Abstract
The N1,N1,N3,N3-tetramethyl –N1,N3-diphenylpropane-1,3-diaminium dichloride ionic liquid (ILc) is an environmentally friendly catalyst for oxidative–extractive desulfurization of gas oil (sulfur content = 2400 ppm) in the presence of H2O2 as an oxidizing agent. The precise structure of the prepared IL was confirmed using FT-IR spectroscopy, and1H-NMR. The reaction temperature, IL ratios, H2O2 dosage, and reaction time were studied to assess their effects on the desulfurization efficiency. The thermodynamic parameters of the oxidation reaction were determined. A desulfurization efficiency of 84.7% was obtained after the extractive desulfurization process using acetonitrile as an organic solvent at a solvent to feed ratio of 1:1 (v/v). Furthermore, the prepared IL may be reused for at least six cycles without any significant change in its desulfurization performance or chemical structure, which confirms its high reusability.
Similar content being viewed by others
Introduction
Sulfur compounds in fossil fuels present a significant challenge for petroleum refineries1. Sulfur oxides (SOx) formed during the combustion of sulfur-containing fossil fuels are key contributors to serious air pollution, particularly acid rain and hazy weather2. Hydrodesulfurization (HDS) is an important process in oil refining. It is commonly used for oil desulfurization, employing metal catalysts to convert organic sulfur in fuels to hydrogen sulfide and related hydrocarbons3,4,5. HDS is widely used in industry to effectively remove sulfides with low boiling points and no steric hindrance such as thioethers and mercaptans6,7. However, this technique require large hydrogen consumption, expensive catalysts, and extremely hard reaction conditions8,9. Efficient desulfurization can be achieved by multistage extraction desulfurization (EDS)10,11; however, the process costs are high because of the high amount of extractant used and the regeneration problems that may occur during the process1,12,13. Large amounts of catalysts are required for oxidative desulfurization (ODS)14,15,16,17. Moreover, regeneration difficulties and poor repeatability are caused by the loss of catalytic active sites during the process. Thus, it is essential to develop new catalysts and extractants with high desulfurization efficiency18,19,20,21. The oxidation of aromatic sulfides to generate their corresponding sulfones followed by their subsequent removal by extraction in a typical ODS process15,22,23,24. H2O2 is the most used oxidant in ODS because of its strong reactivity, low cost, and environmental compatibility25,26,27,28. Flammable and volatile organic solvents are typically used as extractants, which may generate further safety and environmental problems. The development of EDSmethods is constrained by the requirement of a high solvent-to-oil ratio and the lack of environmentally friendly extraction solvents2,29,30. Organic solvents can be used as extraction media in EDS; however, they have significant limitations due to their high volatility, low selectivity toward sulfur compounds, and high toxicity31. Therefore, new environmentally friendly i.e., biodegradable, nonvolatile, and nontoxic, extraction solvents must be developed. Using ionic liquids (ILs) for EDSis an environmentally friendly method that is increasingly used to remove refractory S-compounds8. ILs are salts with low melting points, usually with melting points less than 100 °C. ILs exhibit unique characteristics such as controllable physicochemical characteristics, strong thermal stability, low volatility, and long-term stability. Because of their unique properties, they are used as green solvents for chemical synthesis, fuel desulfurization, and bio-separation32,33. Furthermore, ILs have a high ability to form complexes with aromatic sulfur compounds and are immiscible with fuel oils34. Zhang et al. 200435 employed l-alkyl-3-methylimidazolium [AMIM] tetrafluoroborate, hexafluorophosphate, and trimethylamine hydrochloride, (TMAC) in (AlCl3–TMAC) as ionic liquids. EMIMBF4 (E = ethyl), BMIMPF6 (B = butyl), BMIMBF4, and the heavier AMIMPF6 exhibited good selectivity, particularly toward aromatic sulfur and nitrogen compounds, in extractive desulfurization and denitrogenation of transportation fuels. The used ionic liquids are easily regenerated by distillation or water displacement of the absorbed molecules. The aromatic S-containing compounds that were absorbed can be also quantitatively recovered. Organic compounds with a greater aromatic π electron density are absorbed more efficiently. As a result of a steric effect, the alkyl substitution on the aromatic rings significantly decreases the absorption capacity. The size and structure of cations and anion in ILs affect their absorption capacity of aromatic compounds. Without mutual hindrance, the extraction of S- and N-containing compounds can be obtained at low concentrations. Typically, AlCl3-TMAC ILs exhibit high absorption capacities for aromatic compounds. To eliminate sulfur compounds from light oils, Lo et al.36 used room temperature ILs (RTILs), i.e., 1-butyl-3-methylimidazolium tetrafluoroborate and 1-butyl-3-methylimidazolium hexafluorophosphate, through a combination of solvent extraction and chemical oxidation. In light oils, sulfur compounds can be extracted using RTILs, and the corresponding sulfones can be then produced through S-oxidation (H2O2-acetic acid) in a one-pot operation. The simultaneous oxidation and extraction of sulfur compounds from light oil increase the desulfurization yield. RTILs can be then reused and recycled without losing their activity.
In this work, N1,N3-dibenzyl-N1,N1,N3,N3-tetramethylpropane-1,3-diaminium chloride was prepared, and its structure was confirmed using several characterization techniques such as Fourier transform infrared spectroscopy (FT-IR) and 1H nuclear magnetic resonance (1H-NMR). In the ODS of gas oil, the developed IL was used as a catalyst in the occurrence of H2O2 as the oxidant, and the optimal composition of IL was determined. The optimum conditions of the desulfurization process were obtained by investigating the effects of various operating parameters, including the reaction time, temperature, IL to gas oil volume ratio, and oxidant dosage, on the process. The efficiency of IL toward sulfur removal from gas oil and its recyclability were also investigated, and the thermodynamic parameters of the ODS reaction were determined.
Experimental methods
Materials
Benzyl chloride (99%), N,N,N,N-tetramethyl-1,3-propanediamine, and H2O2 (50 wt%) were obtained from Sigma Aldrich. Ethyl alcohol and acetonitrile (HPLC grade) were obtained from Morgan and Merck chemicals, respectively. All chemicals were of analytical grade and were used directly without further treatments. Gas oil was collected from Cairo oil refining company, Egypt.
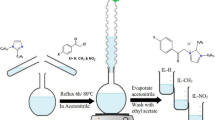
Preparation of the N1,N3-dibenzyl-N1,N1,N3,N3-tetramethylpropane-1,3-diaminium dichloride ILc
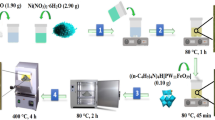
N,N,N,N-tetramethyl-1,3-propanediamine (0.01 mol) was dissolved in acetonitrile. Benzyl chloride (0.02 mol) was then added after that the mixture was refluxed at 80 °C for 2 h. Product crystallization was performed 3 times using ethanol with a yield of 80% at a melting point of 70 °C. Figure 1 shows a summary of the preparation process. The structure of the synthesized compound was confirmed using FT-IR spectroscopic analysis with KBr pellets on Perkin Elmer in Egyptian Petroleum Research Institute. 1H-NMR spectroscopy was carried out in dimethyl sulfoxide (DMSO) using a Varian Gemini-200 MHz system.
Desulfurization experiments
At atmospheric pressure, the experiments were conducted in a closed round-bottle flask with a magnetic stirrer and a thermometer. Gas oil (25 ml) was used with different volumes of H2O2 (5–20 ml) to study their effects on the process. Similarly, the effect of ILc dose of 0.1–0.5 g, a reaction temperature range of 30–80 °C, and a reaction time of 30–240 min on the desulfurization efficiency was investigated. After each treatment, the phase separation was achieved using two layers, an aqueous layer and an oil layer, in a separating funnel37, and the treated oil phase was extracted using acetonitrile at a 1:1 (v/v) ratio.
The oil and solvent phases were separated, and the desulfurization efficiency (R) was calculated using Eq. (1).
where Ci (ppm) and Cf (ppm) are the initial and final sulfur concentrations in the gas oil, respectively.
Analytical methods
Samples in the upper oil phase were collected for the analysis at different time intervals (30–240 min). A viscometer (Stabinger, Model SVM 3001, Anton Paar) was used for the determination of dynamic viscosity, kinematic viscosity, and density of the samples before and after the treatment according to ASTM D 7042, ASTM D 445, and ASTM D 4052, respectively. The total sulfur concentration of gas oil was determined using a sulfur analyzer according to the standard test method for sulfur in petroleum oil and petroleum products by energy dispersive X-ray fluorescence spectrometry (ASTM D 4294).
Figure 2 reports the different stages involved in the preparation of ionic liquid ILc and desulfurization process in the present work.
Results and discussion
The main objective of this study is to remove sulfur compounds, which are usually attached to the aromatic compounds in petroleum fractions, from gas oil by a new IL using an extractive–catalytic ODS process. The physicochemical properties of the gas oil were examined according to the ASTM standard test methods. The results are tabulated in Table 1.
Confirmation of the ILc structure
FT-IR
The infrared spectra of the purified ILc prepared in this study are presented in Fig. 3. The characteristic FT-IR bands of Fig. 3 are listed in Table 2. We noted that for ILc band appear at 3001 and 3050 could be due to Aromatic (–CH–) groups. The peak at about 2969 cm−1 due to Aliphatic (–CH–) groups. The peak at 1634 cm−1 is assigned to the stretching vibration of C=C Aromatic. In addition to peak appear at 1220 cm−1 is assigned to C–N. Finally, the FT-IR analysis indicates the presence of the IR bands that related to the chemical structures of new ILc.
1H-NMR
The chemical structure of the novel investigated ILc was determined by 1H NMR spectroscopy. The detailed spectra shown in Fig. 4 agreed with the designed structure. Table 3 lists the changes in chemical shifts for several sorts of protons in the novel investigated ILc. Aromatic protons, (A, B and C), appeared at (9.07, 7.81 and 7.73) respectively. Aliphatic proton (D, E, F and G) appears at (4.75, 3.40, 3.38 and 3.10). No impurities were observable in 1H spectra.
Effect of the reaction temperature
The impact of temperature on the desulfurization efficiency is a critical factor in defining the potential of the synthesized ILc. Typically, increasing the temperature accelerates the decomposition of H2O2 in water to generate nascent oxygen atoms, or (OH) ions, which oxidize sulfur compounds to varying degrees. To investigate the effect of the reaction temperature on the desulfurization efficiency, experiments were carried out at 30 °C, 50 °C, 60 °C, 70 °C, and 80 °C (Fig. 5 and Table S1). The increase in the temperature from 30 to 80 °C was accompanied by a decrease in the yield from 97.31 to 91.5 wt%, respectively (Fig. 5a). Density and refractive indices decreased (Fig. 5b,c)39, and on the other hand diesel indices increased because of the increase of the aromatic recoveries (Fig. 5e). The aromatic and sulfur recoveries exhibited different degrees of increase. At an operation temperature of 30 °C, the desulfurization efficiency could only reach 47.54%, which indicates that the ILc could not catalyze the reaction efficiently at a relatively low temperature. However, when the temperature increased to 50 °C, 60 °C, and 70 °C, the desulfurization efficiency remarkably increased to 61.58%, 70.42%, and 84.58%, respectively, (Fig. 5d). Sulfur removal was restricted by the unproductive decomposition of H2O2 at elevated temperatures. The loss of H2O2 due to its decomposition to H2O and O2 at high temperatures40 may restrict the effective formation of active oxidation species. Similarly, the H2O impurity generated by the thermal decomposition of H2O2 hindered the catalytic activity of ILc, leading to a reduction in the desulfurization efficiency41. Therefore, the desulfurization efficiency did not significantly improve when the temperature increased from 70 to 80 °C. Thus, the optimal reaction temperature was selected to be 70 °C.
Effect of reaction time
The selectivity of the oxidation process determines the utility of the desulfurization system. The sulfur compounds should be rapidly and selectively oxidized. Thus, the oxidation time of the gas oil was determined. To determine the minimum reaction time for complete desulfurization of sulfur compounds at 70 °C, the performance of the ODS process using ILc was assessed at different times (0.5, 1, 2, 3, and 4 h). Figure 6 and Table S2 show the desulfurization efficiencies and their associated species yields, densities, refractive indices, and diesel indices of the oxidized gas oil. H2O2 (10 ml) and ILc (0.5 g) were used for this test at an oxidation temperature of 70 °C, which is the optimal temperature selected in the previous step. The results presented in Fig. 6 indicate that the increase in the reaction time from 0.5 to 3 h was associated with a continuous sharp decrease in the yield, refractive indices, and density (Fig. 6a–c). This was accompanied by a continuous increase in the desulfurization efficiency and diesel indices (Fig. 6d,e). This can be attributed to the continuous increase in the reaction time, which can increase the π–π interaction between the ILc molecules and sulfur compounds in the reaction media. This enhanced the extraction of the sulfur compounds and enable the oxidizing agent (H2O2) to produce the prior species in the media to complete the ODS process. Further elongation of the reaction time to 4 h showed no significant increase in the desulfurization efficiency or the associated characteristics. Therefore, 3 h was considered a suitable reaction time.
Effect of the ILc dosage
Figure 7 and Table S3 show the effect of the ILc dosage on the process of extractive–ODS process and its subsequent relevant changes in the yield, physical characteristics, desulfurization efficiency, and diesel indices of the produced gas oil. Four doses of ILc (0.1, 0.3, 0.5, and 1 g) were used during this step. The optimal conditions, i.e., reaction temperature = 70 °C and reaction time = 3 h, selected in the previous steps were employed in the current test using H2O2 (10 ml). The increase in the ILc dosage can be an efficient strategy to improve the desulfurization efficiency. The desulfurization efficiency significantly increased from 70.53 to 73.73% (Fig. 7d) when the ILc dosage increased from 0.1 to 0.5 g. Because ILc was used as both a catalyst and an extractant, the increase in the ILc dosage could enhance the extraction efficiency and increase the catalytic active sites, which significantly improve sulfur removal. Nevertheless, no significant differences were observed in the characteristics of the produced gas oil at higher ILc dosage (1 g). That is, nearly the same percentages of desulfurization efficiency were observed. Thus, a lower ILc dosage (0.5 g) was considered the optimal dosage for industrial applications from an economic point of view.
Effect of the H2O2 dose
H2O2 is a potent, environmentally friendly, and inexpensive oxidizing agent. The impact of using H2O2 was also tested (Fig. 8 and Table S4). The experiments in this stage were carried out under the optimal conditions selected above (ILc dosage = 0.5 g, reaction time = 3 h, and oxidation temperature = 70 °C). Four H2O2 dosages (5, 10, 15, and 20 ml) were evaluated in this step. Figure 8 indicates that when the oxidizing agent dose increased from 5 to 10 ml, the yield (Fig. 8a) significantly decreased, showing a clear improvement in the refractive indices and density (Fig. 8b,c). This was accompanied by a simultaneous increase in the desulfurization efficiency (Fig. 8d) and diesel indices (Fig. 8e) until approximately fixed values were detected when the H2O2 dose increased from 10 to 15 then to 20 ml. This verifies that the excessive use of H2O2 is not desirable because it results in the dilution of ILc and increases the cost. Therefore, a H2O2 dose of 10 ml was chosen as the optimal dose in this study.
Thermodynamics of the oxidative desulfurization of diesel fuel
The thermodynamic parameters of the ODS reaction were estimated as follow:
The standard entropy change (ΔS°, J/(mol K)), standard enthalpy change (ΔH°, kJ/mol), and standard free energy change (ΔG°, kJ/mol)42 were calculated using Eqs. (2) and (3).
where T is the absolute temperature (K), Kd is the distribution coefficient (L/g), and R is the perfect gas constant = 8.314 J/mol K.
The thermodynamic parameters of the reaction can be calculated using Eqs. ((2) and (3)), the results are listed in Table 4 and Fig. 9 show thermodynamic behavior of the oxidative desulfurization reaction.
As shown in Table 4, the values of ΔH°, ΔS°, and ΔG° are positive, suggesting that the reaction is endothermic with increase in the temperature. Thus, the efficiency of the oxidation and randomness of the reaction increased, and the reaction was nonspontaneous. The low values of the standard free energy mean that the reaction can proceed easily43.
Recycling of the ILc
From environmental and economic standpoints, ILc regeneration and recycling are vital processes. Repeated trials of eliminating sulfur compounds from heavy gas oil under optimal conditions were conducted to verify the recycling performance of ILc. A biphasic system was observed in the reactor after repeating each cycle. The upper phase was carefully removed from the system by decanting, and the ILc phase was dried under vacuum to remove any remaining water and H2O2. The reactor was then charged with fresh gas oil and H2O2 for the next cycle.
After six repeated cycles, the desulfurization efficiency only dropped from 84.71 to 83.97% (Fig. 10). This slight drop could be attributed to one of two factors. First, trace losses of ILc during the separation and drying processes are unavoidable. Second, the oxidative products of the sulfur compounds concentrated in the system and coated the surface of ILc, reducing its phase transfer capabilities. Nonetheless, based on these results, ILc shows good recyclability through a low-cost regeneration approach and may be suitable for industrial applications. In addition, FT-IR analyses of both fresh and recycled ILc (Fig. 11) were performed to investigate the stability of ILc during the desulfurization process. There was no discernible difference between before and after the reactions.
Desulfurization mechanism
Gas oil is desulfurized through a very effective and selective process using the developed Gimini ionic liquid ILc as a catalyst (Fig. 12). That the ILc contains two positive active sites, which combine with two molecules of hydrogen peroxide and produce active species, which perform their beneficial role in completing the oxidation process. The effect of ILc increases the rate of decomposition of H2O2 to 2OH*, which is unstable and undergo O–H bond break to generate H2O* and O*, which are the most active species for an ODS reaction because of their low activation energy. The effectiveness of the desulfurization process is significantly impacted by the π–π interactions between the aromatic sulfur compounds and aromatic ILc44.
Our Gemini ionic liquid ILc performs more catalytically than its mono-cationic version and this difference can be related to the fact that Gemini ionic liquids have two basic sites, whilst mono-cationic ionic liquids only have one. Gemini ionic liquids have two basic sites that are close to one another and work well in tandem to increase the catalytic efficiency. As Gemini ionic liquids have stronger basic sites than mono-cationic ILc, oxidation process proceed more quickly. They display greater stability and more potent alkalinity because of their more powerful and concentrated basic sites.
In Table 5, the performance of the novel introduced N1,N1,N3,N3-tetramethyl-N1,N3-diphenylpropane-1,3-diaminium dichloride was compared with certain previously referred ILs for further studs.
In addition to the declared factors, the type of the catalyst itself would be effective as well. In Table 5, the novel ILc is contrasted with existing types of ionic liquids employed in the same application—oxidative desulfurization process—to show off its excellent catalytic performance. As a consequence, Gemini ionic liquid ILc performed superior than the other ionic liquid types described in terms of both results and efficiency. So, for the oxidative desulfurization process, the ILc with the best and most competitive performance (84.7%) for real gas oil (2400 ppm sulphur content) is chosen. This is due to the using of a very little amount of the catalyst ratio—oil:ILc = 1:0.02—and the ILc helped on a huge reduction in the amount of the oxidizing agent—H2O2:Oil = 0.4:1—which makes the process very cost-effective when applied in the industrial field.
Conclusion
In this study a novel Gemini IL (N1,N1,N3,N3-tetramethyl-N1,N3-diphenylpropane-1,3-diaminium dichloride) was successfully synthesized and characterized using 1H-NMR and FT-IR spectroscopy. It was then used as a catalyst in ODS of real gas oil with a sulfur content of 2400 ppm. The N1,N1,N3,N3-tetramethyl-N1,N3-diphenylpropane-1,3-diaminium dichloride IL exhibited a high desulfurization efficiency of 84.7% under optimal conditions (H2O2:Oil = 0.4:1, IL:Oil = 0.02:1, reaction temperature = 70 °C, and reaction time = 3 h). Based on the thermodynamic analysis of the ODS process, the values of ΔH° indicated that the reaction is endothermic with increase the temperature. The IL can be directly reused, and they exhibited good recyclability for six times.
Data availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
Butt, H. S., Lethesh, K. C. & Fiksdahl, A. Fuel oil desulfurization with dual functionalized imidazolium based ionic liquids. Sep. Purif. Technol. 248, 116959. https://doi.org/10.1016/j.seppur.2020.116959 (2020).
Wang, Q., Zhang, T., Zhang, S., Fan, Y. & Chen, B. Extractive desulfurization of fuels using trialkylamine-based protic ionic liquids. Sep. Purif. Technol. 231(2019), 115923. https://doi.org/10.1016/j.seppur.2019.115923 (2020).
Chandra Srivastava, V. An evaluation of desulfurization technologies for sulfur removal from liquid fuels. RSC Adv. 2(3), 759–783. https://doi.org/10.1039/c1ra00309g (2012).
Gao, S. et al. A combination desulfurization method for diesel fuel: Oxidation by ionic liquid with extraction by solvent. Fuel 224, 545–551. https://doi.org/10.1016/j.fuel.2018.03.108 (2018).
Rezvani, M. A., Hadi, M. & Rezvani, H. Synthesis of new nanocomposite based on ceramic and heteropolymolybdate using leaf extract of Aloe vera as a high-performance nanocatalyst to desulfurization of real fuel. Appl. Organomet. Chem. 35(5), 1–15. https://doi.org/10.1002/aoc.6176 (2021).
Shang, H., Du, W., Liu, Z. & Zhang, H. Development of microwave induced hydrodesulfurization of petroleum streams: A review. J. Ind. Eng. Chem. 19(4), 1061–1068. https://doi.org/10.1016/j.jiec.2012.12.044 (2013).
Mohumed, H., Rahman, S., Imtiaz, S. A. & Zhang, Y. Oxidative-extractive desulfurization of model fuels using a pyridinium ionic liquid. ACS Omega 5(14), 8023–8031. https://doi.org/10.1021/acsomega.0c00096 (2020).
Kianpour, E., Azizian, S., Yarie, M., Zolfigol, M. A. & Bayat, M. A task-specific phosphonium ionic liquid as an efficient extractant for green desulfurization of liquid fuel: An experimental and computational study. Chem. Eng. J. 295, 500–508. https://doi.org/10.1016/j.cej.2016.03.072 (2016).
Tian, Y., Yao, Y., Zhi, Y., Yan, L. & Lu, S. Combined extraction-oxidation system for oxidative desulfurization (ODS) of a model fuel. Energy Fuels 29(2), 618–625. https://doi.org/10.1021/ef502396b (2015).
Jiang, W. et al. Synergistic effect of dual Brønsted acidic deep eutectic solvents for oxidative desulfurization of diesel fuel. Chem. Eng. J. https://doi.org/10.1016/j.cej.2020.124831 (2020).
Jiang, W. et al. In situ fabrication of hollow silica confined defective molybdenum oxide for enhanced catalytic oxidative desulfurization of diesel fuels. Fuel 305, 121470. https://doi.org/10.1016/j.fuel.2021.121470 (2021).
Li, J. J., Xiao, H., Tang, X. D. & Zhou, M. Green carboxylic acid-based deep eutectic solvents as solvents for extractive desulfurization. Energy Fuels 30(7), 5411–5418. https://doi.org/10.1021/acs.energyfuels.6b00471 (2016).
Song, Z. et al. Screening of ionic liquids for solvent-sensitive extraction -with deep desulfurization as an example. Chem. Eng. Sci. 129, 69–77. https://doi.org/10.1016/j.ces.2015.02.023 (2015).
Rezvani, M. A., Khandan, S. & Rahim, M. Synthesis of (Gly)3PMo12O40@MnFe2O4 organic/inorganic hybrid nanocomposite as an efficient and magnetically recoverable catalyst for oxidative desulfurization of liquid fuels. Int. J. Energy Res. 46(3), 2617–2632. https://doi.org/10.1002/er.7332 (2022).
Harutyunyan, R., Rezvani, M. A. & Heravi, M. M. H5[PMo10V2O40] as a green, reusable, and highly efficient catalyst for the oxidation of dithiols in intermolecular reactions using permanganate as an oxidizing reagent. Synth. React. Inorganic Met. Nano-Metal Chem. 41(1), 94–99. https://doi.org/10.1080/15533174.2010.538019 (2011).
Rezvani, M. A. & Khalafi, N. Deep oxidative desulfurization of real fuel and thiophenic model fuels using polyoxometalate-based catalytic nanohybrid material. Mater. Today Commun. 22(2019), 100730. https://doi.org/10.1016/j.mtcomm.2019.100730 (2020).
Rezvani, M. A. & Imani, A. Ultra-deep oxidative desulfurization of real fuels by sandwich-type polyoxometalate immobilized on copper ferrite nanoparticles, Fe6W18O70⊂ CuFe2O4, as an efficient heterogeneous nanocatalyst. J. Environ. Chem. Eng. 9(1), 105009. https://doi.org/10.1016/j.jece.2020.105009 (2021).
Yu, G. et al. Desulfurization of diesel fuel by one-pot method with morpholinium-based Brønsted acidic ionic liquid. Fuel 296(2020), 120551. https://doi.org/10.1016/j.fuel.2021.120551 (2021).
Wang, X., Han, M., Wan, H., Yang, C. & Guan, G. Study on extraction of thiophene from model gasoline with brønsted acidic ionic liquids. Front. Chem. Eng. China 5(1), 107–112. https://doi.org/10.1007/s11705-010-0539-0 (2011).
Salem, H. M. & Mohmed, D. Nanoarchitectonics of copper tungsten–mesoporous silica with a new template for photo oxidative-desulfurization of dibenzothiophene. J. Inorg. Organomet. Polym. Mater. https://doi.org/10.1007/s10904-022-02363-4 (2022).
Rezvani, M. A., Hadi, M. & Mirsadri, S. A. Synthesis of new nanocomposite based on nanoceramic and mono substituted polyoxometalate, PMo11Cd@MnFe2O4, with superior catalytic activity for oxidative desulfurization of real fuel. Appl. Organomet. Chem. 34(10), 1–14. https://doi.org/10.1002/aoc.5882 (2020).
Mirhoseini, H. & Taghdiri, M. Extractive oxidation desulfurization of sulfur-containing model fuel using hexamine-phosphotungstate hybrid as effective heterogeneous catalyst. Fuel 167, 60–67. https://doi.org/10.1016/j.fuel.2015.11.042 (2016).
Yang, H. et al. Polymeric cation and isopolyanion ionic self-assembly: Novel thin-layer mesoporous catalyst for oxidative desulfurization. Chem. Eng. J. 317, 32–41. https://doi.org/10.1016/j.cej.2017.01.135 (2017).
Rezvani, M. A., Shaterian, M., Aghbolagh, Z. S. & Akbarzadeh, F. Synthesis and characterization of new inorganic-organic hybrid nanocomposite PMo11Cu@MgCu2O4@CS as an efficient heterogeneous nanocatalyst for ODS of real fuel. ChemistrySelect 4(20), 6370–6376. https://doi.org/10.1002/slct.201900202 (2019).
Jiang, X. et al. Deep desulfurization of fuels catalyzed by surfactant-type decatungstates using H2O2 as oxidant. Fuel 88(3), 431–436. https://doi.org/10.1016/j.fuel.2008.11.010 (2009).
Ribeiro, S. O. et al. Catalytic oxidative/extractive desulfurization of model and untreated diesel using hybrid based zinc-substituted polyoxometalates. Fuel 166, 268–275. https://doi.org/10.1016/j.fuel.2015.10.095 (2016).
Options, M. A. Highly Efficient Catalytic Oxidative Desulfurization of Gasoline 23–26 (2022).
Rezvani, M. A. & Jafari, N. Synthesis and characterization of new substituted sandwich-type polyoxometalate-based inorganic-organic hybrid nanocomposites for catalytic oxidative desulfurization of real gasoline. Ind. Eng. Chem. Res. 60(20), 7599–7610. https://doi.org/10.1021/acs.iecr.1c01167 (2021).
Player, L. C., Chan, B., Lui, M. Y., Masters, A. F. & Maschmeyer, T. Toward an understanding of the forces behind extractive desulfurization of fuels with ionic liquids. ACS Sustain. Chem. Eng. 7(4), 4087–4093. https://doi.org/10.1021/acssuschemeng.8b05585 (2019).
Li, J. et al. Acid dicationic ionic liquids as extractants for extractive desulfurization. Energy Fuels 33(5), 4079–4088. https://doi.org/10.1021/acs.energyfuels.9b00307 (2019).
Saha, B., Sengupta, S. & Selvin, R. Comparative studies of extraction ability of organic solvents to extract thiophene from model fuel. Sep. Sci. Technol. 55(6), 1123–1132. https://doi.org/10.1080/01496395.2019.1580292 (2020).
Vekariya, R. L. A review of ionic liquids: Applications towards catalytic organic transformations. J. Mol. Liq. 227, 44. https://doi.org/10.1016/j.molliq.2016.11.123 (2017).
Moghadam, F. R. et al. Extractive desulfurization of liquid fuel by using a green, neutral and task specific phosphonium ionic liquid with glyceryl moiety: A joint experimental and computational study. Fuel 208, 214–222. https://doi.org/10.1016/j.fuel.2017.07.025 (2017).
Jian-long, W. A., Zhao, D. S., Zhou, E. P. & Zhi, D. O. Desulfurization of gasoline by extraction with N-alkyl-pyridinium-based ionic liquids. Ranliao Huaxue Xuebao/J. Fuel Chem. Technol. 35(3), 293–296. https://doi.org/10.1016/S1872-5813(07)60022-X (2007).
Zhang, S., Zhang, Q. and Zhang C. Extractive Desulfurization and Denitrogenation of Fuels Using Ionic Liquids. Ind. Eng. Chem. 43, 614–622 (2004).
Lo, W. H., Yang, H. Y. & Wei, G. T. One-pot desulfurization of light oils by chemical oxidation and solvent extraction with room temperature ionic liquids. Green Chem. 5(5), 639–642. https://doi.org/10.1039/b305993f (2003).
Mello, P. D. A. et al. Ultrasound-assisted oxidative process for sulfur removal from petroleum product feedstock. Ultrason. Sonochem. 16(6), 732–736. https://doi.org/10.1016/j.ultsonch.2009.03.002 (2009).
Deyab, M. A., Moustafa, Y. M., Nessim, M. I., Fatthallah, N. A. & Asaad Bagato, N. M. New series of ionic liquids based on benzalkonium chloride derivatives: Synthesis, characterizations, and applications. J. Mol. Liq. 313, 113566. https://doi.org/10.1016/j.molliq.2020.113566 (2020).
Mostafa, H. Y., El Naggar, A. M. A., Elshamy, E. A., Farag, A. S. & Hashem, A. I. Microwave-assisted extraction for refining of petroleum wax distillate feedstock. J. Clean. Prod. 228, 1034–1047. https://doi.org/10.1016/j.jclepro.2019.04.263 (2019).
Pędziwiatr, P., Mikołajczyk, F., Zawadzki, D., Mikołajczyk, K. & Bedka, A. Decomposition of hydrogen perodixe-kinetics and review of chosen catalysts. Acta Innov. 26, 45–52. https://doi.org/10.32933/ActaInnovations.26.5 (2018).
Abdelrahman, A. A., Salem, H. M., Mohammed, H. A. & Rabie, A. M. Facial synthesis of mesoporous {Mo132}/BiOCl for the efficient oxidative desulfurization of fuel. Inorg. Chem. Commun. 141, 1–9. https://doi.org/10.1016/j.inoche.2022.109568 (2022).
Aravind Kumar, J. et al. One pot Green Synthesis of Nano magnesium oxide-carbon composite: Preparation, characterization and application towards anthracene adsorption. J. Clean. Prod. https://doi.org/10.1016/j.jclepro.2019.117691 (2019).
Zaidi, Z. & Sorokhaibam, L. G. Manganese modified multifunctional carbon material for desulfurization of transportation fuel and CO2 sequestration. J. Environ. Chem. Eng. 9(4), 105378. https://doi.org/10.1016/j.jece.2021.105378 (2021).
Li, H. et al. H2O2 decomposition mechanism and its oxidative desulfurization activity on hexagonal boron nitride monolayer: A density functional theory study. J. Mol. Graph. Model. 84, 166–173. https://doi.org/10.1016/j.jmgm.2018.07.002 (2018).
Zhao, D. S., Sun, Z. M., Li, F. T. & Shan, H. D. Optimization of oxidative desulfurization of dibenzothiophen using acidic ionic liquid as catalytic solvent. Ranliao Huaxue Xuebao/J. Fuel Chem. Technol. 37(2), 194–198. https://doi.org/10.1016/s1872-5813(09)60015-3 (2009).
Li, H. et al. Deep oxidative desulfurization of fuels in redox ionic liquids based on iron chloride. Green Chem. 11(6), 810–881. https://doi.org/10.1039/b901127g (2009).
Akbari, A. & Haji Andevary, H. Catalytic oxidative-extractive deep desulfurization of diesel fuel by N-methyl-2-pyrrolidone-based protic acidic ionic liquids (Pails). Iran. J. Chem. Chem. Eng. 39(3), 81–92 (2020).
Abro, R. et al. Oxidative desulfurization of gasoline by ionic liquids coupled with extraction by organic solvents. J. Braz. Chem. Soc. 27(6), 998–1006. https://doi.org/10.5935/0103-5053.20150355 (2016).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
H.Y.M.: conceptualization, data curation, formal analysis, investigation, methodology, resources, validation, visualization, writing original draft, review and editing, H.A.M.: formal analysis, methodology, writing—review and editing, D.M.A.E.: thermodynamics calculation, writing—review and editing, A.M.A.: methodology, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohammed, H.A., Mostafa, H.Y., El-Aty, D.M.A. et al. Novel Gemini ionic liquid for oxidative desulfurization of gas oil. Sci Rep 13, 6198 (2023). https://doi.org/10.1038/s41598-023-32539-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32539-y
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.