononchoides and Neodiplogaster. These three genera share a characteristic stomatal composition, i.e., cheilostomatal rugae and telostegostomatal cuticular tube1,2. However, Onthodiplogaster n. gen. is distinguished from Mononchoides by the number of rugae, 12 versus various, but often more than 12, presence versus absence of receptaculum seminis and tail shape, absence versus presence of filiform tip1,2,36,37,40. The new genus is also distinguished from Neodiplogaster by its amphid shape, large ellipsoidal versus small pore-like, number of rugae, 12 versus 18, and presence versus absence of receptaculum seminis1,2,6,41.
Onthodiplogaster japonica n. gen., n. sp
https://zoobank.org/NomenclaturalActs/D9F9DCEA-BCFE-4656-9A07-95B03B8C0191
Figures 2, 3, 4, 5, 6, 7, 8, 9 and 10; Supplementary Data 1–4.
Onthodiplogaster japonica n. gen., n. sp. (a) Entire body of female in right lateral view; (b) entire body of male in right lateral view; (c) surface of lip region of male in left lateral view; (d,e) stomatal region of female feeding on Botrytis cinerea in left (d) and right (e) lateral views; (f,g) stomatal region of female feeding on Acrobeloides sp. in left (f) and right (g) lateral views; (h) pharyngeal region of female in left lateral view; (i) Body surface showing striation, lateral field and deirid in right lateral view. Left subventral ridge (with small triangular shape), right subventral and dorsal teeth (both form flag-like shape combined with telostegostomatal cylinder, and dorsal tooth with dorsal pharyngeal gland) are separately drawn below each subfigure (d–g).
Light micrographs of stomatal region of Onthodiplogaster japonica n. gen., n. sp. (a) Right lateral view of male from fungal culture in four different focal planes; (b,c) left (b) and right (c) lateral views of predating female in three different focal planes; (d,e) left (d) and right (e) lateral views of bacteria feeding female in three different focal planes; (f,g) left (f) and right (g) lateral views of fungal feeding female in three different focal planes. Labels are as follows: am = amphid; cs = cephalic sensilla; d = dorsal tooth; lsv = left subventral ridge; ls = labial sensilla; rsv = right subventral tooth.
Light micrographs of anterior region and female characters of Onthodiplogaster japonica n. gen., n. sp. (a) Left lateral view of metacorpus to posterior pharynx region in two different focal planes; (b) right lateral view of basal bulb region in two different focal planes; (c) ventral view of vulval region in four different focal planes; (d) left lateral view of vulval region in two different focal planes; (e) left lateral view of anal region in two different focal planes. Labels are as follows: a = anus; bb = basal bulb; d = dorsal anal gland; dr = deirid; ep = secretory-excretory pore; lf = lateral field; lsv = left subventral anal gland; m = vulval muscle; mb = median bulb (metacorpus); ph = phasmid; rs = receptaculum seminis; v = vulva; vg = vagina.
Light micrographs of male tail characters of Onthodiplogaster japonica n. gen., n. sp. (a) Right lateral view of entire tail in four different focal planes; (b) posterior region in two different focal planes; (c) flattened spicule and gubernaculum in two different focal planes. Labels are as follows: ph = phasmid; v + number, ad, pd = genital papillae according to the terminology by Sudhaus & Fürst von Lieven1.
Scanning electron micrographs of stomatal region in left subventral view. (a) Male; (b) female with everted gymnostom and stegostom. Labels are as follows: am = amphid; cs = cephalic sensilla; dpgo = dorsal pharyngeal gland orifice; dt = dorsal tooth; g = gymnostom; ls = labial sensilla; rsvt = right subventral tooth; ? = an indentation with unknown function.
Scanning electron micrographs of male tail characters. (a,b) Entire tail in right lateral view of two different individuals; (c) ventral view of cloacal region; (d) right subventral view of posterior region. Labels are as follows: ph = phasmid; v + number, ad, pd = genital papillae according to the terminology by Sudhaus & Fürst von Lieven1; vh = ventral hook (ventral single papilla). Tail tip is out of the range of the micrograph in Fig. 9a.
Species epithet is derived from its type locality, Japan.
Measurements
See Table 1.
Description
To avoid redundancy with previous diplogastrid species descriptions, a short description on the diagnostic characters is presented here, and a detailed description in traditional telegraph style is provided as Supplementary text S1. In addition, stomatal elements mentioned in the generic and species descriptions and Figs. 2 and 5 are illustrated in Suppl. Fig. S2.
Adults
Medium-to-small sized species as the family, body length 450–589 μm in males and 528–723 μm in females. Body shape, body surface structure and stomatal structure as described for generic characteristics, but a circular or horseshoe-shaped indentation is observed on ventral side at the level of amphid. Pharynx composed by muscular anterior part with cuticular inner lining and glandular posterior part. Nerve ring at the level of mid-isthmus. Secretory–excretory pore at level of basal bulb to cardia. Deirids slightly posterior to secretory–excretory pore. Lateral glands not observed.
Males
Body ventrally arcuate, strongly ventrally curved at tail region when killed by heat. Testis single, on the right ventral of intestine, with typical structure of the family. Postdeirid around the anterior end of vas deferens. Spicules separate, smoothly curved in ventral view; smoothly ventrally arcuate in lateral view, with rounded to roundish squared manubrium; lamina/calomus complex ventrally expanded at one-fifth to one-fourth length from anterior end. Gubernaculum, about half of spicule in length, ear-like shape in lateral view; anterior part forming ventrally curved extension with blunt tip; posterior half dorsally enveloping spicules. Dorsal side of gubernaculum well sclerotised. Tail conical, with a sharply pointed spike which is approximately 1.5–2.5 cloacal body diameter (CBD) in length. In total, 19 papilliform genital papillae, i.e., one small, ventral, single papilla on anterior cloacal lip, and nine pairs, present. Nine pairs of genital papillae and a pair of phasmids present, and arranged as < v1, v2d, v3 / v4, ad, v5–v7, pd > in the terminology of Sudhaus & Fürst von Lieven1, where subventral v1 approximately 1 CBD anterior to cloacal opening (CO); laterally located v2d slightly anterior to CO; subventral P3 almost adcloacal; subventral P4 less than one-third CBD posterior to CO, i.e. v2d, v3, CO and v4 are close to each other; cloacal slit and P4 are close to each other; laterally located at approximately 1 CBD posterior to CO; v5–v7 forming triplet, and the central one (v6) slightly more ventrally located than the other two; subdorsally directed pd located at level of or slightly posterior to v7. Anterior five pairs (v1–ad) almost equal in size, rather large and conspicuous; v5 and v6 very small; and v7 and pd small but larger than v5 and v6. Phasmid conspicuous, forming ellipsoidal pore, located slightly posterior to ad.
Females
Slightly and smoothly arcuate ventrally when killed by heat. Gonad didelphic, amphidelphic with typical structure of the family, but a receptaculum seminis forms an independent branch. Vagina pore-like in ventral view, without flap apparatus. Receptaculum seminis oval or kidney-shaped in lateral view, rounded in ventral view; overlapping the dorsal side of uterus; sometimes harbours tightly packed sperm (spermatophore). Postdeirid at the level of the reflection of posterior gonad. Phasmid located less than one anal body diameter posterior to anus. Tail smoothly tapering or slightly elongate conical, with pointed terminus.
Type host and locality
New species was isolated from Onthophagus sp., collected from a rotten mushroom, Boletus violaceofuscus, in the experimental stand of the Kansai Research Center, Forestry and Forest Products Research Institute, Kyoto, Japan (GPS: 34.94166666 N, 135.77361111 E, 77 m a.s.l.) in July 2021.
Type material
Holotype male (slide number: T-775t) four paratype males (T-7718p and T-7721p) and five paratype females (T-7722p and T-7726p) deposited in the USDA Nematode Collection, Beltsville, MD, USA; five paratype males (Onthodiplogaster japonica M01–05) and five paratype females (Onthodiplogaster japonica F01–05), deposited in the Forest Pathology Laboratory Collection at Forestry and Forest Products Research Institute, Tsukuba, Japan.
Discussion
Onthodiplogaster n. gen. is described based on its morphological characteristics: (1) presence of oval or kidney-shaped receptaculum seminis, (2) stoma with 12-plated cheilostomatal rugae, gymnostom with serrated and notched anterior edge and stegostomatal cuticular cylinder, and (3) large and ellipsoidal amphid. The new genus is described based on the combination of the characters, i.e., without a clear genus-specific apomorphy. Therefore, in some cases, the justification of the generic status could be typologically challenging, e.g., the species could be placed into the typologically closest genus, Mononchoides. However, considering its phylogenetic status, i.e., clearly separable from this typologically similar genus, and the paraphyly of the genus Mononchoides (Fig. 1, Suppl. Fig. S1), we consider that the new species, O. japonica n. gen., n. sp. is suitable for classification as a separate genus to avoid confusion in future systematic studies.
Interestingly, each of these morphological characteristics, has been reported in genera phylogenetically separate from the clade , although the gymnostomatal shape (dorsal notch) has not been confirmed in other genera. This suggests that these characteristics can occur independently in different clades of diplogastrids.
The oval or kidney-shaped receptaculum seminis is a generic diagnostic characteristic of Acrostichus1,2,42, and is confirmed in several Diplogasteroides s. l. species previously considered an independent genus, Pseudodiplogaster43,44. Also, although the shape is different, a long and slender receptaculum seminis is present in several Diplogasteroides (previous Rhabdontolaimus Fuchs) and Pseudodiplogasteroides45,46. Considering their phylogenetic relationships, i.e., Onthodiplogaster n. gen., Acrostichus, Diplogasteroides (Pseudodiplogaster), Diplogasteroides (Rhabdontolaimus), and Pseudodiplogasteroides are phylogenetically separated (not monophyletic)4 (Fig. 1, Suppl. Fig. S1), these characteristics occurred independently at least five times. In all cases, the structure harbours tightly packed sperm (spermatophore)44,47,48. Therefore, the structure, as represented in its morphological terminology, receives the spermatophore during copulation, and stores it until the sperm migrate to spermatheca. However, the adaptive significance of the structure is unknown. For example, close relatives (= phylogenetically neighbouring genera) do not have this structure1,4.
The composition of the stoma of the new genus is shared with its relatively close relatives, Mononchoides and Neodiplogaster, i.e., narrow cheilostomatal plates (rugae), teeth and ridges in metastegostom and telostegostomatal cylinder. Because the new genus is relatively basal to the clade, and members of the clade have highly variable stomatal forms—e.g., a cheilostom ring in Sachsia, Tylopharynx and Cutidiplogaster1,38,39,49, and plates in Paroigolaimella1,50—the stomatal shape shared by Onthodiplogaster n. gen., Mononchoides and Neodiplogaster could be a plesiomorphic character of the clade containing these genera. Further developmental and ecological studies are necessary to understand the evolution of stomatal morphology.
A large amphid is shared by the new genus, some Mononchoides species, Sachsia, Demaniella and Goffartia; Demaniella belongs to a different clade than the new genus (Fig. 1, Suppl. Fig. S1), and the molecular phylogenetic status of Goffartia is unknown. In addition, several Allodiplogaster species associated with dung beetles, e.g., A. henrichae (Sachs) Paramonov & Sobolev, and several freshwater species, e.g., A. carinata (Zullini) Kanzaki, Ragsdale & Giblin-Davis, have relatively large amphids45,51. An amphid is an opening of the chemosensory organ that enables perception of water-soluble substances52,53,54; therefore, the large opening of the amphid suggests that the nematode is highly sensitive to chemical (environmental) cues under humid conditions. The genera and species with large amphids are commonly found in nutrient-rich or humid environments, i.e., rotten plant materials (compost and humus), dung, carrion, and polluted water1,36,45,55. Therefore, the large amphid is hypothesised to be an adaptation to heterologous environments with high nutrient loads and humidity. This is because the substrates, e.g., dung and carrion, encompass heterologous conditions, contain many microbes and nematodes and are decomposed quickly56,57. Accordingly, these nematodes need to recognise environmental changes and respond to them by effectively receiving chemical signals to distinguish nutrients (foods) and natural enemies using their large amphids. However, several other nematode groups living in similar conditions do not have large amphids, and so may have different ways of adapting to the environment. Onthodiplogaster japonica n. gen., n. sp. showed little conspecific predation (cannibalism) in co-culture with Acrobeloides sp. The diplogastrid nematode Pristionchus pacificus Sommer, Carta, Kim & Sternberg shows stomatal dimorphism and avoids cannibalism by chemically recognising the species9,58. Similarly, the large amphid of O. japonica n. gen., n. sp. may enable recognition of conspecific individuals for mating and avoiding cannibalism.
A circular or horseshoe-shaped indentation was observed at the amphid level (Fig. 8). This structure was clearer in females and could not be confirmed by light microscopy. Also, the presence or absence of the structure on the dorsal side was not confirmed. Because such a structure has not been reported in other diplogastrid nematodes, its function is unknown, and further examinations of the structure, including whether it is present or an artefact, are necessary.
An interesting biological characteristic of O. japonica n. gen., n. sp. is omnivory, i.e., the species feeds on fungi, bacteria and other nematodes. Omnivory has also been reported in other diplogastrid species, including the bacteriophagous stenostomatous and predatory eurystomatous forms of Pristionchus spp.5, and fungal feeding stenostomatous and predatory eurystomatous forms of Neodiplogaster spp.6. In addition, an aphelenchoidid fungal feeder, Bursaphelenchus sinensis Marinari Palmisano, Ambrogioni, Tomiczek & Brandstetter, and its close relatives show stylet dimorphism to produce a predatory form59,60,61,62. However, O. japonica n. gen., n. sp. is the only omnivore that feeds on three food sources (fungi, bacteria and nematodes) with a nearly (or totally) monomorphic stoma. If the species is truly monomorphic, that could be an adaptation to heterologous and short-lived substrates, i.e., the species can feed on various foods without stomatal dimorphism, which requires at least one generation to change the stomatal morphology. A similar pattern of omnivory has been reported in Mononchoides kanzakii Mahboob, Bashir, Asif, Nazir, Jahan & Tahseen and its close relative M. composticola Steel, Moens, Scholaert, Boshoff, Houthoofd & Bert. These bacteriophagous/predatory nematodes are found in nutrient-rich environments, e.g., humus and manure, in India, and sometimes associated with dung beetles63. Their feeding pattern could be a similar adaptation to habitat as in O. japonica n. gen., n. sp.
Interestingly, O. japonica n. gen., n. sp. fed on, but the feeding was not active, and did not propagate on, Mucorales fungus. The hyphae of Mucorales fungus may contain repellent and/or toxic substance(s) for the nematode. Mucorales fungi generally grow rapidly and consume nutrients. Therefore, the nematode’s feeding on the fungus could damage hyphae and thereby constrain fungal growth.
Further investigation of the stomatal structural morphology, development and genetics of O. japonica n. gen., n. sp. will provide insight into its adaptation and phenotypic plasticity for comparison with Pristionchus, a model for developmental plasticity.
Data availability
The molecular sequences generated during the current study are available in the GenBank database with accession numbers, LC721118 and LC721119. The accession numbers of the other sequences phylogenetically analyzed in the current study are summarized in Supplementary Table S1. This publication and its nomenclatural acts have been registered in Zoobank (www. zoobank.org), the official registry of the International Commission on Zoological Nomenclature. The LSID (Life Science Identifier) of the article is http://zoobank.org/References/01E97499-085D-454C-A10F-6385D7E3BF56.
References
Sudhaus, W. & Fürst von Lieven, A. A phylogenetic classification and catalogue of the Diplogastridae (Secernentea, Nematoda). J. Nematode Morph. Syst. 6, 43–90 (2003).
Kanzaki, N. & Giblin-Davis, R. M. Pristionchus pacificus a nematode model for comparative and evolutionary biology. Nematol. Monogr. Perspect. 11, 43–76. https://doi.org/10.1163/9789004260306_004 (2015).
Ragsdale, E. J. et al. A developmental switch coupled to the evolution of plasticity acts through a sulfatase. Cell 155, 922–933. https://doi.org/10.1016/j.cell.2013.09.054 (2013).
Susoy, V. et al. Large-scale diversification without genetic isolation in nematode symbionts of figs. Sci. Adv. 2, e1501031. https://doi.org/10.1126/sciadv.1501031 (2016).
Sommer, R. J. The future of evo-devo: Model systems and evolutionary theory. Nat. Rev. Genet. 10, 416–422. https://doi.org/10.1038/nrg2567 (2009).
Kanzaki, N. Stomatal dimorphism of Neodiplogaster acaloleptae (Diplogastromorpha: Diplogastridae). PLoS ONE 11, e0155715. https://doi.org/10.1371/journal.pone.0155715 (2016).
Sommer, R. J. Pristionchus pacificus a nematode model for comparative and evolutionary biology. Nematol. Monogr. Perspect. 11, 19–41. https://doi.org/10.1163/9789004260306_003 (2015).
Sternberg, P. W. Pristionchus pacificus a nematode model for comparative and evolutionary biology. Nematol. Monogr. Perspect. 11, 1–17. https://doi.org/10.1163/9789004260306_002 (2015).
Lightfoot, J. W. et al. Small peptide mediated self-recognition prevents cannibalism in predatory nematodes. Science 364, 86–89. https://doi.org/10.1126/science.aav9856 (2019).
Bui, L. T. & Ragsdale, E. J. Multiple plasticity regulators reveal targets specifying an induced predatory form in nematodes. Mol. Biol. Evol. 36, 2387–2399. https://doi.org/10.1093/molbev/msz171 (2019).
Kanzaki, N. Simple methods for morphological observation of nematodes. Nematol. Res. 43, 15–17. https://doi.org/10.3725/jjn.43.15 (2013).
Minagawa, N. & Mizukubo, T. A simplified procedure of transferring nematodes to glycerol for permanent mounts. Jpn. J. Nematol. 24, 75. https://doi.org/10.3725/jjn1993.24.2_75 (1994).
Hooper, D. J. Laboratory Methods for Work with Plant and Soil Nematodes 59–80 (Her Majesty’s Stationery Office, 1986).
Kanzaki, N. et al. Seinura caverna n. sp. (Tylenchomorpha: Aphelenchoididae) an androdioecious species isolated from bat guano in a calcareous cave. Nematology 21, 207–225. https://doi.org/10.1163/15685411-00003207 (2019).
Kanzaki, N. et al. Three Seinura species from Japan with a description of S. shigaensis n. sp. (Tylenchomorpha: Aphelenchoididae). PLoS ONE 16, e0244653. https://doi.org/10.1371/journal.pone.0244653 (2021).
Kanzaki, N. et al. Description of Neodiplogaster crenatae sp. N. and N. acaloleptae sp. n. (Nematoda: Diplogastridae) from Japan. Nematology 10, 545–560. https://doi.org/10.1163/156854108784513833 (2008).
Kikuchi, T. et al. A rapid and precise diagnostic method for detecting the pinewood nematode Bursaphelenchus xylophilus by loop-mediated isothermal amplification (LAMP). Phytopathology 99, 1365–1369. https://doi.org/10.1094/PHYTO-99-12-1365 (2009).
Tanaka, R. et al. Simple and quick methods for nematode DNA preparation. Appl. Entomol. Zool. 47, 291–294. https://doi.org/10.1007/s13355-012-0115-9 (2012).
Ye, W. et al. Phylogenetic relationships among Bursaphelenchus species (Nematoda: Parasitaphelenchidae) inferred from nuclear ribosomal and mitochondrial DNA sequence data. Mol. Phylogenet. Evol. 43, 1185–1197. https://doi.org/10.1016/j.ympev.2007.02.006 (2007).
Ekino, T. et al. Transmission electron microscopic observation of body cuticle structures of phoretic and parasitic stages of Parasitaphelenchinae nematodes. PLoS ONE 12, e0179465. https://doi.org/10.1371/journal.pone.0179465 (2017).
Kanzaki, N. et al. Bursaphelenchus carpini n. sp., B. laciniatae n. sp. and B. cryphali okhotskensis n. subsp. (Nematoda: Aphelenchoididae) isolated from Japan. Nematology 21, 361–388. https://doi.org/10.1163/15685411-00003220 (2019).
Kanzaki, N. & Futai, K. A PCR primer set for determination of phylogenetic relationships of Bursaphelenchus species within the xylophilus group. Nematology 4, 35–41. https://doi.org/10.1163/156854102760082186 (2002).
Kanzaki, N. et al. Acrostichus ziaelasi n. sp. (Nematoda: Diplogastridae) isolated from the beetle Ziaelas formosanus, a tenebrionid symbiont of the termite Odontotermes formosanus with remarks on the genus Acrostichus Rahm, 1928. Zool. Anz. 286, 20–30. https://doi.org/10.1016/j.jcz.2020.03.002 (2020).
Gonzalez, R. et al. Nematode epibionts on skin of the Florida manatee, Trichechus manatus latirostris. Sci. Rep. 11, 1211. https://doi.org/10.1038/s41598-020-79879-7 (2021).
Katoh, K. et al. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30, 3059–3066. https://doi.org/10.1093/nar/gkf436 (2002).
Kuraku, S. et al. aLeaves facilitates on-demand exploration of metazoan gene family trees on MAFFT sequence alignment server with enhanced interactivity. Nucleic Acids Res. 41, W22–W28. https://doi.org/10.1093/nar/gkt389 (2013).
Kumar, S. et al. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. https://doi.org/10.1093/molbev/msw054 (2016).
Huelsenbeck, J. P. & Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17, 1754–1755. https://doi.org/10.1093/bioinformatics/17.8.754 (2001).
Ronquist, F. et al. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. https://doi.org/10.1093/sysbio/sys029 (2012).
Larget, B. & Simon, D. L. Markov chain Monte Carlo algorithms for the Bayesian analysis of phylogenetic trees. Mol. Biol. Evol. 16, 750–759. https://doi.org/10.1093/oxfordjournals.molbev.a026160 (1999).
Fürst von Lieven, A. Functional morphology, origin and phylogenetic implications of the feeding mechanism of Tylopharynx foetida (Nematoda: Diplogastrina). Russ. J. Nematol. 10, 11–23 (2002).
Fürst von Lieven, A. Koerneria sudhausi n sp (Nematoda: Diplogastridae); a hermaphroditic diplogastrid with an egg shell formed by zygote and uterine components. Nematology 10, 27–45. https://doi.org/10.1163/156854108783360087 (2008).
Kanzaki, N. et al. Revision of the paraphyletic genus Koerneria Meyl, 1960 and resurrection of two other genera of Diplogastridae (Nematoda). Zookeys 442, 17–30. https://doi.org/10.3897/zookeys.442.7459 (2014).
Fürst von Lieven, A. The genus Oigolaimella Paramonov, 1952 (Nematoda: Diplogastridae) and description of O. kruegeri n. sp. and O. ninae n. sp. Nematology 5(583–600), 2003. https://doi.org/10.1163/156854103322683300 (1952).
Andrássy, I. Klasse Nematoda (Ordnungen Monhysterida, Desmoscolecida, Araeolaimida, Chromadorida, Rhabditida) (Fischer, 1984).
Steel, H. et al. Mononchoides composticola n. sp. (Nematoda: Diplogastridae) associated with composting processes: morphological, molecular and autecological characterisation. Nematology 13, 347–363. https://doi.org/10.1163/138855410X523023 (2011).
Troccoli, A. M. et al. (Nematoda: Rhabditidae): nematode associates of Rhynchophorus ferrugineus (Oliver) (Coleoptera: Curculionidae) in Italy. Nematology 17(953–966), 2015. https://doi.org/10.1163/15685411-00002916 (1985).
Körner, H. Die Nematoden fauna des vergehenden Holzes und ihre Beziehungen zu den Insekten. Zool. Jahrb. (Systematik) 82, 245–353 (1954).
Fürst von Lieven, A. et al. Cutidiplogaster manati n. gen., n. sp. (Nematoda: Diplogastridae) from skin lesions of a West Indian manatee (Sirenia) from the Okinawa Churaumi Aquarium. Nematology 13, 51–59. https://doi.org/10.1163/138855410X500082 (2011).
Goodrich, M. et al. Mononchoides changi n. sp., and M. bollingeri n. sp. (Nematoda: Diplogasterinae), from a waste treatment plant. Nematologica 14, 25–36. https://doi.org/10.1163/187529268X00615 (1968).
Hechler, H. C. Redescription of Neodiplogaster tropica Cobb and N. pinicola Steiner (Nematoda: Diplogasteridae). J. Nematol. 3, 341–348 (1971).
Rahm, G. Alguns nematodes parasitas e semiparasitas das plantas culturaes do Brasil. Arch. Inst. Biol. 1, 239–251 (1928).
Takaki, S.-I. Studies on a nematode parasitic on the common beetle, Anomala testaceipes. J. Coll. Agric. / Imper. Univ. Tokyo 15, 1–11 (1941).
Kiontke, K. et al. Redescription of Diplogasteroides nasuensis Takaki, 1941 and D. magnus Völk, 1950 (Nematoda: Diplogastrina) associated with Scarabaeidae (Coleoptera). Nematology 3, 817–832. https://doi.org/10.1163/156854101753625317 (2001).
Fürst von Lieven, A. Vergleichendeund funktionelle Morphologie der Mundhöhle der Diplogastrina (Nematoda) mit einemersten Entwurf der Phylogeniedieses Taxons (Freie Universität Berlin, 2001).
Fürst von Lieven, A. & Sudhaus, W. Comparative and functional morphology of the buccal cavity of Diplogastrina (Nematoda) and a first outline of the phylogeny of this taxon. J. Zool. Syst. Evol. Res. 38, 37–63. https://doi.org/10.1046/j.1439-0469.2000.381125.x (2000).
Kanzaki, N. & Ide, T. Diplogasteroides luxuriosae n. sp. associated with Acalolepta luxuriosa (Cerambycidae) from Japan. Nematology 18, 221–233. https://doi.org/10.1163/15685411-00002955 (2016).
Kanzaki, N. et al. Diplogasteroides nix n. sp. (Nematoda: Diplogastridae), a cryptic species related to D. andrassyi, isolated from Monochamus urussovii (Coleoptera: Cerambycidae) from Hokkaido, Japan, with remarks on the body surface structures. Nematology 18, 753–773. https://doi.org/10.1163/15685411-00002990 (2016).
Wu, J. H. et al. Description of a new species of the genus Tylopharynx (Nemata: Diplogasteroidea), T. clariamphida sp. n., with a redescription of T. foetida (Bütschli, 1874) Goffart, 1930. J. Nematol. 33, 83–90 (2001).
Paramonov, A. A. Opyt ekologicheskoi klassifikatsii fitonematod. Trudy Gelmintol. Laboratorii Akad. Nauk SSSR (Moskva) 6, 338–369 (1952).
Kühne, R. Nematoden mit phoretischer Bindung an Dung vergrabende Geotrupes-Käfer: ein morphologischer Vergleich von Diplogaster henrichae Sachs, 1950, D. hirschmannae Sachs, 1950, und D. labiomorphus sp. n. (Nematoda: Diplogasteridae). Nematologica 41, 7–34. https://doi.org/10.1163/003925995X00026 (1995).
Baldwin, J. G. & Perry, R. N. Nematode morphology, physiology and ecology. Nematol. Adv. Perspect. 1, 175–257. https://doi.org/10.1079/9780851996455.0175 (2004).
Bumbarger, D. J. et al. Three-dimensional reconstruction of the amphid sensilla in the microbial feeding nematode, Acrobeles complexus (nematoda: Rhabditida). J. Comp. Neurol. 512, 271–281. https://doi.org/10.1002/cne.21882 (2009).
Hong, R. L. et al. Evolution of neuronal anatomy and circuitry in two highly divergent nematode species. Elife 8, e47155. https://doi.org/10.7554/eLife.47155 (2019).
Kanzaki, N. & Giblin-Davis, R. M. Acrostichus palmarum n. sp., a cryptic species separated from A. rhynchophori by molecular sequences and hybridization tests. Nematology 20, 751–768. https://doi.org/10.1163/15685411-00003173 (2018).
Slade, E. M. et al. Disentangling the ‘brown world’ faecal – detritus interaction web: Dung beetle effects on soil microbial properties. Oikos 125, 629–635. https://doi.org/10.5061/dryad.4q230 (2015).
Hilal, M. G. et al. Exploring microbial communities, assessment methodologies and applications of animal’s carcass decomposition: A review. FEMS Microbiol. Ecol. 97, fib098. https://doi.org/10.1093/femsec/fiab098 (2021).
Sommer, R. J. et al. Morphological, genetic and molecular description of Pristionchus pacificus sp. n. (Nematoda, Diplogastridae). Fundam. Appl. Nematol. 19, 511–521 (1996).
Braasch, H. & Braasch-Bidasak, R. First record of the genus Bursaphelenchus Fuchs, 1937 in Thailand and description of B. thailandae sp. N. (Nematoda: Parasitaphelenchidae). Nematology 4, 853–863. https://doi.org/10.1163/156854102760402621 (2002).
Marinari Palmisano, A. et al. Bursaphelenchus sinensis sp. N. and B. thailandae Braasch et Braasch-Bidasak in packaging wood from China. Nematol. Mediterr. 32, 57–65 (2004).
Kanzaki, N. & Giblin-Davis, R. M. The genus Berntsenus Massey, 1974 is a junior synonym of Bursaphelenchus Fuchs, 1937. Nematology 22, 677–695. https://doi.org/10.1163/15685411-00003332 (2020).
Kanzaki, N. et al. Feeding dimorphism in a mycophagous nematode, Bursaphelenchus sinensis. Sci. Rep. 9, 13956. https://doi.org/10.1038/s41598-019-50462-z (2019).
Mahboob, M. et al. Molecular and phenotypic characterization of two cryptic species of the predatory genus Mononchoides Rahm, 1928 (Rhabditida: Diplogastridae) and their congeneric affinities. J. Helminthol. 96, e41. https://doi.org/10.1017/S0022149X22000323 (2022).
Acknowledgements
We thank Dr. Robin M. Giblin-Davis, Professor Emeritus in University of Florida, for writing corrections and providing insights on diplogastrid nematodes, and Ms. Yoshiko Shimada, Kansai Research Center, FFPRI for assistance in preparation of permanent type specimens and morphometrics. This study was supported in part by the Grants-in-Aid for Scientific Research (B), No. 17H03831 and 19K06145 from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Contributions
N.K. and R.S. designed the research; N.K. and Y.I. performed the research; N.K. and Y.I. analyzed the data; and N.K., Y.I., and R.S. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Video 1.
Supplementary Video 2.
Supplementary Video 3.
Supplementary Video 4.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kanzaki, N., Ikeda, Y. & Shinya, R. Onthodiplogaster japonica n. gen., n. sp. (Rhabditida: Diplogastridae) isolated from Onthophagus sp. (Coleoptera: Scarabaeidae) from Japan. Sci Rep 13, 6470 (2023). https://doi.org/10.1038/s41598-023-33586-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-33586-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.