Abstract
Background/Objectives
To investigate if the visual and anatomic response to the first dexamethasone implant (DEX) predicts the 12-month clinical outcome after shifting to fluocinolone acetonide (FAc) implant in patients with diabetic macular oedema (DMO).
Methods
Retrospective cohort study including pseudophakic patients with previously treated DMO, undergone one or more DEX injections before FAc. Functional and morphologic response to DEX was defined based on the best-corrected visual acuity (BCVA) and central macular thickness (CMT) changes after the first DEX, respectively. Steroid-response was defined as intraocular pressure (IOP) elevation ≥5 mmHg or IOP > 21 mmHg after any previous DEX exposure. Pairwise comparisons for BCVA, CMT, and IOP after FAc were performed with linear mixed models and a repeated-measure design.
Results
Forty-four eyes of 33 patients were included. Patients were shifted to FAc after a mean ± standard deviation of 4.6 ± 3.2 DEX injections. Overall, BCVA and CMT improved during the first 12 months after switching to FAc (p = 0.04 and p < 0.001, respectively). Only eyes with a good morphologic response to DEX had a significant CMT reduction after FAc (p < 0.001), while no significant relationship was found between BCVA improvement after DEX and after FAc. IOP elevation occurred in 9 eyes (20%) following DEX implant. These eyes carried a 20-fold increased risk of having an IOP rise after FAc (p < 0.001), with a non-linear relationship between the IOP increase after DEX and the one after FAc.
Conclusion
The response to previous DEX may anticipate the morphologic response to subsequent FAc. Eyes with steroid-induced IOP elevation after DEX are at a high risk of IOP increase after FAc. The visual response after FAc was not associated with the visual response to previous steroids, indicating that FAc may have a role also in patients refractory to DEX implant.
Similar content being viewed by others
Introduction
Anti-vascular endothelial growth factor (VEGF) agents are currently the first-line therapeutic approach for diabetic macular oedema (DMO) [1]. In the subset of eyes not responding to anti-VEGF [2] or in the presence of systemic contraindications to anti-angiogenic treatment, a switch to intravitreal corticosteroids is recommended [3].
Two different steroidal drug-delivery systems (DDS) are currently approved for DMO. The dexamethasone (DEX) 700 µg bioerodable implant (OZURDEX®; Allergan, Inc., Irvine, CA) has a high-rate drug release in the first 2 months, followed by a lower-dose release for 4 months [4]. The fluocinolone acetonide (FAc) 0.19 mg non-bioerodable implant (ILUVIEN®; Alimera Sciences, Inc., Alpharetta, GA, USA), by contrast, exhibits zero-order release kinetics with a low-peak concentration and a steady-state for 36 months.
FAc implant has been approved in Europe for DMO unresponsive to previous agents; therefore, it represents a second- or third-line therapy [5]. FAc implant yields good visual and anatomical outcomes, regardless of the nature and the number of previous DMO treatments administered [6,7,8]. Intraocular pressure (IOP) rise and cataract worsening are the most common side effects of FAc. It is known that the IOP response can be anticipated by the exposure to previous steroids [9,10,11]. Nevertheless, it is still unknown whether the clinical response to previous DEX predicts the clinical outcome to FAc.
This study was designed to investigate whether the visual and anatomic response to the first DEX implant predicts the 12-month clinical outcome after shifting to fluocinolone acetonide (FAc) implant in patients with diabetic macular oedema (DMO). The secondary aim was to examine the IOP changes following FAc as a function of previous IOP changes following DEX implant.
Methods
Retrospective study of patients who were switched to FAc implant after at least one DEX injection at the Medical Retina Unit of the Department of Ophthalmology, Ospedale San Raffaele (Milan, Italy). The study followed the tenets of the Declaration of Helsinki for research involving human subjects and received the approval of the San Raffaele Hospital IRB; all the subjects signed a written consent at the time of FAc administration.
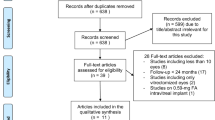
Electronic charts of all patients who underwent FAc implant for DMO between July 2017 and April 2019 were reviewed. As this was a retrospective study, switching criteria were not set in advance, and the FAc implant was administered according to the following general principles: DMO with a suboptimal response to previous strategies (focal/grid laser, anti-VEGF, or DEX); patient preference for less intense injection schedule; or contraindications to alternative treatments. All eyes were pseudophakic with no history of progressing glaucoma or uncontrolled IOP, as per the Italian reimbursement norms [12].
Inclusion criteria for this study were: (1) age ≥ 18 years; (2) diagnosis of diabetes mellitus (DM), either type 1 or type 2; (3) treatment with at least one DEX implant before the first FAc injection; (4) minimum follow-up of 12 months after the first FAc injection. Exclusion criteria were: (1) macular oedema secondary to causes other than DMO; (2) corneal, lens, or vitreous opacity preventing good-quality optical coherence tomography (OCT) during the follow-up; (3) history of ocular trauma or surgery ≤6 months before FAc injection, (4) intravitreal anti-VEGF (bevacizumab, ranibizumab, or aflibercept) <1 month or DEX implant <4 months before FAc administration. For patients with bilateral DMO, both eyes were included in the study if all the inclusion and exclusion criteria were fulfilled.
The baseline visit was set at the time of receiving the FAc implant. Patients receiving FAc were followed every two months to monitor the clinical response and potential adverse events; the follow-up scheme was intensified if deemed necessary by the clinician treating the patient. In the case of non-resolving or recurrent DMO after FAc implant, additional anti-VEGF injections were administered with a pro-re-nata approach. In the case of IOP elevation judged above target by the treating clinician, glaucoma treatment was initiated.
At each visit, best-corrected visual acuity (BCVA) measured on decimal charts, intraocular pressure (IOP) measured by Goldmann applanation tonometry, and spectral-domain OCT (SD-OCT) (Spectralis HRA, Heidelberg Engineering; Heidelberg, Germany) were performed. The BCVA was converted into LogMAR scale for statistical calculations. Central macular thickness (CMT) was obtained through 19 horizontal lines centered on the fovea, each with 9 averaged OCT B-scans-1024 A-scans per line at 240 μm intervals. The number of IOP-lowering medications or procedures across the follow-up was recorded.
At the baseline visit, two trained ophthalmologists (M.V.C and L.C.) evaluated the following features on a horizontal SD-OCT B-scan line passing through the fovea: (1) subfoveal disruption of the ellipsoid zone (EZ) and/or external limiting membrane (ELM); (2) presence of disorganization of the inner retinal layers (DRIL); (3) presence of epiretinal membrane (ERM); (4) presence and number of hyper-reflective intraretinal spots (HRS); and (4) presence of neurosensory detachment (subfoveal fluid, SF). The EZ and ELM were defined as disrupted or absent if partially or completely interrupted, respectively [13]. DRIL was defined as loss of clear demarcation between the ganglion cell–inner plexiform layer complex, the inner nuclear layer, and the outer plexiform layer. HRS were manually counted and a cut-off of 20 was arbitrarily set [14]. As all eyes presented intraretinal fluid at the time of FAc injection, this parameter was not considered in further analysis.
Patients’ group classification
The first DEX injection was used as a reference to evaluate the functional and the anatomic response, while any DEX injection was considered to evaluate the steroid-induced IOP elevation. A classification regression tree (CART) was used to find the best cut-off to predict the 12-month BCVA and CMT values as a function of the visual and anatomic changes occurring after the first DEX injection, respectively. The CART algorithm is a binary recursive tree structure that divides the parent nodes (all data) into two child nodes while searching for all possible variables and all possible values to find the best split [15]. A similar approach was used to identify the best cut-off predicting the IOP response after FAc.
-
Good functional response was defined as at least 0.22 LogMAR improvement in BCVA recorded 1 month after the first DEX implant injection.
-
Good morphologic response was defined as at least 95-μm decrease in CMT 1 month after the first DEX implant injection.
-
Steroid-induced IOP elevation (steroid-response) was defined as at least a 5-mmHg increase in IOP or IOP > 21 mmHg recorded after any intravitreal DEX implant.
All analyses were repeated with an alternative definition of morphologic response, with patients divided in good and poor responders whether CMT decreased by ≥10% and <10% following DEX implant, respectively [16].
Statistical analysis
Statistical calculations were performed with the open-source programming language R [17]. Descriptive statistics were reported as mean ± standard deviation [SD] for continuous variables or frequency and percentage for categorical variables. The last observation carried forward method was used to impute missing data.
Generalized mixed models were fitted to compare the baseline demographic and clinical variables between patients according to their response (good vs. poor) to previous DEX. To evaluate the visual and morphologic response to FAc, a repeated-measure design was applied, where the various time points and group effect (functional or morphological response to the first DEX, respectively) were included as fixed factors and the random effect had a nested structure, with the patient and eye identification numbers being the outer and inner levels, respectively, to account for within-subject (two eyes of the same patient) and within-eye (multiple observations from the same eye) correlations. A similar analysis was run to investigate the IOP response. Pairwise comparisons of BCVA, CMT, and IOP between each pair of time points within the model were investigated, applying a Tukey correction for multiple contrasts. The increase in IOP after DEX was correlated with the increase in IOP after FAc with Pearson’s correlation test. The effects of being steroid-responder to DEX on the risk of IOP increase and the chance of being treated with IOP-lowering medications or procedures in the first year after FAc injection were studied with logistic mixed model, accounting for the patient ID as a random effect.
The cutoff point for statistical significance was set at p < 0.05.
Results
Forty-four eyes of 33 patients were included in the study. Patients were shifted to FAc after a mean of 4.6 ± 3.2 DEX injections. Mean follow-up after FAc injection was 14 ± 1.2 months; all the eyes received only one FAc implant. Main demographic and baseline clinical features are listed in Table 1.
Nine eyes (20%) of 9 patients had a good functional outcome after the first DEX; good visual responders had a slightly longer duration of DMO (p = 0.04) and were more likely to have previously undergone focal laser (p < 0.001) than poor visual responders. Twenty-nine eyes (66%) of 22 patients had a good morphologic response; a greater proportion of eyes with good morphologic response had DRIL (p < 0.001) and EZ/ELM disruption (p < 0.001) on SD-OCT. The good morphologic responders’ group had a minor rate of self-reported systemic hypertension (p < 0.001) and type 1 diabetes (p = 0.04). There was also a greater proportion of eyes with NPDR (p = 0.8), previous vitrectomy (p = 0.8), and peripheral (p = 0.5) and macular laser (p = 0.4) among good morphologic responders; however, these differences were non-significant compared to poor morphologic DEX responders.
No difference was found between good and poor responders to the first DEX in terms of the number of intravitreal DEX injections administered before shifting to FAc (p = 0.8 and p = 0.4 for the visual and the morphologic response, respectively).
Visual and morphologic response to DEX implant
The BCVA before the first DEX was significantly worse in good responders compared to poor responders (0.96 ± 0.48 vs. 0.57 ± 0.43 LogMAR, p = 0.02) (Table 2). Overall, BCVA improved by 0.09 ± 0.2 LogMAR one month after the first DEX implant injection, with good responders having greater improvement than poor responders (0.38 ± 0.22 vs. −0.01 ± 0.09 LogMAR, p < 0.001). Pre-DEX CMT was significantly greater in good responders than poor responders (593.8 ± 135.5 vs. 431.9 ± 126 μm, p = 0.002). Overall, mean CMT decreased by 192.6 ± 150.7 μm; good responders had greater CMT reduction compared to poor responders (270.8 ± 122 vs. 41.5 ± 54.6 μm, p < 0.001).
Visual and morphologic response to FAc implant
Overall, BCVA and CMT values significantly improved during the first 12 months after switching to long-term steroids (p = 0.04 and p < 0.001, respectively). At the 12 month post-FAc visit, poor responder had slightly worse BCVA compared to good reponders, albeit the difference between the two groups was non-significant (p = 0.9). Within each group, the BCVA did not significantly change during the follow-up (Supplementary Table 1A). Conversely, the CMT after FAc significantly decreased in eyes that had a good morphologic response to DEX (at 2, 4, 6, 8, 10, and 12 months, p < 0.001, Supplementary Table 1B) but not in eyes who were poor responders to the first DEX injection. Similar results were obtained by dividing the morphologic response to DEX between patients who had ≥10% reduction in baseline CMT after the first injection and those who had <10% reduction in baseline CMT after the first injection (Supplementary Fig. 1).
Eleven eyes (25%) underwent additional anti-VEGF in the first year after FAc implant injection, with a mean of 1.5 ± 0.8 doses of anti-VEGF administered. The proportion of patients receiving additional treatments was not different between good and poor visual responders to DEX (33% vs. 23%, respectively; p = 0.8). On the other hand, a greater proportion of poor morphologic responders to DEX underwent supplemental anti-VEGF injections compared to good morphologic responders (34% vs. 20%, p = 0.006).
Safety
IOP elevation following any DEX injection occurred in 9 eyes (20%) of 8 patients. Pre-DEX IOP values did not differ significantly between patients with and without subsequent steroid-induced IOP elevation (p = 0.9). The mean IOP increase after DEX was 8.7 ± 1.9 mmHg in steroid-responders and 1 ± 1.6 mmHg in non steroid-responders (p < 0.001); all eyes returned to their pre-DEX IOP values before receiving FAc (Fig. 1C).
A Boxplots illustrating the changes in best-corrected visual acuity (BCVA) before and after DEX and up to 12 months after fluocinolone acetonide (FAc) implant. B Boxplots illustrating the changes in central macular thickness (CMT) before and after DEX and after FAc implant. C Boxplots illustrating the changes in IOP before and after DEX and up to 12 months after FAc implant. The bold horizontal line inside the box represents the median; the upper and the lower box limit represent the first and the third quartile, respectively. Outliers are plotted as individual points. For quantitative cut-offs refer to the methods section.
Overall, the IOP increased by 3.7 ± 6.2 mmHg 12 months after FAc, and the magnitude of increase was greater in those who had an IOP-related event after DEX (8.6 ± 5.3 mmHg vs. 2.5 ± 5.9 mmHg, p = 0.03)(Fig. 2A). After switching to FAc, 12 eyes (27%) had an IOP > 21 mmHg. Of note, 5 eyes (11%) had steroid-induced IOP elevation to FAc in the absence of any previous steroid-response to DEX.
A Upper panel: Spaghetti plot exploring the longitudinal changes in IOP after receiving the FAc implant. Patients were divided into steroid-responders (i.e., experiencing at least 5 mmHg increase in IOP or IOP > 21 mmHg after any previous DEX implant injection) and non steroid-responders. Lower panel: Bar plot indicating the number of IOP-lowering medications prescribed to patients before DEX, after DEX, and one year after FAc implant injection. B Correlation between the relative increase in IOP after DEX and the relative increase in IOP after FAc. A non-linear relationship was clear after a cut-off value of 5 mmHg for delta IOP increase after DEX.
The increase in IOP after long-term steroidal implant correlated with the mean increase in IOP after DEX (Pearson r = 0.29, p = 0.04), but the visual inspection of the bivariate plot suggested a non-linear relationship between the two variables. Graphically, the curve changed its slope at 5 mmHg on the x-axis (delta IOP after DEX)(Fig. 2B).
Before FAc injection, 4 eyes (9%) and 2 (5%) eyes were treated with one and two anti-glaucoma medications, respectively. At 12 months, 2 eyes (5%) were taking one medication, 8 eyes (18%) took 2 meds, 3 eyes (7%) 3 meds, and 2 eyes (4%) four meds (Fig. 2A). One eye (2%) underwent laser trabeculoplasty, and two eyes (5%) underwent surgical trabeculectomy. Eyes with previous steroid-induced IOP increase after previous DEX had markedly increased risk of having an IOP rise of at least 5 mmHg after FAc (OR = 19.8, 95% CI = 10.1–44.6, p < 0.001) and being under IOP-lowering treatment (OR = 11.8, 95% CI = 2.33–90.3, p = 0.006). No significant association between steroid-induced IOP increase after previous DEX and likelihood of undergoing glaucoma laser or surgical treatment was found (OR = 9.7, 95% CI = 0.8–227 p = 0.05), however, this specific analysis was greatly underpowered because of the small number of patients requiting glaucoma laser or surgical treatment.
Discussion
In our study, we found that the morphologic changes occurring after FAc were predicted by looking back to the first injection of DEX intravitreal implant. Specifically, patients with a greater macular thinning after DEX (i.e., ≥95 µm CMT reduction on SD-OCT) had a greater CMT reduction in the first year post-FAc injection. Conversely, eyes with poor morphologic response to the first DEX (i.e., <95 µm reduction in DMO or increased fluid on SD-OCT) experienced a non-significant change in the CMT values after switching to FAc. Patients who had steroid-induced IOP elevation after DEX (i.e., at least a 5-mmHg increase in IOP or IOP > 21 mmHg) were more likely to have IOP elevation at 12 months after FAc. There was a non-linear relationship between IOP spike after DEX and IOP increase after FAc . The visual response after FAc was not associated with the functional response to previous steroids, indicating that FAc may have a role also in patients refractory to DEX implant.
The efficacy of FAc in terms of visual function and anatomic improvement has been reported in randomized clinical trials [18, 19] and real-life studies [7, 9,10,11, 20,21,22,23,24]. Our cohort experienced sustained visual and morphologic improvement at one-year follow-up, with positive effects in eyes previously unresponsive or only partially responding to one or repeated DEX. We hypothesize that the difference in the pharmacokinetics of the two implants may account for this dissimilar behavior. In detail, the continuous release of a lower amount of FAc over a longer period may be more suitable to subside the local inflammatory milieu occurring in DMO [25]. Previous studies have shown that FAc implant causes less CMT fluctuation [26, 27], compared to the classic saw-tooth pattern seen after DEX [28, 29].
Patients undergoing FAc have usually received multiple treatments for DMO, including focal laser, anti-VEGF agents, and intravitreal steroids [9, 10, 19,20,21, 24]. Rehak et al. showed that eyes previously treated with DEX implant experienced similar outcomes after FAc injection to those shifted directly from anti-VEGF agents [6]. The authors concluded that switching from anti-VEGF straight to FAc might reduce the global treatment burden. Our study showed that a trial with DEX after an unsatisfactory course of anti-VEGF agents could help to some extent in predicting the clinical outcome after FAc. Our group previously demonstrated that a poor response to the anti-VEGF monthly loading dose might foresee the need of switching to intravitreal corticosteroids [2]. Subsequent real-life studies have conjectured that early switch in DMO eyes with insufficient response to anti-VEGF therapy may lead to better therapeutic results compared to patients maintained on anti-angiogenic agents [2, 30]. The present data showed that patients with a greater retinal thinning after exposure to DEX had a better response to the long-term steroidal implant. We cannot exclude that other baseline anatomic factors may contribute to determining the differential response to long-term intravitreal steroids, including the baseline CMT. However, the differential morphologic outcome of these patients one year after FAc might further support the existence of two different subsets of DMO, being one more inflammatory than the other [25].
Patients with a poor anatomic response after exposure to DEX had a higher proportion of systemic hypertension and PDR. Systemic hypertension has been recognized as a risk factor for macular oedema in patients with diabetes; [31, 32] our data suggest a worse anatomic response to intravitreal therapies in patients with high blood pressure. On the other hand, in the setting of PDR, higher intravitreal and intraretinal levels of VEGF may maintain macular oedema, reducing the efficacy of any given anti-DMO treatment. A higher figure of DEX-responding patients had previously undergone vitrectomy, which might suggest more inflammatory DMO pathogenesis in eyes that underwent intraocular surgery [33]. Finally, 10% of DEX responders showed indirect signs of macular ischemia (DRIL) [34] and nearly 80% had outer retinal disruption on SD-OCT, leading to a thinner retina when macular oedema resolved.
Our study showed that eyes with a good visual response to the first DEX did not experience greater functional changes after switching to FAc. In fact, the relationship between the visual acuity and baseline characteristics in diabetic eyes is complex and not fully understood. Older age, glycaemic control, the severity of DR, the duration and the extent of DMO, and the baseline visual function may all affect the final visual outcome [35]. Data from randomized clinical trials using anti-VEGF demonstrated that patients with high baseline BCVA generally achieve the highest visual acuity at the study endpoint. Conversely, patients with poor initial BCVA experience worse clinical outcomes, despite relatively higher visual gains after treatment [36]. In light of these considerations, we recommend considering FAc even in patients refractory to DEX implant.
Our study confirms the utility of a steroid challenge to assess the risk of a steroid-response to FAc injection; however, we learnt from our data that the IOP response to DEX -although indicative- did not fully predict the IOP changes after FAc. In our cohort, the number of eyes experiencing an IOP-related adverse event (27%) was slightly lower compared to the FAME trial [37], and more similar to real-life registries [9, 10]. Eyes that had a prior steroid-induced IOP elevation had a higher chance to experience IOP elevation after FAc, in accordance with the Medisoft audit [9] and the USER study [11]. The relative increase in IOP after FAc showed a non-linear relationship with the IOP increase after DEX. A qualitative inspection of the IOP curve showed that the curve changed its slope at 5 mmHg, suggesting that it might be a possible cut-off to predict the IOP response after FAc (Fig. 2B). Nevertheless, we did not perform a formal statistical analysis to identify the best IOP value discriminating steroid-responders from non steroid-responders. Noteworthily, an IOP-related adverse event was recorded in 5 patients with no previous history of steroid-response; this might be explained either as a delayed steroid-response (as previously described after repeated DEX injections) [38] or might suggest differences in the pharmacodynamic of the two steroidal molecules at the level of the trabecular meshwork [39].
Limitations of this study include the short follow-up and the analysis after the first injection of DEX implant only. As a consequence, the study does not account for patients who had a delayed effect to intravitreal DEX implant, as well as late steroid-response after cumulative exposure to intravitreal steroids. Being a retrospective data collection, we had a complete case analysis only after the first DEX injection, while the data were fragmented afterwards. We did not include information regarding the perfusion state of the macula and its relationship with the response to the FAc implant. Although useful for this task, OCT angiography does not represent the standard of care for following DMO, and the included eyes did not have serial OCT angiography exams before DEX and FAc, respectively. As we do not have any control group and we run the analyses on non-random samples (i.e., good responders, poor responders), this study is potentially vulnerable to regression to the mean.
In conclusion, a good morphologic response to previous DEX predicted a greater anatomic improvement after shifting to the FAc implant. The functional response to previous steroids was not associated with the final visual outcomes, indicating that FAc still have a role in patients refractory to DEX implant. More aggressive anti-glaucomatous treatments after the FAc injection should be anticipated eyes who had a steroid-response after DEX, but IOP-related adverse events also occurred in eyes with no previous steroid response.
What was known before
-
FAc implant has been approved in Europe for DMO unresponsive to previous agents.
-
FAc implant yields good visual and anatomical outcomes, regardless of the nature and the number of previous DMO treatments.
-
The IOP response can be anticipated by the exposure to previous steroids.
What this study adds
-
The anatomic outcome to FAc implant can be predicted by the anatomic response to the first DEX.
-
The intraocular pressure increase after FAc is non-linearly related to the IOP increase after DEX.
Change history
01 March 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41433-021-01469-2
References
Bandello F, Cicinelli MV, Parodi MB. Anti-VEGF molecules for the management of diabetic macular edema. Curr Pharm Des. 2015;21:4731–7.
Cicinelli MV, Cavalleri M, Querques L, Rabiolo A, Bandello F, Querques G. Early response to ranibizumab predictive of functional outcome after dexamethasone for unresponsive diabetic macular oedema. Br J Ophthalmol. 2017;101:1689–93.
Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, Berg K, Chakravarthy U, Gerendas BS, et al. Guidelines for the management of diabetic macular edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica. 2017;237:185–222.
Whitcup SM, Cidlowski JA, Csaky KG, Ambati J. Pharmacology of corticosteroids for diabetic macular edema. Invest Ophthalmol Vis Sci. 2018;59:1–12.
Boyer DS, Yoon YH, Belfort R Jr, Bandello F, Maturi RK, Augustin AJ, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:1904–14.
Rehak M, Busch C, Unterlauft JD, Jochmann C, Wiedemann P. Outcomes in diabetic macular edema switched directly or after a dexamethasone implant to a fluocinolone acetonide intravitreal implant following anti-VEGF treatment. Acta Diabetol. 2020;57:469–78.
Vaz-Pereira S, Castro-de-Sousa JP, Martins D, Prates Canelas J, Reis P, Sampaio A, et al. The outcomes of switching from short- to long-term intravitreal corticosteroid implant therapy in patients with diabetic macular edema. Ophthalmic Res. 2020;63:114–21.
Singh P, Chedid A, Deuchler SK, Kohnen T, Muller M, Koch FH. The efficacy and safety outcomes of the 0.19 mg fluocinolone acetonide implant after prior treatment with the 0.7 mg dexamethasone implant in patients with diabetic macular edema. Int Med Case Rep J. 2018;11:265–9.
Bailey C, Chakravarthy U, Lotery A, Menon G, Talks J, Medisoft Audit G. Real-world experience with 0.2 mug/day fluocinolone acetonide intravitreal implant (ILUVIEN) in the United Kingdom. Eye. 2017;31:1707–15.
Chakravarthy U, Taylor SR, Koch FHJ, Castro de Sousa JP, Bailey C, Group IRSSI. Changes in intraocular pressure after intravitreal fluocinolone acetonide (ILUVIEN): real-world experience in three European countries. Br J Ophthalmol. 2019;103:1072–7.
Eaton A, Koh SS, Jimenez J, Riemann CD. The USER study: a chart review of patients receiving a 0.2 microg/day fluocinolone acetonide implant for diabetic macular edema. Ophthalmol Ther. 2019;8:51–62.
Riclassificazione del medicinale per uso umano “Iluvien”, ai sensi dell’articolo 8, comma 10, della legge 24 dicembre 1993, n. 537. (Determina n. 33/2017). https://www.federfarmalombardia.it/cat_authority/normativa/page/34/.
Panozzo G, Cicinelli MV, Augustin AJ, Battaglia Parodi M, Cunha-Vaz J, Guarnaccia G, et al. An optical coherence tomography-based grading of diabetic maculopathy proposed by an international expert panel: The European School for Advanced Studies in Ophthalmology classification. Eur J Ophthalmol. 2020;30:8–18.
Framme C, Schweizer P, Imesch M, Wolf S, Wolf-Schnurrbusch U. Behavior of SD-OCT-detected hyperreflective foci in the retina of anti-VEGF-treated patients with diabetic macular edema. Invest Ophthalmol Vis Sci. 2012;53:5814–8.
Song YY, Lu Y. Decision tree methods: applications for classification and prediction. Shanghai Arch Psychiatry. 2015;27:130–5.
Pieramici DJ, Wang PW, Ding B, Gune S. Visual and anatomic outcomes in patients with diabetic macular edema with limited initial anatomic response to ranibizumab in RIDE and RISE. Ophthalmology. 2016;123:1345–50.
R Development Core Team. R: a language and enviroment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2010.
Campochiaro PA, Brown DM, Pearson A, Ciulla T, Boyer D, Holz FG, et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118:626–35 e2.
Campochiaro PA, Brown DM, Pearson A, Chen S, Boyer D, Ruiz-Moreno J, et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119:2125–32.
Alfaqawi F, Lip PL, Elsherbiny S, Chavan R, Mitra A, Mushtaq B. Report of 12-months efficacy and safety of intravitreal fluocinolone acetonide implant for the treatment of chronic diabetic macular oedema: a real-world result in the United Kingdom. Eye. 2017;31:650–6.
Fusi-Rubiano W, Mukherjee C, Lane M, Tsaloumas MD, Glover N, Kidess A, et al. Treating Diabetic Macular Oedema (DMO): real world UK clinical outcomes for the 0.19 mg Fluocinolone Acetonide intravitreal implant (Iluvien) at 2 years. BMC Ophthalmol. 2018;18:62.
Coelho J, Malheiro L, Melo Beirao J, Meireles A, Pessoa B. Real-world retrospective comparison of 0.19 mg fluocinolone acetonide and 0.7 mg dexamethasone intravitreal implants for the treatment of diabetic macular edema in vitrectomized eyes. Clin Ophthalmol. 2019;13:1751–9.
Carneiro A, Meireles A, Castro Sousa J, Teixeira C. Clinical impact of the 0.2 µg/day fluocinolone acetonide intravitreal implant: outcomes from the ILUVIEN® clinical evidence study in Portugal. Ther Adv Ophthalmol. 2020;12:2515841420917768.
Augustin AJ, Bopp S, Fechner M, Holz F, Sandner D, Winkgen AM, et al. Three-year results from the retro-IDEAL study: real-world data from diabetic macular edema (DME) patients treated with ILUVIEN((R)) (0.19 mg fluocinolone acetonide implant). Eur J Ophthalmol. 2020;30:382–91.
Chakravarthy U, Yang Y, Lotery A, Ghanchi F, Bailey C, Holz FG, et al. Clinical evidence of the multifactorial nature of diabetic macular edema. Retina. 2018;38:343–51.
Schechet SA, Adams OE, Eichenbaum DA, Hariprasad SM. Macular thickness amplitude changes when switching from discontinuous to continuous therapy for diabetic macular oedema. BMJ Open Ophthalmol. 2019;4:e000271.
Riemann CD, Eaton AM, Cutino A. Reduction in retinal thickness fluctuations after treatment with fluocinolone acetonide implant for DME: a post-hoc analysis of the USER Study. Ophthalmic Surg Lasers Imaging. Ophthalmic Surg Lasers Imaging Retina. 2020;51:298–306.
Danis RP, Sadda S, Li XY, Cui H, Hashad Y, Whitcup SM. Anatomical effects of dexamethasone intravitreal implant in diabetic macular oedema: a pooled analysis of 3-year phase III trials. Br J Ophthalmol. 2016;100:796–801.
Adams OE, Schechet SA, Hariprasad SM. Discontinuous to continuous therapy for persistent diabetic macular edema leads to reduction in treatment frequency. Eur J Ophthalmol. 2020;1120672120901691.
Busch C, Fraser-Bell S, Iglicki M, Lupidi M, Couturier A, Chaikitmongkol V, et al. Real-world outcomes of non-responding diabetic macular edema treated with continued anti-VEGF therapy versus early switch to dexamethasone implant: 2-year results. Acta Diabetol. 2019;56:1341–50.
Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW. Diabetic retinopathy: more than meets the eye. Surv Ophthalmol. 2002;47:S253–62.
Lopes de Faria JM, Jalkh AE, Trempe CL, McMeel JW. Diabetic macular edema: risk factors and concomitants. Acta Ophthalmol Scand. 1999;77:170–5.
Chatziralli I, Dimitriou E, Theodossiadis G, Chatzirallis A, Kazantzis D, Theodossiadis P. Treatment of macular edema after pars plana vitrectomy for idiopathic epiretinal membrane using intravitreal dexamethasone implant: long-term outcomes. Ophthalmologica. 2019;242:16–21.
Moein HR, Novais EA, Rebhun CB, Cole ED, Louzada RN, Witkin AJ, et al. Optical coherence tomography angiography to detect macular capillary ischemia in patients with inner retinal changes after resolved diabetic macular edema. Retina. 2018;38:2277–84.
Sadda SR, Campbell J, Dugel PU, Holekamp NM, Kiss S, Loewenstein A, et al. Relationship between duration and extent of oedema and visual acuity outcome with ranibizumab in diabetic macular oedema: a post hoc analysis of Protocol I data. Eye. 2020;34:480–90.
Dugel PU, Hillenkamp J, Sivaprasad S, Vogeler J, Mousseau MC, Wenzel A, et al. Baseline visual acuity strongly predicts visual acuity gain in patients with diabetic macular edema following anti-vascular endothelial growth factor treatment across trials. Clin Ophthalmol. 2016;10:1103–10.
Parrish RK II, Campochiaro PA, Pearson PA, Green K, Traverso CE, Group FS. Characterization of intraocular pressure increases and management strategies following treatment with fluocinolone acetonide intravitreal implants in the FAME trials. Ophthalmic Surg Lasers Imaging Retina. 2016;47:426–35.
Zarranz-Ventura J, Sala-Puigdollers A, Velazquez-Villoria D, Figueras-Roca M, Copete S, Distefano L, et al. Long-term probability of intraocular pressure elevation with the intravitreal dexamethasone implant in the real-world. PLoS One. 2019;14:e0209997.
Nehme A, Lobenhofer EK, Stamer WD, Edelman JL. Glucocorticoids with different chemical structures but similar glucocorticoid receptor potency regulate subsets of common and unique genes in human trabecular meshwork cells. BMC Med Genomics. 2009;2:58.
Acknowledgements
The International Retina Collaborative is an independent research branch of the International Retina Panel, which receives travel support from Allergan.
On behalf of the International Retina Collaborative:
Maria Vittoria Cicinelli1,2, Amir Rosenblatt3,4, Marco Nassisi6,7, Anna Tan8,9,10, Rhianon Reynolds11, Sumit Randhir Singh12, Kaivon P. Vaezi13, Audrey Giocanti-Aurégan14, Tamir Weinberg15, Livia Faes16, Roy Schwartz17,18,19, Denis Yurani Anaya Reyes20, Alfonso Savastano21,22, Sara Touhami23, Humberto Ruiz Garcia24, Dominika Pohlmann25, Adam Plant26, Camila V. Ventura27,28, Irini Chatziralli29, Nopasak Phasukkijwatana30, Jorge Ruiz-Medrano31,32, Miner Yuan33, İhsan Yılmaz34, Denise Vogt35, Daniel Shu Wei Ting8,9,10, Mushawiahti Mustapha36, Marcos Mendaro37
Funding
MVC, LC, PZ, AR, DG: No financial disclosures. RL: Allergan (Irvine, California, USA), Novartis (Basel, Switzerland), Bayer Shering-Pharma (Berlin, Germany), SIFI (Aci Sant’Antonio, Italy). AR reports grant/fees from Allergan Inc (Irvine, California, USA), Bayer Shering-Pharma (Berlin, Germany), Novartis (Basel, Switzerland). AL reports grant/fees from: Allergan Inc (Irvine, California,USA), Bayer Shering-Pharma (Berlin, Germany), Novartis (Basel, Switzerland), Notal-Vision, Sensor, Syneos Health, Beyeonics, Roche, Oxurion Oculis, Pres-by, Xbran, WebMD. FB consultant for: Allergan Inc (Irvine, California, USA), Bayer Shering-Pharma (Berlin, Germany), Hoffmann-La-Roche (Basel, Switzerland), Novartis (Basel, Switzerland), Sanofi-Aventis (Paris, France), Thrombogenics (Heverlee, Belgium), Zeiss (Dublin, USA), Boehringer-Ingelheim, Fidia Sooft, Ntc Pharma, SIFI (Aci Sant’Antonio, Italy).
Author information
Authors and Affiliations
Consortia
Contributions
All the authors contributed to the conception or design of the work, the acquisition, analysis and interpretation of data, drafting the work, revising it critically for important intellectual content.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Members of the International Retina Collaborative are listed below acknowledgements.
The original online version of this article was revised: In some instances, “edema” was changed to “oedema” and “DME” to “DMO” to be consistent throughout the article.
Supplementary information
Rights and permissions
About this article
Cite this article
Cicinelli, M.V., Rosenblatt, A., Grosso, D. et al. The outcome of fluocinolone acetonide intravitreal implant is predicted by the response to dexamethasone implant in diabetic macular oedema. Eye 35, 3232–3242 (2021). https://doi.org/10.1038/s41433-020-01373-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-01373-1
This article is cited by
-
Factors associated with the response to fluocinolone acetonide 0.19 mg in diabetic macular oedema evaluated as the area-under-the-curve
Eye (2023)
-
Fluocinolone Acetonide Implant Injected 1 Month after Dexamethasone Implant for Diabetic Macular Oedema: the ILUVI1MOIS Study
Ophthalmology and Therapy (2023)
-
Pathogenesis and current therapies for non-infectious uveitis
Clinical and Experimental Medicine (2022)
-
Intraocular Pressure Changes After Intravitreal Fluocinolone Acetonide Implant: Results from Four European Countries
Ophthalmology and Therapy (2022)