Abstract
Diet is the primary factor affecting host nutrition and metabolism, with excess food intake, especially high-calorie diets, such as high-fat and high-sugar diets, causing an increased risk of obesity and related disorders. Obesity alters the gut microbial composition and reduces microbial diversity and causes changes in specific bacterial taxa. Dietary lipids can alter the gut microbial composition in obese mice. However, the regulation of gut microbiota and host energy homeostasis by different polyunsaturated fatty acids (PUFAs) in dietary lipids remains unknown. Here, we demonstrated that different PUFAs in dietary lipids improved host metabolism in high-fat diet (HFD)-induced obesity in mice. The intake of the different PUFA-enriched dietary lipids improved metabolism in HFD-induced obesity by regulating glucose tolerance and inhibiting colonic inflammation. Moreover, the gut microbial compositions were different among HFD and modified PUFA-enriched HFD-fed mice. Thus, we have identified a new mechanism underlying the function of different PUFAs in dietary lipids in regulating host energy homeostasis in obese conditions. Our findings shed light on the prevention and treatment of metabolic disorders by targeting the gut microbiota.
Similar content being viewed by others
Introduction
Diet is the primary factor in host nutrition and metabolism; however, excess food intake causes dysregulation of energy balance and leads to metabolic disorders such as obesity and type II diabetes1. Excess food intake, especially in high-calorie diets, such as high-fat (HFD) and high-sugar diets, is considered the greatest risk factor in the development of obesity2. Obesity is a complex disease associated with increased inflammatory markers, leading to chronic low-grade systemic inflammation, which is implicated in the development of insulin resistance3. Recently, gut microbiota has also been proposed to be involved in the development of metabolic disorders4. Lipopolysaccharides (LPS), also known as endotoxins, are membrane components of gram-negative bacteria that induce inflammation by activating toll-like receptor (TLR) 4, which is expressed on immune cells such as macrophages as well as on other cell types including hepatocytes and adipocytes. LPS can also trigger HFD-induced insulin resistance5. Additionally, mucosal TLR activation contributes to hepatic steatosis via the TLR adaptor MYD88 expressed in the intestine6. Mice with intestinal epithelial cell-specific MYD88 deletion fed an HFD have improved glucose homeostasis and decreased hepatic lipid content compared with wild-type mice6. Moreover, obesity is associated with LPS leakage across the intestinal epithelial barrier, resulting in the potent induction of systemic inflammation7.
Dietary lipids can alter gut microbial compositions in obese individuals and mouse models. Comparing mice fed a variety of diets (low-fat diet and diet containing high levels of either saturated lipids, ω6 polyunsaturated fatty acids (PUFAs) or ω3 PUFAs) revealed that diets with saturated lipids or ω6 PUFAs induced weight gain, but only saturated lipids caused increased insulin resistance, colonic permeability, and mesenteric fat inflammation8. The gut microbiota composition differed from other groups in mice fed a low-fat diet or a ω3 PUFA diet, but was similar in mice fed either a saturated fat diet or a ω6 PUFA diet. Another study showed that ω3 PUFA-enriched flaxseed oil reduced the ratio of Firmicutes/Bacteroidetes in type 2 diabetic rats9. In addition, the fish oil administration induced beneficial bacteria, such as Lactobacillus, Akkermansia muciniphila, and Bifidobacterium, and gut bacteria differences contributed to obesity phenotypic alteration in fish-oil-fed mice compared with lard-fed mice10. Lard is rich in saturated fatty acids, while fish oil is enriched in the ω3 PUFAs, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). Recently, Qin et al. reported that fish oil extracted from Coregonus peled facilitated the growth of Bifidobacterium and Adlercreutzia, thereby improving recurrent obese phenotypes in mice11.
Furthermore, PUFAs as free fatty acids (FFAs), including DHA and EPA, may promote glucagon-like peptide-1 (GLP-1) secretion and attenuate chronic inflammation in peripheral tissues12,13. Colon-specific delivery of DHA– and EPA–induced GLP-1 and insulin release with subsequent reductions in glucose concentrations14. Moreover, long-term intracolonic DHA administration for 4 weeks increased plasma GLP-1 levels and decreased non-fasting blood glucose concentrations15. In humans with abdominal obesity and insulin resistance, ingestion of a Mediterranean diet rich in olive oil for 28 days resulted in significantly higher postprandial GLP-1 blood concentrations16. Compared to a diet enriched in saturated fatty acids, consumption of the Mediterranean diet also improved insulin sensitivity, which could be responsible for the decreased insulin secretion as well as fasting and postprandial blood glucose concentrations15. Moreover, recent research suggests that DHA can improve insulin signaling in HFD-fed mice by affecting the gut-adipose axis17. Recently, concerns have emerged that a ω6 PUFA-containing diet exacerbated the risk of intestinal diseases18. These concerns also stem from evidence that traditional dietary habits and lifestyle unique to the Mediterranean diet lower the incidence of chronic diseases and improve longevity19. However, the mechanisms underlying the beneficial effects of PUFA-enriched oils, but not FFAs in obesity needed to be investigated.
In this study, we investigated whether a PUFA-enriched diet could prevent HFD-induced obesity by modulating the gut microbiota. We compared phenotypical and biochemical parameters in obese mice fed diets enriched in either soybean oil, linseed oil, fish oil, or olive oil. Moreover, we performed 16S rRNA analysis to explore the role of the gut microbiome in these beneficial functions of PUFAs. Our study will aid the prevention and treatment of metabolic disorders by targeting the gut microbiota.
Results
Polyunsaturated fatty acids improve glucose metabolism via GLP-1 secretion
We first examined the effects of long-chain fatty acids (LCFAs)-containing dietary lipids on GLP-1 secretion and glucose homeostasis. We investigated whether LCFAs induce GLP-1 secretion using the mouse intestinal secretin tumor-cell line, STC-1 cells. We observed that linoleic acid (LA), oleic acid (OA), DHA, and EPA significantly induced GLP-1 secretion. Additionally, α-linolenic acid (α-LNA), cis-9, trans-11 conjugated linoleic acid (CLA1), as well as trans-10, cis-12 conjugated linoleic acid (CLA3) tended to induce GLP-1 secretion. However, trans-9, trans-11 conjugated linoleic acid (CLA2), stearic acid (SA), and palmitic acid (PA) had no effects on GLP-1 secretion (Fig. 1a). The incretin GLP-1, a gut hormone that stimulates glucose-induced insulin secretion and inhibits food intake, is secreted from enteroendocrine L cells, which are primarily found in the ileum and colon20. We previously reported that the oral administration of the gut microbial PUFA-metabolite, 10-hydroxy-cis-12 octadecenoic acid (HYA), strongly induced GLP-1 secretion and improved glucose homeostasis after 1–2 h21. We found that plasma GLP-1 levels were significantly induced 2 h after LCFAs (mainly α-LNA, DHA, and EPA) administration (Fig. 1b,c). Hence, 2 h after GLP-1 levels had elevated, we performed an intraperitoneal glucose tolerance test (GTT) and found that administration of DHA and EPA significantly suppressed the increase in blood glucose levels compared to that observed in the control mice (Fig. 1d). Our findings indicated that acute administration of LCFAs, mainly DHA and EPA, promoted GLP-1 secretion and improved glucose homeostasis.
Acute administration of PUFAs improved glucose metabolism by promoting GLP-1 secretion. (a) GLP-1 secretion in the STC-1 cells treated with each LCFA (n = 6 per group). **P < 0.01; *P < 0.05, vs. Control (Dunn’s test). (b) Experimental scheme. (c) Each mouse was administered LCFAs by oral gavage. After 2 h, the plasma GLP-1 levels were measured (n = 8 mice for each group). **P < 0.01; *P < 0.05, vs Control (Dunn’s test). (d) Each mouse was administered LCFAs by oral gavage. ipGTT was evaluated (left panel) and area under the curve (AUC) of ipGTT was calculated (right panel) after 2 h (n = 7–8 mice for each group). **P < 0.01; *P < 0.05, vs. Control (Dunn’s test for 0, 15, and 30 min and Dunnett test for 60, 90, 120 min, and AUC). Results are presented as means ± standard error.
Polyunsaturated fatty acid-containing dietary lipids improve host metabolism
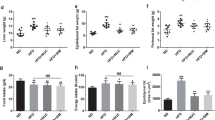
We examined the effects of PUFA-containing dietary lipids on host energy regulation in a mouse model of HFD-induced obesity. We used 8-week-old mice and fed them different PUFA-containing diets as follows; NC (normal chow), HFD (soybean oil), Linseed (Soybean oil in HFD was replaced with linseed oil), Fish (Soybean oil in HFD was replaced with fish oil), and Olive (Soybean oil in HFD was replaced with olive oil), for 8 weeks (Supplementary Table 1). We observed significantly lower body weight of Fish-fed mice compared with HFD-fed mice during development (Fig. 2a). In addition, the white adipose tissue (WAT) fat mass was significantly lower in Fish-fed mice than in HFD-fed mice at 16 weeks of age (Fig. 2a). However, the food intake was similar between HFD-fed and Fish-fed mice (data not shown). Moreover, the HFD-induced impaired glucose tolerance, as determined by GTT, was significantly attenuated in Fish-fed mice compared with HFD-fed mice (Fig. 2b). Furthermore, the blood glucose level of Fish-fed mice was significantly lower than those of HFD-fed mice (Fig. 2c). Additionally, plasma triglycerides (TGs), and non-esterified fatty acids (NEFAs) levels were significantly lower than those of HFD-fed mice, whereas plasma total cholesterol (T-Cho) levels were similar between control and Fish-fed mice (Fig. 2d, upper panel). Moreover, we observed significantly higher plasma GLP-1 and significantly lower plasma insulin levels in Fish-fed mice compared to those of HFD-fed mice, whereas plasma PYY levels were similar between control and Fish-fed mice (Fig. 2d, lower panel). However, these effects were not observed in Linseed- and Olive-fed mice, suggesting that fish oil replacement improved glucose metabolism, thereby inducing greater resistance to HFD-induced obesity in the presence of DHA and EPA in dietary lipids, but not α-LNA or OA.
Polyunsaturated fatty acid-containing dietary lipids prevent high fat diet-induced obesity. (a–d) C57BL/6J male mice were fed either normal chow (NC), a high fat diet (HFD), or a modified HFD diet [Soybean oil in HFD was replaced with either linseed oil (Linseed), fish oil (Fish), or olive oil (Olive)] for 8 weeks. (a) Changes in body and tissue weights. epi epididymal, peri perirenal, sub subcutaneous, WAT white adipose tissue. **P < 0.01; *P < 0.05, vs, NC. ##P < 0.01; #P < 0.05, vs, HFD (Dunn’s test for 8, 15, and 16 weeks of age, and tissue weight and Tukey–Kramer test for 9–14 weeks of age). (b) ipGTT was evaluated at 15 weeks of age. **P < 0.01; *P < 0.05, vs, NC. ##P < 0.01, vs, HFD (Dunn’s test for 0, 30, 90, and 120 min and Tukey–Kramer test for 15 and 60 min). (c,d) After 5 h fasting, blood glucose, plasma triglycerides, non-esterified fatty acids, total cholesterol, GLP-1, insulin, and PYY levels were measured at the end of the experimental period (n = 7–10 mice for each group). **P < 0.01; *P < 0.05 (Dunn’s test for blood glucose, plasma triglycerides, NEFAs, total cholesterol, GLP-1, and insulin and Tukey–Kramer test for PYY). Results are presented as means ± standard error.
Fish oil replacement regulates systemic inflammation
Obesity, which is a feature of metabolic syndrome, was associated with chronic inflammation in obese subjects and mice22. Next, we examined whether fish oil replacement affected adipose and colonic inflammation. We found a significant decrease in WAT adipocyte size in Fish-fed mice (Fig. 3a, left). Adipocyte differentiation and increased adipose size are correlated in obesity23,24. Next, the mRNA expressions of Pparg and Fabp4 were also decreased in Fish-fed mice (Fig. 3a, right). Moreover, we observed significantly decreased mRNA expressions of the inflammatory markers tumor necrosis factor α (Tnfα), F4/80, and monocyte chemoattractant protein 1 (Mcp1) in Fish-fed mice as compared with HFD-fed mice (Fig. 3b). Additionally, the mRNA expression of colonic inflammation marker (Tnfα) was also suppressed in Fish-fed mice compared to HFD-fed mice (Fig. 3c, left). Ingestion of an HFD increases plasma LPS levels from gram-negative bacteria in the gut, and several studies have shown that HFD-induced obesity is associated with a breach in the intestinal barrier, leading to increases in LPS levels25,26. Thus, we investigated the effects of Fish-fed mice on intestinal permeability and barrier function. Fish-fed mice showed significant improvements in intestinal epithelial barrier permeability, as measured by plasma LPS levels (Fig. 3c, right). Additionally, the expression of Ocln, encoding occludin that plays a key role in maintain the barrier function of tight junction, in the colon was comparable between NC-fed and Fish-fed mice, suggesting a possible role of occludin in influencing gut permeability (Fig. 3d). Moreover, the mRNA expression of Gcg (encoding an enteroendocrine cell marker) in the colon was decreased in HFD-fed, Linseed-fed, Fish-fed, and Olive-fed mice compared to the NC-fed mice (Fig. 3d). Altogether, these findings suggested mitigation of increased intestinal permeability, systemic inflammation, and increased GLP-1 secretion in the gut of Fish-fed mice via enhanced intestinal barrier function.
Fish oil suppressed WAT inflammation by enhancing intestinal barrier function. (a–d) C57BL/6J male mice were fed either normal chow (NC), a high fat diet (HFD), or a modified HFD diet (Soybean oil in HFD was replaced with either linseed oil (Linseed), fish oil (Fish), or olive oil (Olive)) for 8 weeks. (a) Hematoxylin–eosin (H&E)–stained epididymal WAT and the mean area of adipocytes (n = 4 mice for each group). Scale bar; 400 μm. The mRNA expression of Pparg and Fabp4 in the epididymal WAT (n = 8 mice for each group). **P < 0.01; *P < 0.05 (Tukey–Kramer test for adipocytes size and Fabp4, and Dunn’s test for Pparg). (b) The mRNA expression of Tnfα, F4/80, and Mcp-1 in the epididymal WAT (n = 8 mice for each group). **P < 0.01; *P < 0.05 (Dunn’s test). (c) The mRNA expression of Tnfα in the colon (left panel) and plasma LPS levels (right panel) were measured at the end of the experimental period (n = 7–10 mice for each group). **P < 0.01; *P < 0.05 (Tukey–Kramer test for plasma LPS and Dunn’s test for Tnfα). (d) The mRNA expression of Ocln, and Gcg in the colon (n = 7–8 mice for each group). **P < 0.01; *P < 0.05 (Tukey–Kramer test for Ocln and Dunn’s test for Gcg). Results are presented as means ± standard error.
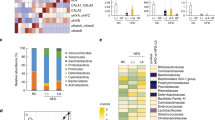
Fish oil alters the gut microbial composition
To assess how dietary lipid sources affect the gut microbial composition, we fed mice isocaloric diets that differed only in lipid composition (either soybean or fish oil) (Supplementary Table 1) for 8 weeks. We analyzed the gut microbial composition using the 16S rRNA gene in the feces of these mice and observed dramatic changes in the microbial ecology based on the dietary lipid type (Fig. 4a–d). The 16S rRNA gene sequencing confirmed that Fish-fed mice had altered the relative abundance of the major phyla constituting the gut microbiota (Fig. 4a). Specifically, in agreement with a previous study27, we observed increased Firmicutes and decreased Bacteroidota levels in HFD-fed mice; however, Fish-fed mice had decreased Firmicutes and increased Bacteroidota levels (Fig. 4b). The Chao1 and Shannon indexes were used to evaluate the microbiota richness and diversity. Fish-fed mice had slightly increased alpha diversity compared with HFD-fed mice (Fig. 4c). Additionally, we confirmed that Fish-fed mice had altered gut microbiota composition as compared with HFD-fed mice, indicated by principal coordinate analysis (PCoA) based on taxonomic datasets (Fig. 4d).
Fish oil altered the gut microbiota composition. (a–d) C57BL/6J male mice were fed either a high fat diet (HFD) or a modified HFD diet (Soybean oil in HFD was replaced with fish oil (Fish)) for 8 weeks. Gut microbial composition was evaluated for the determination of the relative abundance of microbial phyla (a), Firmicutes to Bacteroidota ratio (b), Chao1 and Shannon index at the OTU level (c), and principal coordinate analysis (PCoA) at the phylum level (d) of mice fed HFD or a modified HFD (Fish) in feces (n = 7–8 mice for each group). *P < 0.05 (Mann–Whitney test).
The metabolic effects of fish oil were abolished in antibiotics-treated mice
Finally, to determine whether the metabolic effects of the Fish oil-containing diet depend on the gut microbiota, we further assessed the effects of the Fish oil-containing diet in antibiotics-treated mice (Abx.). Consistent with the findings of a previous study28,29, we observed an increase in cecal tissue weight and a decrease in the total bacterial levels in HFD- and Fish-fed, Abx.-treated mice (Supplementary Fig. 1). Similar to Figs. 2 and 3, HFD induced an increase in body and tissue weight, glucose intolerance, and colonic inflammation, while Fish-fed mice restored these metabolic parameters and inflammation (data not shown). However, body and tissue weight, glucose tolerance, and blood glucose levels were comparable between HFD- and Fish-fed in Abx.-treated mice (Fig. 5a–c). Additionally, levels of the plasma metabolic parameters (TGs, NEFAs, T-Cho, GLP-1, and insulin) were similar between HFD- and Fish-fed in Abx.-treated mice (Fig. 5d). Moreover, by modulating colonic inflammation and permeability, mRNA expressions of Tnfα and occludin, and plasma LPS levels were comparable between HFD- and Fish-fed in Abx.-treated mice (Fig. 5e). In white adipose tissues, adipocyte size and mRNA expressions of Pparg, Fabp4, Tnfα, F4/80, and Mcp1 were comparable between HFD-fed and Fish-fed in Abx.-treated mice (Supplementary Fig. 2). Thus, these results suggested that the metabolic benefits of fish oil depend partially upon the gut microbiota.
Fish oil improved metabolic conditions were attenuated in antibiotic-treated mice. (a–e) C57BL/6J male mice were fed either a high fat diet (HFD) or a modified HFD diet (Soybean oil in HFD was replaced with fish oil (Fish)) and treated with antibiotics (Abx.) for 8 weeks. (a) Changes in body and tissue weights. epi epididymal, peri perirenal, sub subcutaneous, WAT white adipose tissue. (b) ipGTT was evaluated at 15 weeks of age. (c,d) After 5 h fasting, blood glucose, plasma triglycerides, non-esterified fatty acids, total cholesterol, GLP-1, insulin, and PYY levels were measured at the end of the experimental period. (e) The mRNA expression of Tnfα and Ocln in the colon (upper panel) and plasma LPS levels (lower panel) were measured at the end of the experimental period (n = 7–9 mice for each group). Results are presented as means ± standard error. NS not significant.
Discussion
Polyunsaturated fatty acids, especially DHA and EPA, improve glucose tolerance and obesity. However, the mechanisms underlying the beneficial effects of dietary PUFA-enriched oil but not FFAs on obesity remain unclear. In this study, we report that PUFA containing a high level of fish oil was efficacious against body and tissue weights by improving glucose metabolism and modulating gut microbiota. These metabolic benefits of fish oil-enriched PUFA were associated with the gut microbial composition and their effects on promoting intestinal barrier function (Supplementary Fig. 3).
PUFAs as FFAs exert several beneficial effects, improving tight junction permeability, facilitating epithelial cell proliferation, changing gut microbial composition, and gut hormone secretion in the colon21,30. Additionally, numerous studies demonstrated that PUFAs, mainly DHA and EPA, promote the secretion of GLP-112,14,31. Our data suggested that DHA and EPA-stimulated STC-1 cells and Fish-fed mice specifically promoted GLP-1 secretion. Although α-linolenic acid and oleic acid also tended to promote GLP-1 secretion in STC-1 cells, Linseed (rich in α-linolenic acid) and Olive (rich in oleic acid)-fed mice did not exhibit increased GLP-1 secretion. FFAs in dietary lipids are produced from lipid precursors by the action of lipases. Increased circulating LPS suppresses lipase activity, favoring the accumulation of triglycerides in the intestine and other organs32. Moreover, the lipid type entering the small intestine and its absorption through the stomach wall directly affects the amount and type of lipid entering the colon. Indeed, unsaturated FAs are more easily absorbed than saturated FAs. The mechanisms by which dietary fatty acids affect the gut microbiota are not clear. Although most consumed fatty acids are absorbed in the small intestine, a small amount passes through the gastrointestinal tract and may therefore directly modulate colonic microbiota composition. Moreover, lipid digestion in the stomach and absorption into the small intestine affects the amount and type of lipids entering the colon. The mechanistic details of how dietary fatty acids affect the gut microbiota are not known. The basal levels of the incretin hormone, GLP-1 are elevated in mice lacking gut microbiota, suggesting that in the absence of gut microbiota, increased colonic-derived GLP-1 slows intestinal transit, allowing more time for nutrient absorption33. In contrast, gut microbial metabolites (e.g., SCFAs, secondary bile acids, and HYA) promote GLP-1 secretion21,34,35. These findings exemplify how microbial contribution to systemic energy supply affects host gene expression and physiology in the gut. Therefore, it is necessary to examine the location where the LCFAs in ingested dietary lipids are converted to FFAs thereby, affecting physiological functions.
Chronic HFD resulting in obesity leads to a leaky intestinal epithelial barrier25,26. Consequently, LPS can enter the systemic circulation and induce chronic inflammation that leads to glucose intolerance36. We showed that fish oil feeding reduced circulating LPS levels and systemic inflammation, suggesting that fish oil might be conducive to maintaining intestinal epithelial barrier function and gut microbial homeostasis, thus improving glucose tolerance. Moreover, the 16S rRNA gene sequencing analysis confirmed that the gut microbial composition in Fish-fed mice was altered compared to that in the HFD-fed mice. Supplemental Table 1 shows that saturated fatty acids that are enriched in lard exhibit toxic influences the inhibiting bacterial growth, antibacterial activity such as lysis and solubilization of bacterial cell membranes, and inhibition of ATP production37,38,39. On the other hand, a comparison of mice on a variety of diets (low-fat diet and diets containing high levels of saturated fatty acids, ω6 PUFA, or ω3 PUFA) showed that diets with saturated fatty acids or ω6 PUFAs induced weight gain, however, increased insulin resistance, colonic permeability, and mesenteric fat inflammation were only induced saturated fats8. The gut microbiota composition of mice fed a low-fat diet and a ω3 PUFA diet differed from the other groups, whereas the gut microbiota of mice fed a saturated fat diet and a ω6 PUFA diet were similar. Therefore, gut microbial functions and FFA composition in dietary lipids associated between diet, gut microbiota structure, and dyslipidemia should be studied in large human cohorts to develop therapeutic strategies for treatment of metabolic disorders.
In conclusion, DHA and EPA-rich fish oil could ameliorate HFD-induced obesity and glucose intolerance partly by regulating the gut microbiome, modulating gut permeability, and promoting GLP-1 secretion. DHA- and EPA-stimulated GLP-1 secretion in enteroendocrine cells might play an important role in linking diet, gut microbiome, and host metabolism. This study provides a new mechanism underlying the function of DHA and EPA in decreasing blood glucose levels in obese individuals.
Materials and methods
Cell culture
STC-1 cells (murine enteroendocrine cells) were purchased from American Type Culture Collection (ATCC; Manassas, VA, USA) and cultured in Dulbecco’s modified Eagle medium (DMEM) containing 5% fetal bovine serum (FBS) and 15% horse serum and were maintained at 37 °C with 5% CO2. For measurement of GLP-1 secretion, cells were plated in 24-well plates (3 × 105 cells/well) and cultured for 48 h before inducing GLP-1 secretion. After 48 h, the culture media was collected, and serum-free media was added followed by incubation for 90 min. Each well was treated with FAs [200 μM; linolenic acid (LA), oleic acid (OA), α-linolenic acid (α-LNA), docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), cis-9, trans-11-conjugated linoleic acid (CLA1), trans-9, trans-11-conjugated linoleic acid (CLA2), trans-10, cis-12-conjugated linoleic acid (CLA3), stearic acid (SA), or palmitic acid (PA)] for 1 h, and the supernatant was collected in the presence of a dipeptidyl peptidase IV (DPP-IV) inhibitor.
Animal study
C57BL/6J male mice were purchased from Japan SLC (Shizuoka, Japan) and housed under a 12-h light–dark cycle. All experimental procedures involving mice were performed according to protocols approved by the Committee on the Ethics of Animal Experiments of the Tokyo University of Agriculture and Technology (permit No. R4-147) and the Animal Welfare Act Regulations and Guide for the Care and Use of Laboratory Animals. The study has been reported in accordance with the ARRIVE guidelines40.
The glucose tolerance test (GTT) and GLP-1 secretion measurement were performed after acute administration of FAs (LA, OA, α-LNA, DHA, or EPA). For GTT, 7-week-old C57BL/6J male mice were administered FAs (1 g/kg of body weight) dissolved in 0.5% CMC (Carboxymethyl cellulose ammonium, Nacalai Tesque, Kyoto, Japan) by gavage following a 16-h fast period21. After 2 h, mice were administered glucose (2 g/kg of body weight) intraperitoneally. Plasma glucose concentration in the tail vein was monitored before glucose injection and at 15-, 30-, 60-, 90-, and 120-min post-injection. For GLP-1 measurement, 8-week-old C57BL/6J male mice were administered FFAs (1 g/kg of body weight) dissolved in 0.5% CMC by gavage following a 16-h fast period, and plasma samples were collected from the vena cava after 2 h.
For long-term treatment, 7-week-old C57BL/6 J mice were acclimatized for 1 week with a normal chow (NC, CE-2; CLEA, Tokyo, Japan). After acclimatization, mice were randomly allocated into each treatment group, and fed either NC, an HFD (D12492: Research Diets, New Brunswick, NJ, USA), or a modified HFD diet [Soybean oil in HFD was replaced with either linseed oil (Sigma-Aldrich), fish oil (Sigma-Aldrich), or olive oil (Sigma-Aldrich)] for 8 weeks. The compositions of the diets are provided in Supplementary Table 1. Antibiotic-treated mice were given a cocktail of antibiotics [0.5 g/L ampicillin (Wako Pure Chemical Co. Ltd., Osaka, Japan), 0.2 g/L vancomycin (Wako), 0.5 g/L neomycin (Wako) and 0.5 g/L metronidazole (Wako)] in their drinking water. Diets and water were provided ad libitum throughout the experiment41. After 7 weeks of treatment, mice were made to fast for 16 h followed by the GTT.
Biochemical analyses
Blood glucose concentrations were measured using a One Touch Ultra device (LifeScan, Milpitas, CA, USA). The concentrations of plasma triglycerides (LabAssay Triglyceride; Wako), total cholesterol (LabAssay Cholesterol; Wako), non-esterified fatty acid (LabAssay NEFA; Wako), Peptide YY (Mouse/Rat PYY ELISA Kit, Wako Pure Chemical Co. Ltd., Osaka, Japan), GLP-1 (active) ELISA kit (Merck Millipore, Billerica, MA, USA), and insulin ELISA Kit (Shibayagi, Gunma, Japan) were measured according to manufacturer instructions. For GLP-1 measurement, plasma samples and culture media were treated with a DPP-IV inhibitor (Merck Millipore, Billerica, MA, USA) to prevent degradation of active GLP-1. Bacterial endotoxin levels were evaluated by a limulus amebocyte lysate chromogenic endpoint assay, according to the manufacturer’s instructions (Hycult Biotech, Wayne, PA, USA).
Histology
Epididymal white adipose tissues were fixed in 10% neutral-buffered formalin, embedded in paraffin, and sectioned (7 μm). Hematoxylin and eosin staining was performed using standard techniques42, with minor modifications. To measure adipocyte area, the diameters of ≥ 20 cells from 4 sections in each group were measured using ImageJ software (National Institutes of Health, Bethesda, MD, USA). The results were expressed as the average of > 10 fields examined.
RNA extraction and qRT-PCR
Total RNA was isolated from epididymal WAT and colon using a RNAiso Plus reagent (TaKaRa, Shiga, Japan). cDNA was transcribed using RNA as templates and Moloney murine leukemia virus reverse transcriptase (Invitrogen, Carlsbad, Cam USA). Quantitative reverse transcriptase (qRT)-PCR analysis was performed using the StepOnePlus real-time PCR system (Applied Biosystems, Foster City, CA, USA) with TB Green Premix Ex Taq II (TaKaRa). The reaction was performed at 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s, 58 °C for 30 s and 72 °C for 1 min. The dissociation stage was analyzed at 95 °C for 15 s, followed by 1 cycle of 60 °C for 1 min and 95 °C for 15 s. Primer sequences were as follows: Pparg, 5′-TCAGCTCTGTGGACCTCTCC-3′ (forward) and 5′-ACCCTTGCATCCTTCACAAG-3′ (reverse); Fabp4, 5′-GATGCCTTTGTGGGAACCTGG-3′ (forward) and 5′-CTGTCGTCTGCGGTGATTTC-3’(reverse); Tnfa, 5′-TCGTAGCAAACCACCAAGTG-3′ (forward) and 5′-CTTTGAGATCCATGCCGTTG-3′ (reverse); F4/80, 5′-GGAGGATGGGAGATGGACAC-3′ (forward) and 5′-ACAGCACGACACAGCAGGAA-3′ (reverse); Ocln, 5′-CACACTTGCTTGGGACAGAG-3′ (forward) and 5′-TAGCCATAGCCTCCATAGCC-3′ (reverse); Mcp1, 5′-AATCTGAAGCTAATGCATCC-3′ (forward) and 5′-GTGTTGAATCTGGATTCACA-3′ (reverse); Gcg, 5′-TGGACTCCCGCCGTGCCCAA-3′ (forward) and 5′-CGACTTCTTCTGGGAAGTCTCGCCT-3′ (reverse); and 18S, 5′-ACGCTGAGCCAGTCAGTGTA-3′ (forward) and 5′-CTTAGAGGGACAAGTGGCG-3′ (reverse).
Gut microbial composition
Fecal DNA was extracted from frozen samples using the FastDNA SPIN kit for feces (MP Biomedicals, Santa Ana, CA, USA) according to manufacturer instructions20. Partial 16 S rRNA gene sequences were amplified targeting the hypervariable regions v4 using primers 515 F (5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGGTGYCAGCMGCCGCGGTAA-3′) and 806 R (5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGGACTACHVGGGTWTCTAAT-3′). Amplicons generated from each sample were subsequently purified using AMPure XP (Beckman Coulter, Brea, CA, USA). The 16S rRNA sequence data generated by the MiSeq sequencer (Illumina, San Diego, CA, USA) using the MiSeq Reagent kit (version 3.0; 600 cycles) were processed by the quantitative insights into microbial ecology (QIIME 2. 2022.2.0; http://qiime.org/) pipeline. Data analysis was performed using MiSeq Reporter software with the SILVA database (Illumina). Diversity analyses were performed using the QIIME script core_diversity_analyses.py. The statistical significance of sample groupings was assessed using permutational multivariate analysis of variance (QIIME script compare_categories.py). The 16S rRNA sequencing data have been deposited into the DDBJ under the accession No. DRA015131.
Quantitative PCR analysis was performed with using SYBR Premix Ex Taq II (TAKARA) and StepOneTM real time PCR system (Applied Biosystems) as previously described41. Bacterial primer sequences are as follows; Universal, 5′-CRAACAGGATTAGAACCCT-3′ (forward) and 5′-GGTAAGGTTCCTCGCGTAT-3′ (reverse).
Statistical analysis
All values are presented as the mean ± standard error. Data were analyzed using commercially available statistical software: Prism version 9.4.1 (GraphPad Software, La Jolla, CA, www.graphpad.com). Data normality was checked by using the Shapiro–Wilk test. For normal data, equal-variance test was carried out by F test (for comparing two groups) or Bartlett’s test (for comparing more than 3 groups). When comparing 2 groups, either 2-tailed unpaired t-test (for parametric data) or Mann–Whitney test (for nonparametric data) was used. When comparing more than 3 groups, one-way analysis of variance (ANOVA) (for parametric data) or Kruskal–Wallis test (for nonparametric data) was carried out, followed by post-hoc multiple comparisons tests either by Dunnett, Tukey–Kramer, or Dunn’s test. P-values from these multiple comparison tests were corrected and reported as adjusted P values. Statistical significance was set at P < 0.05.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The 16S rRNA sequencing data have been deposited into the DDBJ under the Accession No. DRA015131 (https://ddbj.nig.ac.jp/resource/sra-submission/DRA015131).
References
Christ, A., Lauterbach, M. & Latz, E. Western diet and the immune system: An inflammatory connection. Immunity 51, 794–811 (2019).
Kahn, S. E., Hull, R. L. & Utzschneider, K. M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444, 840–846 (2006).
Makki, K., Froguel, P. & Wolowczuk, I. Adipose tissue in obesity-related inflammation and insulin resistance: Cells, cytokines, and chemokines. ISRN Inflamm. 2013, 139239 (2013).
Marchesi, J. R. et al. The gut microbiota and host health: A new clinical frontier. Gut 65, 330–339 (2016).
Delzenne, N. M. & Cani, P. D. Gut microbiota and the pathogenesis of insulin resistance. Curr. Diab. Rep. 11, 154–159 (2011).
Everard, A. et al. Intestinal epithelial MyD88 is a sensor switching host metabolism towards obesity according to nutritional status. Nat. Commun. 5, 5648 (2014).
de Git, K. C. G. & Adan, R. A. H. Leptin resistance in diet-induced obesity: The role of hypothalamic inflammation. Obes. Rev. 16, 207–224 (2015).
Lam, Y. Y. et al. Effects of dietary fat profile on gut permeability and microbiota and their relationships with metabolic changes in mice. Obesity 23, 1429–1439 (2015).
Zhu, L. et al. Dietary flaxseed oil rich in omega-3 suppresses severity of type 2 diabetes mellitus via anti-inflammation and modulating gut microbiota in rats. Lipids Health Dis. 19, 20 (2020).
Caesar, R., Tremaroli, V., Kovatcheva-Datchary, P., Cani, P. D. & Bäckhed, F. Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metab. 22, 658–668 (2015).
Qin, N. et al. Fish oil extracted from Coregonus peled improves obese phenotype and changes gut microbiota in a high-fat diet-induced mouse model of recurrent obesity. Food Funct. 11, 6158–6169 (2020).
Zhuang, P. et al. Eicosapentaenoic and docosahexaenoic acids attenuate hyperglycemia through the microbiome-gut-organs axis in db/db mice. Microbiome 9, 185 (2021).
Bhaswant, M., Poudyal, H. & Brown, L. Mechanisms of enhanced insulin secretion and sensitivity with n-3 unsaturated fatty acids. J. Nutr. Biochem. 26, 571–584 (2015).
Morishita, M., Tanaka, T., Shida, T. & Takayama, K. Usefulness of colon targeted DHA and EPA as novel diabetes medications that promote intrinsic GLP-1 secretion. J. Control. Release 132, 99–104 (2008).
Shida, T., Kamei, N., Takeda-Morishita, M., Isowa, K. & Takayama, K. Colonic delivery of docosahexaenoic acid improves impaired glucose tolerance via GLP-1 secretion and suppresses pancreatic islet hyperplasia in diabetic KK-Ay mice. Int. J. Pharm. 450, 63–69 (2013).
Paniagua, J. A. et al. A MUFA-rich diet improves posprandial glucose, lipid and GLP-1 responses in insulin-resistant subjects. J. Am. Coll. Nutr. 26, 434–444 (2007).
Zhuang, P. et al. Eicosapentaenoic and docosahexaenoic acids differentially alter gut microbiome and reverse high-fat diet-induced insulin resistance. Mol. Nutr. Food Res. 64, e1900946 (2020).
Pickens, C. A., Albuquerque Pereira, M. D. F. & Fenton, J. I. Long-chain ω-6 plasma phospholipid polyunsaturated fatty acids and association with colon adenomas in adult men: A cross-sectional study. Eur. J. Cancer Prev. 26, 497–505 (2017).
Romagnolo, D. F. & Selmin, O. I. Mediterranean diet and prevention of chronic diseases. Nutr. Today 52, 208–222 (2017).
Greiner, T. U. & Bäckhed, F. Microbial regulation of GLP-1 and L-cell biology. Mol. Metab. 5, 753–758 (2016).
Miyamoto, J. et al. Gut microbiota confers host resistance to obesity by metabolizing dietary polyunsaturated fatty acids. Nat. Commun. 10, 1–5 (2019).
Stȩpień, M. et al. Obesity indices and inflammatory markers in obese non-diabetic normo- and hypertensive patients: A comparative pilot study. Lipids Health Dis. 13, 29 (2014).
Emont, M. P. et al. A single-cell atlas of human and mouse white adipose tissue. Nature 603, 926–933 (2022).
Kazak, L. et al. Genetic depletion of adipocyte creatine metabolism inhibits diet-induced thermogenesis and drives obesity. Cell Metab. 26, 660–671 (2017).
Cani, P. D. et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57, 1470–1481 (2008).
Luck, H. et al. Regulation of obesity-related insulin resistance with gut anti-inflammatory agents. Cell Metab. 21, 527–542 (2015).
Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006).
Maertens, B., Gagnaire, A., Paerewijck, O., De Bosscher, K. & Geldhof, P. Regulatory role of the intestinal microbiota in the immune response against Giardia. Sci. Rep. 11, 10601 (2021).
Ge, X. et al. Antibiotics-induced depletion of mice microbiota induces changes in host serotonin biosynthesis and intestinal motility. J. Transl. Med. 15, 13 (2017).
Miyamoto, J. et al. A gut microbial metabolite of linoleic acid, 10-hydroxy-cis-12-octadecenoic acid, ameliorates intestinal epithelial barrier impairment partially via GPR40-MEK-ERK pathway. J. Biol. Chem. 290, 2902–2918 (2015).
Rodriguez-Echevarria, R. et al. Diet switch and omega-3 hydroxy-fatty acids display differential hepatoprotective effects in an obesity/nonalcoholic fatty liver disease model in mice. World J. Gastroenterol. 24, 461–474 (2018).
Kobyliak, N., Virchenko, O. & Falalyeyeva, T. Pathophysiological role of host microbiota in the development of obesity. Nutr. J. 15, 43 (2016).
Wichmann, A. et al. Microbial modulation of energy availability in the colon regulates intestinal transit. Cell Host Microbe 14, 582–590 (2013).
Tolhurst, G. et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61, 364–371 (2012).
Thomas, C. et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 10, 167–177 (2009).
Winer, D. A., Luck, H., Tsai, S. & Winer, S. The intestinal immune system in obesity and insulin resistance. Cell Metab. 23, 413–426 (2016).
Jackman, J. A., Yoon, B. K., Li, D. & Cho, N. J. Nanotechnology formulations for antibacterial free fatty acids and monoglycerides. Molecules 21, 305 (2016).
Shilling, M. et al. Antimicrobial effects of virgin coconut oil and its medium-chain fatty acids on Clostridium difficile. J. Med. Food 16, 1079–1085 (2013).
Sheu, C. W. & Freese, E. Lipopolysaccharide layer protection of gram-negative bacteria against inhibition by long-chain fatty acids. J. Bacteriol. 115, 869–875 (1973).
Percie du Sert, N. et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 18, e3000410 (2020).
Miyamoto, J. et al. Ketone body receptor GPR43 regulates lipid metabolism under ketogenic conditions. Proc. Natl. Acad. Sci. U.S.A. 116, 23813–23821 (2019).
Kimura, I. et al. Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science 367, 8429 (2020).
Acknowledgements
This work was supported by research grants from the JSPS KAKENHI (JP22K17771), the Astellas Foundation for Research on Metabolic Disorders, the Institute for Fermentation Osaka, the Nakajima Foundation, the Takeda Science Foundation, the TOYO SUISAN Foundation and the Asahi Glass Foundation (to J.M.).
Author information
Authors and Affiliations
Contributions
Y.H. performed the experiments and wrote the paper; Y.F., M.H., H.T. performed the experiments; Y.T., T.M., M.R. interpreted the data; J.M. supervised the project, performed the experiments, interpreted data, and wrote the paper; J.M. had primary responsibility for the final content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haneishi, Y., Furuya, Y., Hasegawa, M. et al. Polyunsaturated fatty acids-rich dietary lipid prevents high fat diet-induced obesity in mice. Sci Rep 13, 5556 (2023). https://doi.org/10.1038/s41598-023-32851-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32851-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.