Abstract
Macular peeling combined or followed by intravitreal dexamethasone implant (DEX-i) was recommended as an efficacy approach for tractional diabetic macular edema (tDME). Knowing the synergistic effect of cataract surgery and DEX-i one month earlier in eyes with DME, we compared Epiretinal Membrane/Inner Limiting Membrane (ERM/ILM) peeling preceded by DEX-i one month before versus ERM/ILM peeling alone for the treatment of tDME. A retrospective study on patients affected by tDME who underwent ERM/ILM peeling one month after DEX-i (n = 11; Group A) or ERM/ILM peeling alone (n = 10; Group B) was performed. Longitudinal comparison of best-correct visual acuity (BCVA), central retinal thickness (CRT), and intraocular pressure (IOP) between the time of surgery (T0) and each time point (months 1,3,5,6) within and among the groups were assessed. To evaluate the repeated measurements of BCVA, CRT, and IOP, a linear mixed-effects model was used. In Group A, DEX-i significantly improved mean BCVA and CRT (P < 0.001) just after 1 month (T0). After ERM/ILM peeling, mean BCVA and CRT significantly improved from month 1 in Group A and month 3 in Group B. Mixed model revealed a significant difference in BCVA (P ≤ 0.0001) and CRT (P ≤ 0.02) at different time-points among the groups with better results in Group A. Neither complications nor uncontrolled IOP increase was detected. ERM/ILM peeling confirmed its effectiveness in treating tDME. DEX-i performed one month before surgery seemed to be a safe approach and ensured a greater and faster recovery considering functional and tomographic parameters.
Similar content being viewed by others
Introduction
Diabetic macular edema (DME) affects approximately 6.8% of the diabetic population1 and 20% of the patients with diabetic retinopathy2, being the major cause of vision loss in these patients3,4.
The incidence of epiretinal membrane (ERM) in DME patients has been reported to be 13–34% on structural optical coherence tomography (OCT) imaging5,6,7,8 and 46.9% on En-face OCT imaging9.
Vitrectomy may be considered when there is little or no response to non-surgical treatments10,11 or in cases in which DME is associated with vitreomacular traction (VMT) or ERM11,12,13,14. After surgery, the anatomical outcome has been shown to be better than functional recovery15. Evidence from the Diabetic Retinopathy Clinical Research Network (DRCR.net) has demonstrated a reduction of the central retinal thickness (CRT) after vitrectomy and ERM peeling in DME eyes with vitreomacular interface abnormality16,17. However, the efficacy of improving visual acuity was limited16,17 and influenced by predictive factors, including poorer glycemic control18, preoperative visual acuity16, greater retinal thickness18, presence of subretinal fluid19, and lack of integrity of outer retinal layers20,21.
Combining vitrectomy and nonsurgical treatments, including intravitreal triamcinolone and laser photocoagulation, revealed favorable outcomes, but with short-term efficacy and only in non-tractional DME22,23,24.
The intravitreal dexamethasone implant (DEX-i) (Ozurdex®, Allergan Inc, Irvine, CA, USA) provides a sustained release of corticosteroid for up to 6 months thanks to its biodegradable polymer matrix. DEX-i has been shown to have similar pharmacokinetic profiles and safety in both vitrectomized and nonvitrectomized eyes25. Additionally, DEX-i has been associated with different advantages over other sustained-release steroid implants using fluocinolone acetonide or triamcinolone acetonide as a smaller incidence of increase in intraocular pressure (IOP)26 and a complete dissolution within the vitreous cavity that avoid surgical removal of the implant.
Recently, Figueira et al. recommended macular peeling plus DEX-i as first-line therapy for DME when traction is present27. Furthermore, ERM and Inner Limiting Membrane (ILM) peeling performed in combination with intravitreal DEX-i showed longer-lasting treatment effects, especially in tractional DME (tDME)28,29. However, if vitrectomy and simultaneous DEX-i seem to work synergistically, the efficacy of the use of DEX-I one month before ocular surgery, benefiting from its peak of action, was recently observed in patients with DME undergoing cataract surgery30,31.
So, the current study aimed to compare functional and anatomical results of macular peeling alone versus peeling preceded by DEX-i one month before surgery in patients with tDME.
Methods
Study design and objectives
We conducted a retrospective, comparative, single-center cohort study on 21 patients affected by tDME. Between September 2015 and February 2016, at the Eye Clinic of “SS. Annunziata” Hospital in Taranto, Italy, 11 consecutive patients were treated with DEX-i performed 30 ± 5 days before primary vitrectomy combined with ERM and inner limiting membrane (ILM) peeling (Group A) and compared with 10 consecutive patients treated with primary vitrectomy associated with ERM and ILM peeling alone (Group B). Inclusion criteria were tDME defined as central-involved diabetic macular edema (cystoid pattern, sponge-like pattern, or retinal detachment pattern) associated with ERM, non-proliferative diabetic retinopathy, proliferative diabetic retinopathy previously treated with laser photocoagulation or intravitreal anti-Vascular Endothelial Growth Factor (VEGF) injection.
Exclusion criteria included HbA1c > 9% (75 mmol/mol), untreated proliferative diabetic retinopathy; a history of ocular hypertension or glaucoma; previous or concomitant retinal diseases including retinal vein occlusion, age-related macular degeneration and other conditions that could worsen DME; treatment of DME with intravitreal anti-VEGF in the 3 months before surgery; treatment of DME with intravitreal corticosteroid in the 6 months before surgery, apart from DEX-i implant one month before vitrectomy in Group A; cataract surgery within the past 6 months; prior history of vitreoretinal surgery; uncompleted follow-up.
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of the Eye Clinic of “SS. Annunziata” Hospital in Taranto, Italy, approved this study.
The objective of the study was to compare the effect of ERM and ILM peeling performed 1 month after DEX-i with ERM and ILM peeling alone in terms of variation of best corrected visual acuity (BCVA) and CRT.
Surgical procedure
DEX implant 0.7 mg was injected into the vitreous cavity using standard protocol32. Twenty-five Gauge (25G) three ports vitrectomy was performed using the Constellation® Vision System (Alcon Laboratories, Fort Worth, TX). Povidone-iodine 5% preparation (Oftasteril, Alfa Intes Industria Terapeutica Splendore S.r.l., Naples, Italy) was applied to the cornea, conjunctival sac, and periocular skin for 3 min before surgery. Peribulbar anesthesia was performed on all patients. Congiuntival displacement with forceps and three 30° oblique incisions 3.5–4 mm from the limbus were performed to insert three valved cannula trocar systems. For posterior visualization, Oculus BIOM 4 (Oculus Surigcal Inc, FL, USA) and a plano-concave contact lens were used. The central core and peripherical vitrectomy were performed in all cases with 5000 cuts per min (cpm) cut-rate and linear aspiration of 0–650 mmHg.
In Group A, the DEX-i was visible floating in the vitreous chamber during vitrectomy, so a lower aspiration rate and slower maneuver were performed to avoid accidentally cutting the implant with the vitrectome. The use of a triamcinolone-based vitreous stainer was avoided in all surgery. ERM and ILM peeling was performed in all patients, using 25-G internal limiting membrane forceps (Alcon Laboratories) after staining with Trypan Blu (TB) 0.15% + Brilliant Blue G (BBG) 0.05% + Lutein 2%solution (DOUBLE DYNE; Alfa Intes Industria Terapeutica Splendore S.r.l., Naples, Italy). A sclerotomy site suture was performed only when needed due to leakage of the wound.
Assessments
All participants underwent a complete ophthalmic examination at baseline (before intravitreal dexamethasone implant insertion in Group A; before surgery in Group B), including demographic and anamnestic data and HbA1c levels. At the time of surgery (T0) and during the follow-up visits scheduled at months 1,3,5 and 6, BCVA, IOP, and CRT were measured. IOP and BCVA were measured with a Goldmann tonometer and a standardized Early Treatment Diabetic Retinopathy Study protocol, respectively; Early Treatment Diabetic Retinopathy Study Values were converted to the logarithm of the minimum angle of resolution for statistical analysis; CRT was assessed with spectral-domain optical coherence tomography (SD-OCT; CIRRUS, Carl Zeiss, Jena, Germany). CRT was defined as the average thickness of the macula in the central 1-mm Early Treatment Diabetic Retinopathy Study grid. Central involved macular edema was defined as a CRT > 300 microns (Fig. 1).
IOP measurement and all intraoperative and postoperative adverse events were recorded for safety evaluation. Additional non-surgical treatments for postoperative recurrence of DME were identified.
Statistical analysis
To describe patients’ characteristics at baseline, mean ± standard deviation (SD) was used for continuous variables and counts with percentages for categorical variables. Demographic and baseline characteristics of the two samples were compared using Fisher’s Exact test for categorical variables and Mann–Whitney U Test for quantitative ones. In Group A, the effect of DEX-i on outcomes after 1 month was analyzed using the Wilcoxon Test.
A linear mixed model was used to evaluate repeated measurements of BCVA, CRT, and IOP at each time point within each group and among the groups, and the trajectories of BCVA, CRT, and IOP. A P value < 0.05 was considered statistically significant. No formal sample size calculation was performed. All statistical analyses were performed using the software package SAS version 9.1 or higher.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review board as well as the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Institutional Review Board (Eye Clinic of “SS. Annunziata” Hospital, Taranto, Italy) approved the study.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Results
Demographic and data before treatment
Patients’ characteristics are reported in Table 1. The study enrolled 21 patients; 11 were assigned to Group A and 10 to Group B. There were 12 female patients in both groups. The mean age was 71,9 ± 5,1 years in Group A and 73,2 ± 8,1 in Group B; in each group, 1 patient had type 1diabetes. Time from diagnosis of diabetes was similar for the two groups, 16 years and 17,8 years, respectively. No difference in HbA1c was observed between the groups. All patients were pseudophakic. There were no significant differences between the two groups regarding mean BCVA, CRT, and IOP when patients were at baseline.
Follow-up
The comparisons between the mean of BCVA, CRT, and IOP at each follow-up visit (months 1, 3, 5, and 6) to baseline (T0) for each group of treatment were reported.
Best-corrected visual acuity
In Group A, mean BCVA increased significantly from 20/132 (0,82 ± 0,4 LogMar) to 20/53 (0,42 ± 0,19 logMar) at 1 month after DEX-i (T0) (P < 0.01). At T0, visual acuity was significantly better in Group A than in B (P < 0.0001). In Group A, mean BCVA significantly improved at all time points (P < 0.001) after surgery. In Group B, mean BCVA significantly improved only from the third month and in the following months (P < 0.001) after surgery (Fig. 2; Table 2).
The comparison among the groups revealed that the mean BCVA in Group A was significantly lower than Group B at each time point (at 1 month, P < 0.0001; 3 months, P = 0.001; 5 months, P = 0.01; 6 months, P = 0.03) (Table 2).
Central retinal thickness
In Group A, mean CRT decreased from 419,1 ± 81,5 µm to 326,3 ± 63,6 µm at 1 month after DEX-i (T0) (P < 0.001). Then, a progressive thinning to 265,9 ± 84,1 µm at Month 6 after surgery (P < 0.001) was observed. In Group B, mean CRT decreased from 408,5 ± 55,4 µm at T0 to 303,4 ± 69,7 µm at Month 6 after surgery (P < 0.001) (Fig. 3; Table 2).
The comparison among the groups revealed that the mean CRT in Group A was significantly lower than Group B (P < 0.001) at each time point (Table 2).
In Group A, only 1 patient had CRT > 300 µm at month 3, showing a progressive increase of thickness over follow-up. This patient refused rescue therapy over 6 months postoperatively.
In Group B, 5 patients with CRT > 300 µm at month 3 showed a further reduction in thickness at 5 months, and only 3 of these had a new increase of thickness at 6 months. Furthermore, another 2 patients had a recurrence of edema (CRT > 300 µm) at 6 months after surgery. At the last follow-up, a second DEX-i was scheduled for 1 patient in Group A and 5 patients in Group B (P = 0.06).
Intraocular pressure and complications
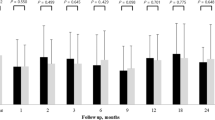
During the study, mean IOP did not significantly increase in both groups, neither significant differences were observed between the groups (Fig. 4). None of the patients showed significant increase in the IOP requiring medical or surgical management.
Comparing the variations of the two groups, no significant differences were found over the follow-up. No surgical complications were detected.
Linear mixed-effects model
The model revealed that the effect of each treatment, time, and interaction between treatment and time on BCVA and CRT was significantly different among the groups. The same was not the case for IOP (Table 2).
Discussion
This study analyzed the efficacy and safety of macular peeling preceded by DEX-i one month before surgery to treat tDME.
Clinical characteristics such as a long duration of diabetes and high HbA1c level at the time of surgery, preoperative presence of ERM, and poor baseline visual acuity have been associated with poor prognosis in patients with DME undergoing vitreoretinal surgery33. So our functional results should be analyzed considering that the mean duration of diabetes mellitus was not different between the groups, ranging from 16 to 18 years, the mean HbA1c level before surgery was 7% in both groups, and the mean visual acuity before any treatment was not significantly different between the groups.
The efficacy and safety of DEX-i for various retinal diseases have been proved in clinical trials and real-life studies34,35,36. Steroids have multiple mechanisms of action, inhibiting different molecules involved in vascular permeability and inflammation processes37. Several authors reported significant anatomical and functional effects of DEX-i in vitrectomized eyes with different conditions but implanted at the time of vitrectomy or after vitrectomy38,39,40,41. To the best of our knowledge, there are no published papers evaluating the impact of ERM and ILM peeling performed during the time-release of DEX-i on eyes affected by tDME. Comparing the changes in outcomes due to the combined approach versus peeling alone, we speculated on the role of DEX-i in inhibiting the inflammatory molecules related to DME and containing the inflammatory stress due to the surgical maneuvers, obtaining better and earlier results than peeling alone, and extending them over time.
In implanted eyes, preoperative CRT ranged from 311 to 610 µm, suggesting the heterogeneity of DME feature as a target for DEX implant, also in tDME. The greatest mean reduction in CRT occurred just after one month from the implant when dexamethasone reaches the highest concentration in the vitreous humor, only partially removed for macular peeling, followed by a stabilization of retinal thickness during the next months, and a late macular thickening (3–35 µm), in line with the known pharmacodynamics of Ozurdex®42. From T0 to 6 months, a significant reduction in CRT was observed in both groups. However, the implanted eyes arrived at the surgery with a thinner central retina than non-implanted eyes and maintained a thinner macula (mean value, < 300 µm) overall follow-up when compared to the non-implanted eyes that reached the lowest mean CRT (295 µm) only at 5 months. Overall, DEX-i combined with ERM/ILM peeling gave a mean reduction of 153 µm vs. 105 µm after peeling alone. Implanted eyes had a progressive and regular reduction of retinal thickness after surgery, while non-implanted eyes achieved a significant reduction only after 3 months, with the greatest change between the first and the third month. The vitreous body, together with the posterior hyaloid and the ILM, may be involved in the pathogenesis of DME43,44. Chronic inflammation due to hypoxia, oxidative stress, and upregulation of VEGF45,46 induces the adhesion of the posterior vitreous cortex to the ILM and promotes the proliferation of Müller cells, myofibroblast-like cells, and macrophages as the main components of ERM associated with DME45,46,47. ERM and ILM peeling may act on macular edema by removing tractional forces on the retina48, improving oxygen diffusion through the vitreous49, and removing the largest reservoir of pro-inflammatory factors50. This way of acting of macular peeling justifies the significant reduction of CRT observed in both groups, as previously reported by several studies on ILM peeling for tDME16,51,52. Furthermore, DEX-i releases active ingredients within the vitreous chamber over 3–6 months, potentially limiting the inflammatory stress of surgical maneuvers on retinal tissue and quickening anatomical recovery.
In Group A, the greater mean visual improvement (0.4 LogMAR) occurred just after one month from the implant, followed by further functional recovery (0.21 LogMAR) up to the sixth month after surgery. In Group B, the mean visual acuity increased only at month 3 after surgery and then remained substantially stable with an overall average gain of 0.37 LogMAR.
The anatomical outcome is better than functional results in eyes with tDME that underwent surgery15,16,17. On a large cohort, between 28 and 49% of eyes had an improvement in visual acuity, whereas between 13 and 31% had a worsening in function after surgery17. However, we observed a visual acuity improvement in 10/11 of implanted eyes and 8/10 of non-implanted eyes at the last follow-up. Only in one eye that underwent peeling alone a worsening in visual acuity was observed.
Probably, the better predictive factors for visual recovery observed in our cohort than that observed in the above-mentioned cohort including the preoperative retinal thickness (median, 364 µm vs. 491µm17) and the preoperative visual acuity (median, 20/66 vs. 20/10017) could explain our better functional results.
In the linear mixed-effects model, we have evaluated the effect of each treatment, the effect of time, and the effect of the interaction between treatment and time. For repeated measurements of BCVA and CRT, significant differences among the treatments were observed for distinct time points. Before and after surgery, the implanted eyes always had better outcomes than non-implanted eyes. At the time of surgery, some predictive factors influencing the efficacy of macular peeling, including preoperative visual acuity and retinal thickness, were significantly better in eyes previously implanted.
ILM peeling appeared to extend the benefit of the DEX-i in eyes with DME53 and tDME28 and reduce the rate of reimplanting28. Furthermore, a recent meta-analysis proved that ILM peeling combined with the removal of ERM could reduce the recurrence rate of ERM54 by eliminating a scaffold for proliferating cells, which could stimulate a leukocyte response in the macular region causing persistent macular edema55. The recurrence of macular edema requiring a second DEX-i was observed for only one patient previously implanted. In contrast, five out of ten patients not previously implanted had a final CRT > 300 µm, so potentially needing rescue therapy with DEX-i.
As known, ILM peeling may cause damage to the Müller cells and it could explain the cases with the increase of macular thickness56, potentially worsened by diabetic retinopathy.
Regarding safety concerns, none of the patients in the DEX-i group showed a significant increase in the IOP requiring medical or surgical management. Additionally, no statistically significant differences in the change of IOP were recorded between the groups throughout the follow-up. Furthermore, the study selection criteria allowed us to select patients with a clear vitreous, so in all implanted eyes, DEX-i was always clearly visible in the vitreous chamber, not hindering the vitrectomy maneuvers.
This study has some limitations that should be noted; among them is its retrospective design. Other limitations are the single-center nature of the study, the limited number of patients, and the absence of analysis of retinal layers integrity and its relationship with visual function. Moreover, another limitation of the study is the absence of adjustments for multiplicity, and as such, all analyses should be regarded as exploratory.
In conclusion, our results seemed to confirm the efficacy of ERM/ILM peeling to treat tDME. DEX-i performed one month before surgery seemed to be a safe approach and ensure a greater and faster recovery considering functional and tomographic parameters.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Yau, J. W. Y. et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 5, 556–564 (2012).
Klein, R., Klein, B. E., Moss, S. E. & Cruickshanks, K. J. (1998) The wisconsin epidemiologic study of diabetic retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology 105, 10 (1801).
Cheung, N., Mitchell, P. & Wong, T. Y. Diabetic retinopathy. Lancet 376, 124–136 (2010).
Bhagat, N., Grigorian, R. A., Tutela, A. & Zarbin, M. A. Diabetic macular edema: Pathogenesis and treatment. Surv. Ophthalmol. 54, 1–32 (2009).
Thomas, D., Bunce, C., Moorman, C. & Laidlaw, A. H. Frequency and associations of a taut thickened posterior hyaloid, partial vitreomacular separation, and subretinal fluid in patients with diabetic macular edema. Retina 25, 883–888 (2005).
Ophir, A., Martinez, M. R., Mosqueda, P. & Trevino, A. Vitreous traction and epiretinal membranes in diabetic macular oedema using spectral-domain optical coherence tomography. Eye (Lond.) 24, 1545–1553 (2010).
Akbar Khan, I., Mohamed, M. D., Mann, S. S., Hysi, P. G. & Laidlaw, D. A. Prevalence of vitreomacular interface abnormalities on spectral domain optical coherence tomography of patients undergoing macular photocoagulation for centre involving diabetic macular oedema. Br. J. Ophthalmol. 99, 1078–1081 (2015).
Arf, S. et al. Spectral domain optical coherence tomography classification of diabetic macular edema: A new proposal to clinical practice. Graefes Arch. Clin. Exp. Ophthalmol. 258, 1165–1172 (2020).
Fujiwara, A. et al. En face image-based classification of diabetic macular edema using swept source optical coherence tomography. Sci. Rep. 11(1), 7665 (2021).
Abdel Hadi, A.M. Evaluation of vitrectomy with planned foveal detachment as surgical treatment for refractory diabetic macular edema with or without vitreomacular interface abnormality. J. ophthalmol. 9246384 (2018).
Vikas, S. J., Agarwal, D., Seth, S., Kumar, A. & Kumar, A. Comparison of anatomical and functional outcomes of vitrectomy with internal limiting membrane peeling in recalcitrant diabetic macular edema with and without traction in Indian patients. Indian J. Ophthalmol. 69(11), 3297–3301 (2021).
Rosenblatt, B.J., Shah, G.K., Sharma, S. & Bakal, J. Pars plana vitrectomy with internal limiting membranectomy for refractory diabetic macular edema without a taut posterior hyaloid. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 243(1), 20–25 (2005).
Nakajima, T., Roggia, M. F., Noda, Y. & Ueta, T. Effect of internal limiting membrane peeling during vitrectomy for diabetic macular edema: Systematic review and meta-analysis. Retina (Philadelphia Pa.) 35(9), 1719–1725 (2015).
Flikier, S., Wu, A. & Wu, L. Revisiting pars plana vitrectomy in the primary treatment of diabetic macular edema in the era of pharmacological treatment. Taiwan J. Ophthalmol. 9(4), 224–232 (2019).
Schmidt-Erfurth, U. et al. Guidelines for the management of diabetic macular Edema by the European society of retina specialists (Euretina). Ophthalmologica 237(4), 185–222 (2017).
Flaxel, C. J. et al. Factors associated with visual acuity outcomes after vitrectomy for diabetic macular edema: Diabetic retinopathy clinical research network. Retina 30, 1488–1495 (2010).
Diabetic Retinopathy Clinical Research Network Writing Committee. Haller, J.A. et al. Vitrectomy outcomes in eyes with diabetic macular edema and vitreomacular traction. Ophthalmology 117, 6, 1087 (2010)
Yamada, Y. et al. Systemic factors influence the prognosis of diabetic macular edema after pars plana vitrectomy with internal limiting membrane peeling. Curr. Eye Res. 38, 1261–1265 (2013).
Shah, S. P., Patel, M., Thomas, D., Aldington, S. & Laidlaw, D. A. Factors predicting outcome of vitrectomy for diabetic macular oedema: Results of a prospective study. Br J. Ophthalmol. 9, 33–36 (2006).
Chhablani, J. K., Kim, J. S., Cheng, L., Kozak, I. & Freeman, W. External limiting membrane as a predictor of visual improvement in diabetic macular edema after pars plana vitrectomy. Graefes Arch. Clin. Exp. Ophthalmol. 250, 1415–1420 (2012).
Murakami, T. et al. Segmentational analysis of retinal thickness after vitrectomy in diabetic macular edema. Invest. Ophthalmol. Vis. Sci. 53, 6668–6674 (2012).
Sakamoto, T. et al. Triamcinolone-assisted pars plana vitrectomy improves the surgical procedures and decreases the postoperative blood-ocular barrier breakdown. Graefes Arch. Clin. Exp. Ophthalmol. 240(6), 423–429 (2002).
Kang, S. W., Park, S. C., Cho, H. Y. & Kang, J. H. Triple therapy of vitrectomy, intravitreal triamcinolone, and macular laser photocoagulation for intractable diabetic macular edema. Am. J. Ophthalmol. 144, 878–885 (2007).
Kim, J. H., Kang, S. W., Ha, H. S. & Kim, J. R. Vitrectomy combined with intravitreal triamcinolone acetonide injection and macular laser photocoagulation for non-tractional diabetic macular edema. Korean J. Ophthalmol. 27, 186–193 (2013).
Chang-Lin, J. E. et al. Pharmacokinetics of a sustained-release dexamethasone intravitreal implant in vitrectomized and nonvitrectomized eyes. Invest. Ophthalmol. Vis. Sci. 52(7), 4605–4609 (2011).
Mehta, H., Gillies, M. & Fraser-Bell, S. Perspective on the role of Ozurdex (dexamethasone intravitreal implant) in the management of diabetic macular oedema. Ther. Adv. Chronic. Dis. 6(5), 234–245 (2015).
Figueira, J. et al. Guidelines for the management of center-involving diabetic macular edema: treatment options and patient monitorization. Clin. Ophthalmol. 15, 3221–3230 (2021).
Kim, K. T. et al. Vitrectomy combined with intraoperative dexamethasone implant for the management of refractory diabetic macular edema. Korean J. Ophthalmol. 33(3), 249–258 (2019).
Lee, D. H., Kim, Y. J. & Yoon, Y. H. Minimally invasive microincision vitrectomy surgery with an intraoperative dexamethasone implant for refractory diabetic macular edema. Ophthalmologica 235, 150–156 (2016).
Kabanarou, S. A. et al. Pre-operative intravitreal dexamethasone implant in patients with refractory diabetic macular edema undergoing cataract surgery. Sci. Rep. 10, 5534 (2020).
Fallico, M. et al. Intravitreal dexamethasone implant one month before versus concomitant with cataract surgery in patients with diabetic macular oedema: The dexcat study. Acta Ophthalmol. 99(1), e74–e80 (2021).
Guigou, S. et al. Efficacy and safety of intravitreal dexamethasone implant in patients with diabetic macular edema. Ophthalmologica 233(3–4), 169–175 (2015).
Nawrocka, Z.A. & Nawrocki, J. Vitrectomy in diabetic macular edema—A swept-source optical coherence tomography (OCT) angiography study. Ophthalmol. Sci. 100207 (2022). In Press.
Maturi, R. K. et al. Intraocular pressure in patients with diabetic macular edema treated with dexamethasone intravitreal implant in the 3-year mead study. Retina 36, 1143–1152 (2016).
Rajesh, B. et al. Safety of 6000 intravitreal dexamethasone implants. Br J. Ophthalmol. 104, 39–46 (2020).
Bonfiglio, V. et al. Widening use of dexamethasone implant for the treatment of macular edema. Drug. Des. Devel. Ther. 11, 2359–2372 (2017).
Iovino, C. et al. Intravitreal dexamethasone implant as a sustained release Drug delivery device for the treatment of ocular diseases: A comprehensive review of the literature. Pharmaceutics 12, 703 (2020).
Boyer, D. S. et al. Dexamethasone intravitreal implant for treatment of diabetic macular edema in vitrectomized patients. Retina 31, 915–923 (2011).
Reibaldi, M. et al. Treatment of persistent serous retinal detachment in vogt-koyanagi-harada syndrome with intravitreal bevacizumab during the systemic steroid treatment. Retina 34, 490–496 (2014).
Iovino, C. et al. Efficacy and safety of combined vitrectomy with intravitreal dexamethasone implant for advanced stage epiretinal membrane. Drug. Des. Devel. Ther. 13, 4107–4114 (2019).
Fallico, M. et al. Epiretinal membrane vitrectomy with and without intraoperative intravitreal dexamethasone implant: A systematic review with meta-analysis. Front. Pharmacol. 12, 635101 (2021).
Chang-Lin, J. E. et al. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Invest. Ophthalmol. Vis. Sci. 52, 80–86 (2011).
Sebag, J. & Balazs, E. A. Pathogenesis of cystoid macular edema: An anatomic consideration of vitreoretinal adhesions. Surv. Ophthalmol. 28(Suppl), 493–498 (1984).
Bhagat, N., Grigorian, R. A., Tutela, A. & Zarbin, M. A. Diabetic macular edema: Pathogenesis and treatment. Surv. Ophthalmol. 54(1), 1–32 (2009).
Chung, Y.R. et al. Role of inflammation in classification of diabetic macular edema by optical coherence tomography. J Diabetes Res. 8164250 (2019).
Tamura, K., Yokoyama, T., Ebihara, N. & Murakami, A. Histopathologic analysis of the internal limiting membrane surgically peeled from eyes with diffuse diabetic macular edema. Jpn. J. Ophthalmol. 56, 280–287 (2012).
Romano, M. R. et al. Vitreous and intraretinal macular changes in diabetic macular edema with and without tractional components. Graefes. Arch. Clin. Exp. Ophthalmol. 257, 1–8 (2019).
Ghassemi, F. et al. Outcomes of vitrectomy, membranectomy and internal limiting membrane peeling in patients with refractory diabetic macular edema and non-tractional epiretinal membrane. J. Curr. Ophthal. 28(4), 199–205 (2016).
Stefánsson, E. The therapeutic effects of retinal laser treatment and vitrectomy. A theory based on oxygen and vascular physiology. Acta Ophthamol. Scand. 79(5), 435 (2001).
Simó-Servat, O., Hernández, C. & Simó, R. Usefulness of the vitreous fluid analysis in the translational research of diabetic retinopathy. Mediat. Inflamm. 1–11 (2012).
Diabetic Retinopathy Clinical Research Network Writing Committee et al. Vitrectomy outcomes in eyes with diabetic macular edema and vitreomacular traction. Ophthalmology 117(6), 1087 (2010).
Bonnin, S., Sandali, O., Bonnel, S., Monin, C. & El Sanharawi, M. Vitrectomy with internal limiting membrane peeling for tractional and nontractional diabetic macular edema: Long-term results of a comparative study. Retina 35, 921–928 (2015).
Franzolin, E., Gusson, E. & Panozzo, G. The effect of pars plana vitrectomy with internal limiting membrane peeling on the durability of the intravitreal dexamethasone implant in the treatment of diabetic macular edema. Am. J. Ophthalmol. Case Rep. 26, 101401 (2022).
Azuma, K., Ueta, T., Eguchi, S. & Aihara, M. Effects of internal limiting membrane peeling combined with removal of idiopathic epiretinal membrane a systematic review of literature and meta-analysis. Retina 37(10), 1813 (2017).
Suzuki, T. et al. Topical dorzolamide for macular edema in the early phase after vitrectomy and epiretinal membrane removal. Clin. Ophthalmol. 7, 549–553 (2013).
Cicinelli, M. V. et al. Associated factors and surgical outcomes of microcystoid macular edema and cone bouquet abnormalities in eyes with epiretinal membrane. Retina (Philadelphia, Pa.) 42(8), 1455–1464 (2022).
Acknowledgements
We gratefully acknowledge the assistance of Rossella Donghia in statistical analysis.
Author information
Authors and Affiliations
Contributions
F.P. was responsible for the conceptualization. F.P., A.N. designed the study methodology. F.P., A.N., M.F., F.P., S.G., A.N. performed formal analysis and investigation. A.N. wrote the main manuscript text. F.P., G.A. found the resources. V.B., M.R., G.A., T.A. supervised the study. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pignatelli, F., Niro, A., Fallico, M. et al. Mid-term safety and effectiveness of macular peeling one month after intravitreal dexamethasone implant for tractional diabetic macular edema. Sci Rep 13, 5990 (2023). https://doi.org/10.1038/s41598-023-32780-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32780-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.