Abstract
In Japan, hepatocellular carcinoma (HCC) is a leading cause of cancer mortality and hepatitis C virus infection is a major cause of HCC. We conducted a systematic review and meta-analysis of published studies evaluating patient response to antiviral therapy for chronic hepatitis C on the risk of HCC occurrence in Japan. Articles were searched using terms determined a priori through PubMed, screened by title and abstract, and selected by full-text assessment according to criteria determined a priori, including HCC occurrence in response to interferon (IFN)-based or IFN-free therapy, Japanese study, and 2 or more years of follow-up. We excluded studies on HCC recurrence. We calculated the pooled estimate of the crude incidence rate ratio with data from the selected studies using the person-years method with Poisson regression model and pooled estimate of the hazard ratio adjusted for potential confounders reported by the studies using a random effects model. A total of 26 studies were identified, all of which examined only IFN-based therapy as a result of the selection process. The pooled estimate (95% confidence interval [CI]) of 25 studies was 0.37 (0.33–0.43) for sustained virologic response (SVR) and 1.70 (1.61–1.80) for non-SVR for the HCC incidence rate per 100 person-years, and 0.22 (0.19–0.26) for the incidence rate ratio (SVR vs. non-SVR). The pooled estimate of the hazard ratio (95% CI) of HCC incidence adjusted for potential confounders of 8 studies was 0.25 (0.19–0.34). SVR to interferon therapy for chronic hepatitis C reduces the risk of HCC occurrence.
Similar content being viewed by others
Introduction
Based on sufficient evidence for hepatocellular carcinoma (HCC) and non-Hodgkin lymphoma and a positive association with cholangiocarcinoma, the International Agency for Research on Cancer has stated that chronic infection with hepatitis C virus (HCV) is carcinogenic to humans1. Through adoption of the health-related goal to combat hepatitis in the Sustainable Development Goals in 20152, the World Health Organization has further strengthened its global hepatitis program for prevention, screening, and antiviral therapy to eliminate viral hepatitis (hepatitis B and hepatitis C) by 2030 as a public health threat3. Globally, an estimated 58 million people had chronic HCV infection in 20214 and HCV infection caused 140,000 cancer cases including HCC in 20185. According to the WHO, improvements have been made to various policies for hepatitis C infection in Asian countries6,7,8,9,10,11,12,13. In Japan, chronic hepatitis C has been the leading cause of HCC, accounting for 60% of HCC incidence in 201514. Further, HCC was the fifth leading cause of cancer mortality in 2019, and prognosis has not sufficiently improved, with the 5-year relative survival rate of liver cancer reported to be 35.8% in the population-based cancer registry (diagnosed in 2009–2011) in Japan15. According to molecular clock analysis using long-term serial samples of HCV in Japan, HCV infection was started around 1882, widely disseminated from the 1930’s, and decreased from 199516. These data are supported by historical episodes: HCV infection is thought to have been expanded by therapy for Schistosoma japonicum from 1923, stimulant use around the second world war and contaminated blood transfusion/products from the 1950’s, and decreased by blood screening with HCV antibody from 1990 after the discovery of HCV in 1989 and the use of disposable medical supplies16,17. Epidemiological studies have shown a decreasing trend in the proportion of HCV carriers by birth year18. In 2015, an estimated 0.9–1.3 million people had chronic HCV infection19. Regarding antiviral therapy for chronic hepatitis C20, interferon (IFN) drugs were approved in 1992 simultaneously with the introduction of HCV screening for patients. The sustained virologic response (SVR) rate of antiviral therapy for genotype 1 of HCV has gradually improved over recent decades: it began at 5% with IFN monotherapy, increased to 30% with IFN combined with ribavirin from 2001, to 40–50% with pegylated IFN (Peg-IFN) combined with ribavirin from 2004, to over 70% with further addition of direct-acting antivirals from 2011, and then to over 95% with IFN-free therapy consisting of direct-acting antivirals from 2014.
A previous meta-analysis that included studies from Japan reported that a SVR to IFN-based therapy for chronic hepatitis C is an indicator of the risk of HCC occurrence21. The effectiveness of IFN-based therapy is determined by host and viral factors, the distributions of which are specific to various racial and regional characteristics, such as interleukin-28B polymorphism22,23,24 and HCV genotype25,26, respectively. While IFN-free therapy has the potential to overcome both determinants of effectiveness, studies on the association between direct acting antivirals and HCC incidence are sparse27,28,29.
Here, we conducted a systematic review and meta-analysis of the response to antiviral therapy for chronic hepatitis C and risk of HCC occurrence in the Japanese population. This work is part of a series of systematic reviews that summarize evidence available from cancer epidemiology and prevention research exclusively on Japanese subjects.
Methods
Study selection
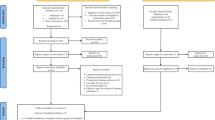
Studies were identified through PubMed as of April 5, 2022, using the following search terms determined a priori: (interferon or daclatasvir or asunaprevir or sofosbuvir or ledipasvir or ombitasvir or paritaprevir or ritonavir or elbasvir or grazoprevir or beclabuvir or glecaprevir or pibrentasvir or velpatasvir or direct acting antiviral) AND (hepatitis c) AND (hepatocellular carcinoma or liver cancer or liver neoplasm) AND (Japan or Japanese). Two investigators (YY and KT) independently screened all article titles and abstracts, and conducted full-text assessment to determine eligibility, according to criteria determined a priori. Inclusion criteria were (1) studies on Japanese populations, (2) examination of HCC incidence related to SVR and non-SVR and/or non-IFN treatment as chronic hepatitis C therapy, and (3) follow-up duration of 2 or more years. The exclusion criteria were (1) examination of HCC recurrence, (2) examination of continuing therapy for chronic hepatitis C, (3) studies including participants with liver transplantation, (4) studies including participants with co-infection by human immunodeficiency virus, and (5) studies with less than 20 total participants or with less than 10 participants with SVR or non-SVR. For overlapping study populations identified by study period and institute, studies that included a more comprehensive population and more complete data were selected. Inconsistencies in study selection between the reviewers were solved by discussion.
Data extraction and assessment of risk of bias
Data were extracted by YY using a spreadsheet developed a priori that included HCC cases with SVR/non-SVR, patients with SVR/non-SVR, follow-up period, hazard ratio (HR) adjusted for covariates, age, sex, advanced fibrosis, HCV genotype, and methods for HCC diagnosis. The data were checked by KT. Risk of bias in the selected studies was assessed by YY using the Newcastle Ottawa Scale for cohort studies, which consists of three items (selection, compatibility, and outcome) with eight sub-items30, and checked by KT. A “good” quality score required 3 or 4 stars in selection, 1 or 2 stars in comparability, and 2 or 3 stars in outcome. A “fair” quality score required 2 stars in selection, 1 or 2 stars in comparability, and 2 or 3 stars in outcome. A “poor” quality score reflected 0 or 1 star in selection, or 0 stars in comparability, or 0 or 1 star in outcome.
Data synthesis and analysis
The crude incidence rate and incidence rate ratio for each study were calculated using the person-years method assuming a Poisson distribution of the observed number of HCC cases with SVR/non-SVR during the follow-up period. Pooled estimates of both measures were derived using a Poisson regression model with a random intercept and a random coefficient for IFN response (SVR vs. non-SVR) according to each study. A pooled estimate of the HR adjusted for potential confounders reported in the studies was calculated using a random effects model with restricted maximum likelihood. Subgroup analysis and sensitivity analysis were conducted on studies that did or did not include/report data on patients with liver cirrhosis, HBsAg, and Peg-IFN use. Heterogeneity among studies was assessed using Q statistics and I2 statistics. Publication bias was tested using funnel plots and Egger’s test. All analyses were performed with Stata 17.0 (Stata Corp LLC, College Station, TX). This systematic review was not registered.
Results
Study selection
A total of 932 articles were identified through PubMed using search terms determined a priori on antiviral therapy for chronic hepatitis C (Fig. 1). Of these, 119 articles were selected by screening titles and abstracts. Full-text assessment according to the inclusion and exclusion criteria led to the exclusion of 93 studies due to unavailable data on HCC (n = 30), studies on HCC recurrence (n = 5), follow-up period less than 2 years (n = 5), population less than 20 (n = 4), duplicate population (n = 40), and incomplete data, including the number of HCC occurrences among those with SVR/non-SVR, patients with SVR/non-SVR, and follow-up period, or adjusted hazard ratio (n = 9). As a result, 26 studies on only IFN-based therapy, but not IFN-free therapy, were selected.
Study characteristics and risk of bias assessment
The characteristics of individual studies are summarized in Table 1. Year of publication, sample size, average follow-up duration and average age ranged from 1997 to 2017, 118 to 4302, 2.4 years to 11.8 years, and 47.2 years to 60 years, respectively. SVR was defined as undetectable HCV RNA at 24 weeks after the end of treatment in all studies, and the SVR rate ranged from 19.6% to 55.3%. In our risk of bias assessment using the Newcastle–Ottawa Scale for individual studies (Table 2), the exposure and outcome were reported based on medical records in most studies as they were hospital-based studies. The mean follow-up period was 5 or more years in 11 studies. The definition of “follow-up period” was inconsistent or unreported. The number lost to follow-up was reported in 6 of 26 studies. The overall study quality was regarded as “good” in 11 studies and “poor” in 15 studies. All eight studies that reported adjusted HRs had a “good” quality score. The magnitude of the association was consistent among studies (Table 3, Fig. 2). Publication bias cannot be ruled out due to asymmetry in the funnel plot (Fig. 3). No effects were observed for small studies using Egger’s test (P = 0.54). Heterogeneity among studies was small (Q = 7.28, P = 0.40; I2 = 12.96%). Further investigation into possible publication bias using a contour-enhanced funnel plot suggested that there may be missing studies in the bottom right-hand side of the plot (Supplementary Fig. 1), where the direction of the effect would be the same as that of the smaller effect magnitude of small studies in the area of non-significance. However, as no effects were observed for small studies using Egger’s test and pooled estimates did not change when fixed-effect and random-effect estimates were compared [HR (95%CI): 0.25 (0.19–0.34) in random-effect model (REML); HR (95%CI): 0.24 (0.19–0.31) in fixed-effect model (inverse-variance)], asymmetry in the funnel plots might indicate the low impact of publication bias or be due to chance.
Funnel plot evaluating the publication bias of 8 studies used to determine the pooled estimate of the hazard ratio of HCC incidence adjusted for potential covariates in patients treated with antiviral therapy (SVR vs. non-SVR). HCC hepatocellular carcinoma, SVR sustained virologic response, CI confidence interval.
Pooled estimates of the crude incidence rate ratio
The crude incidence rate ratio was calculated using data from 25 studies (Table 3). HCC was not observed in patients with SVR in 7 studies. The pooled estimate (95% confidence interval [CI]) was 0.37 (0.33–0.43) in patients with SVR and 1.70 (1.61–1.80) in patients with non-SVR for the HCC incidence rate per 100 person-years, and 0.22 (0.19–0.26) for the incidence rate ratio (SVR vs. non-SVR). In subgroup analysis, pooled estimates of the incidence rate ratio did not differ by inclusion or exclusion of patients with HBsAg, liver cirrhosis, or Peg-IFN use (Table 4).
Pooled estimate of adjusted hazard ratio
Eight studies reported the HR (SVR vs. non-SVR) adjusted for covariates of liver fibrosis including fibrosis stage and platelet count. The pooled estimate of the adjusted HR (95% CI) of HCC incidence was 0.25 (0.19–0.34) (Fig. 2). In subgroup analysis, pooled estimates of the adjusted HR did not differ by the inclusion or exclusion of patients with Peg-IFN use (Fig. 4). In sensitivity analysis, the pooled estimate of the adjusted HR of studies that did and did not include HBsAg-positive patients (n = 7; pooled estimate of adjusted HR = 0.25, 95% CI: 0.19–0.34) did not differ. Patients with liver cirrhosis were included in all 8 studies. Pooled estimates did not change in sensitivity analyses that excluded poor quality studies (incidence rate ratio (95% CI): 0.21 (0.17–0.25) in fair studies (n = 11); HR (95% CI): 0.24 (0.18–0.33) in fair studies (n = 7)). Likewise, pooled estimates did not change in subgroup analyses on study design, study period, follow-up period, sample size, and age (Supplementary Table 1 and 2).
Forrest plot of the pooled estimate of the hazard ratio of HCC incidence adjusted for potential covariates in patients treated with antiviral therapy (SVR vs. non-SVR) by studies that did and did not include Peg-IFN use. HCC hepatocellular carcinoma, SVR sustained virologic response, Peg-IFN pegylated interferon, HR hazard ratio, CI confidence interval.
Discussion
We conducted a systematic review of studies that examined the association between response to antiviral therapy for chronic hepatitis C and risk of HCC occurrence in a Japanese population. Based on pooled estimates of both the crude incidence rate ratio calculated using data from individual studies and the HR adjusted for potential confounders reported by the studies, we concluded that SVR to IFN-based therapy for chronic hepatitis C reduces the risk of HCC occurrence. In subgroup analysis and sensitivity analysis related to HBsAg-positivity, liver cirrhosis, and Peg-IFN use, pooled estimates of each effect size did not change.
A previous meta-analysis of observational studies in Asia, including Japan, Europe and North America reported an effect size of similar magnitude to that reported in the present study, irrespective of whether patients had advanced liver fibrosis: SVR to IFN-based therapy reduced the risk of HCC occurrence overall (pooled HR = 0.24, 95% CI: 0.18–0.31) and in advanced liver fibrosis (pooled HR = 0.23, 95% CI: 0.16–0.35)21.
Eradication of HCV by IFN is thought to reduce HCC occurrence by improving hepatic inflammation, regression of hepatic fibrosis, and the antitumor effects of IFN including tumoricidal, antiproliferative, or immunomodulatory effects31,32,33. These actions are considered unique to IFN compared to IFN-free therapy. Although achievement of SVR to antiviral therapy reduces HCC occurrence, HCC risk can remain during follow-up in patients with SVR. Advanced fibrosis, older age, alcohol intake, and diabetes mellitus have been suggested to increase the risk of HCC34. Strategies to improve lifestyle factors along with surveillance for HCC occurrence in patients with SVR are still needed.
Due to the selection process, this study did not include articles on IFN-free therapy. Although the effect of SVR to IFN-free therapy on HCC occurrence was controversial in earlier studies due to the older age and more advanced fibrosis among patients receiving IFN-free therapy35,36, later studies suggested that SVR to IFN-free therapy did in fact reduce the risk of HCC37. In Japan, Kobayashi et al. reported a cumulative HCC incidence (3-/5-year) of 1.30/3.03% for IFN-free and 1.02/2.19% for IFN-based therapy during the follow-up period (median, 4.0 years and 7.3 years, respectively), with a log-rank test indicating no significant differences in either group27. Similarly, Nagata et al. demonstrated that the 3-year cumulative incidence of HCC occurrence in patients with SVR did not differ by therapy in propensity score-matched analysis (3.3% for IFN-based, 1.4% for IFN-free therapy; P = 0.49, log-rank test)28. Tahata et al. also reported that HCC occurrence in patients with SVR did not differ by therapy in propensity score-matched analysis (cumulative rates of HCC occurrence at 1 year and 2 years: 0.5% and 1.9% in the IFN-based group vs 1.1% and 3.0% in the IFN-free group, P = 0.489; adjusted HR (95% CI) vs IFN-based: 1.134 (0.367–3.498), P = 0.827)38.
Given that the effectiveness of IFN-based therapy is linked to host and viral factors, one strength of this study was that we focused our analysis specifically on the Japanese population. Among host factors such as age, sex, race, and liver fibrosis, a comparison of patient response to Peg-IFN therapy combined with ribavirin in those infected with HCV genotype 1 identified interleukin-28B polymorphisms as a contributing factor22,23,24. The SVR rate was 14% in patients with the unfavorable allele TG/GG at rs809917, and 50% in those with the favorable allele TT39. These polymorphisms are associated with spontaneous clearance of HCV, which is observed in around 30% of those with acute infection with HCV in Japan. The minor allele frequency of rs809917 or rs12979860 is race specific, and the frequency of the favorable allele is higher in Asians compared with Caucasians and African-Americans40,41,42. In terms of viral factors, HCV genotype is related to response to antiviral therapy and the distribution of genotypes differs by region and route of transmission. Genotype 1 and poor response to IFN therapy were found in an estimated 65% of patients with chronic hepatitis C in 2015 in Japan26. Interestingly, evidence suggests that the distribution of genotypes has been altered with recent changes to the route of transmission, such as through intravenous drug abuse and tattoos, leading the prevalence of genotype 2b to be higher in patients born after 1970 regardless their place of birth in Japan43. The reported SVR rate to Peg-IFN therapy combined with ribavirin for genotype 2 is 80%20. Our findings on Japanese patients in this study suggest that eradication of HCV with antiviral therapy could be reducing HCC occurrence. Given the difficulty of comparing SVR and non-SVR to IFN-free therapy due to the high effectiveness of IFN-free therapy, further studies comparing SVR to IFN-free therapy and SVR to IFN are needed to evaluate the effects of SVR to IFN-free therapy on HCC occurrence.
Several limitations of this study should be noted. First, as most studies did not report the number of patients lost to follow-up, we could not rule out some risk of selection bias. Second, the definition of follow-up period was inconsistent or unreported among the selected studies; thus, HCC incidence may have been incorrectly evaluated in this study. Third, adjustment for confounders may have been insufficient: adjustment for alcohol consumption, smoking status, diabetes mellitus, and obesity was not necessarily conducted in each study. Furthermore, the effect size of HCC occurrence in patients with non-SVR to IFN therapy could have been overestimated, since older age and higher fibrosis stage are unfavorable factors for both response to IFN-based therapy and risk of HCC occurrence. Fourth, HCC cases with SVR were not observed and adjusted HRs were not available in earlier studies, possible because the effectiveness of antiviral therapy and technology used to diagnose HCC improved over the study period. Fifth, we used Pubmed only to search for reports during the selection process due to availability. Finally, we could not contact the study authors to clarify any unclear information because the studies were conducted a long time ago.
Future studies should examine the effects of SVR to IFN-free therapy on HCC occurrence by comparing SVR to IFN-free therapy and SVR to IFN. The findings of the present study suggest that eradication of HCV by IFN therapy may reduce HCC occurrence in Japanese patients. However, among the articles selected based on the eligible criteria in this study, only two articles reported outcomes other than HCC by response to antiviral therapy: unchanged incidence of malignancy other than HCC by response to IFN44 and decreased progression to LC in patients without LC before IFN therapy by SVR to IFN45. Outcomes other than HCC including overall-mortality, hepatic decompensation, and liver-related mortality by response to antiviral therapy are also important issues and warrant further study.
In conclusion, our systematic review on the association between response to antiviral therapy for chronic hepatitis C and HCC occurrence in a Japanese population suggests that eradication of HCV using antiviral therapy for chronic hepatitis C reduces HCC occurrence.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to the agreement of the Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan but are available from the corresponding author on reasonable request.
Change history
08 March 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41598-023-31052-6
References
International Agency for Research on Cancer. Biological Agents Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 100B. (2012).
United Nations. Sustainable Development Goal 3: Ensure Healthy Lives and Promote Well-Being for All at all Ages. Target3.3 By 2030, End the Epidemics of AIDS, Tuberculosis, Malaria and Neglected Tropical Diseases and Combat Hepatitis, Water-Borne Diseases and Other Communicable Diseases. (2015).
World Health Organization. Combating Hepatitis B and C to Reach Elimination by 2030. (2016).
World Health Organization. Fact Sheets: Hepatitis C. (2021).
de Martel, C., Georges, D., Bray, F., Ferlay, J. & Clifford, G. M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 8, e180–e190. https://doi.org/10.1016/s2214-109x(19)30488-7 (2020).
World Health Organization. Global Health Sector Strategy on Viral Hepatitis 2016–2021. Towards Ending Viral Hepatitis. (2016).
World Health Organization. Guidelines on Hepatitis B and C Testing. (2017).
World Health Organization. Guidelines for the Care and Treatment of Persons Diagnosed with Chronic Hepatitis C Virus Infection. (2018).
Lee, H. W. et al. Cost-effectiveness of chronic hepatitis C screening and treatment. Clin. Mol. Hepatol. 28, 164–173. https://doi.org/10.3350/cmh.2021.0193 (2022).
Kim, H. L. et al. A cost-effectiveness study of universal screening for hepatitis C virus infection in South Korea: A societal perspective. Clin. Mol. Hepatol. 28, 91–104. https://doi.org/10.3350/cmh.2021.0236 (2022).
Liu, C. H. et al. Sofosbuvir/velpatasvir plus ribavirin for Child–Pugh B and Child–Pugh C hepatitis C virus-related cirrhosis. Clin. Mol. Hepatol. 27, 575–588. https://doi.org/10.3350/cmh.2021.0155 (2021).
Wong, Y. J. et al. The impact of unrestricted access to direct-acting antiviral among incarcerated hepatitis C virus-infected patients. Clin. Mol. Hepatol. 27, 474–485. https://doi.org/10.3350/cmh.2021.0015 (2021).
Huang, C. F. et al. Scaling up the in-hospital hepatitis C virus care cascade in Taiwan. Clin. Mol. Hepatol. 27, 136–143. https://doi.org/10.3350/cmh.2020.0150 (2021).
de Martel, C., Maucort-Boulch, D., Plummer, M. & Franceschi, S. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology 62, 1190–1200. https://doi.org/10.1002/hep.27969 (2015).
Foundation for Promotion of Cancer Research. Cancer Statics in Japan. (2021).
Tanaka, Y. et al. A comparison of the molecular clock of hepatitis C virus in the United States and Japan predicts that hepatocellular carcinoma incidence in the United States will increase over the next two decades. Proc. Natl. Acad. Sci. USA 99, 15584–15589. https://doi.org/10.1073/pnas.242608099 (2002).
Ueno, Y., Sollano, J. D. & Farrell, G. C. Prevention of hepatocellular carcinoma complicating chronic hepatitis C. J. Gastroenterol. Hepatol. 24, 531–536. https://doi.org/10.1111/j.1440-1746.2009.05814.x (2009).
Akita, T. et al. Meta-regression analysis of sex- and birth year-specific prevalence of HBsAg and anti-HCV among un-diagnosed Japanese: Data from the first-time blood donors, periodical health checkup, and the comprehensive health checkup with lifestyle education (Ningen Dock). J. Epidemiol. 30, 420–425. https://doi.org/10.2188/jea.JE20190055 (2020).
Ko, K., Akita, T., Satake, M. & Tanaka, J. Epidemiology of viral hepatitis C: Road to elimination in Japan. Glob. Health Med. 3, 262–269. https://doi.org/10.35772/ghm.2021.01069 (2021).
Tahata, Y., Sakamori, R. & Takehara, T. Treatment progress and expansion in Japan: From interferon to direct-acting antiviral. Glob. Health Med. 3, 321–334. https://doi.org/10.35772/ghm.2021.01083 (2021).
Morgan, R. L. et al. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: A meta-analysis of observational studies. Ann. Intern. Med. 158, 329–337. https://doi.org/10.7326/0003-4819-158-5-201303050-00005 (2013).
Ge, D. et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 461, 399–401. https://doi.org/10.1038/nature08309 (2009).
Suppiah, V. et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat. Genet. 41, 1100–1104. https://doi.org/10.1038/ng.447 (2009).
Tanaka, Y. et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat. Genet. 41, 1105–1109. https://doi.org/10.1038/ng.449 (2009).
Rumi, M. G. et al. Randomized study of peginterferon-alpha2a plus ribavirin vs peginterferon-alpha2b plus ribavirin in chronic hepatitis C. Gastroenterology 138, 108–115. https://doi.org/10.1053/j.gastro.2009.08.071 (2010).
Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol. Hepatol. 2, 161–176. https://doi.org/10.1016/s2468-1253(16)30181-9 (2017).
Kobayashi, M. et al. Sustained virologic response by direct antiviral agents reduces the incidence of hepatocellular carcinoma in patients with HCV infection. J. Med. Virol. 89, 476–483. https://doi.org/10.1002/jmv.24663 (2017).
Nagata, H. et al. Effect of interferon-based and -free therapy on early occurrence and recurrence of hepatocellular carcinoma in chronic hepatitis C. J. Hepatol. 67, 933–939. https://doi.org/10.1016/j.jhep.2017.05.028 (2017).
Nagaoki, Y. et al. The risks of hepatocellular carcinoma development after HCV eradication are similar between patients treated with peg-interferon plus ribavirin and direct-acting antiviral therapy. PLoS One 12, e0182710. https://doi.org/10.1371/journal.pone.0182710 (2017).
Wells, G.A., O'Connell, B.S.D., Peterson, J., Welch, V., Losos, M., & Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses.
Ferrantini, M., Capone, I. & Belardelli, F. Interferon-alpha and cancer: Mechanisms of action and new perspectives of clinical use. Biochimie 89, 884–893. https://doi.org/10.1016/j.biochi.2007.04.006 (2007).
George, P. M., Badiger, R., Alazawi, W., Foster, G. R. & Mitchell, J. A. Pharmacology and therapeutic potential of interferons. Pharmacol. Ther. 135, 44–53. https://doi.org/10.1016/j.pharmthera.2012.03.006 (2012).
Rockey, D. C. & Friedman, S. L. Fibrosis regression after eradication of hepatitis C virus: From bench to bedside. Gastroenterology 160, 1502-1520.e1501. https://doi.org/10.1053/j.gastro.2020.09.065 (2021).
Li, D. K. & Chung, R. T. Impact of hepatitis C virus eradication on hepatocellular carcinogenesis. Cancer 121, 2874–2882. https://doi.org/10.1002/cncr.29528 (2015).
Mettke, F. et al. Interferon-free therapy of chronic hepatitis C with direct-acting antivirals does not change the short-term risk for de novo hepatocellular carcinoma in patients with liver cirrhosis. Aliment Pharmacol. Ther. 47, 516–525. https://doi.org/10.1111/apt.14427 (2018).
Reig, M. et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J. Hepatol. 65, 719–726. https://doi.org/10.1016/j.jhep.2016.04.008 (2016).
Li, D. K. et al. The short-term incidence of hepatocellular carcinoma is not increased after hepatitis C treatment with direct-acting antivirals: An ERCHIVES study. Hepatology 67, 2244–2253. https://doi.org/10.1002/hep.29707 (2018).
Tahata, Y. et al. Hepatocellular carcinoma occurrence does not differ between interferon-based and interferon-free treatment with liver histological assessment. Hepatol. Res. 50, 313–320. https://doi.org/10.1111/hepr.13454 (2020).
Kurosaki, M. et al. Pre-treatment prediction of response to pegylated-interferon plus ribavirin for chronic hepatitis C using genetic polymorphism in IL28B and viral factors. J. Hepatol. 54, 439–448. https://doi.org/10.1016/j.jhep.2010.07.037 (2011).
Thomas, D. L. et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 461, 798–801. https://doi.org/10.1038/nature08463 (2009).
Rauch, A. et al. Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: A genome-wide association study. Gastroenterology 138(1338–1345), 1345.e1331–1337. https://doi.org/10.1053/j.gastro.2009.12.056 (2010).
Tanaka, Y., Nishida, N., Sugiyama, M., Tokunaga, K. & Mizokami, M. lambda-Interferons and the single nucleotide polymorphisms: A milestone to tailor-made therapy for chronic hepatitis C. Hepatol. Res. 40, 449–460. https://doi.org/10.1111/j.1872-034X.2010.00671.x (2010).
Toyoda, H., Kumada, T., Takaguchi, K., Shimada, N. & Tanaka, J. Changes in hepatitis C virus genotype distribution in Japan. Epidemiol. Infect. 142, 2624–2628. https://doi.org/10.1017/s0950268814000478 (2014).
Arase, Y. et al. Effect of type 2 diabetes on risk for malignancies includes hepatocellular carcinoma in chronic hepatitis C. Hepatology 57, 964–973. https://doi.org/10.1002/hep.26087 (2013).
Yoneyama, K. et al. Analysis of background factors influencing long-term prognosis of patients with chronic hepatitis C treated with interferon. Intervirology 45, 11–19. https://doi.org/10.1159/000050082 (2002).
Umehara, Y. et al. Hepatocarcinogenesis is associated with serum albumin levels after sustained virological responses with interferon-based therapy in patients with hepatitis C. Dig. Dis. 35, 548–555. https://doi.org/10.1159/000480147 (2017).
Tada, T. et al. Viral eradication reduces all-cause mortality in patients with chronic hepatitis C virus infection: A propensity score analysis. Liver Int. 36, 817–826. https://doi.org/10.1111/liv.13071 (2016).
Takeuchi, Y. et al. Alpha-fetoprotein before and after pegylated interferon therapy for predicting hepatocellular carcinoma development. World J. Hepatol. 7, 2220–2228. https://doi.org/10.4254/wjh.v7.i19.2220 (2015).
Honda, T. et al. Effect of peginterferon alfa-2b and ribavirin on hepatocellular carcinoma prevention in older patients with chronic hepatitis C. J. Gastroenterol. Hepatol. 30, 321–328. https://doi.org/10.1111/jgh.12703 (2015).
Oze, T. et al. Post-treatment levels of α-fetoprotein predict incidence of hepatocellular carcinoma after interferon therapy. Clin. Gastroenterol. Hepatol. 12, 1186–1195. https://doi.org/10.1016/j.cgh.2013.11.033 (2014).
Ogawa, E. et al. Efficacy of pegylated interferon alpha-2b and ribavirin treatment on the risk of hepatocellular carcinoma in patients with chronic hepatitis C: A prospective, multicenter study. J. Hepatol. 58, 495–501. https://doi.org/10.1016/j.jhep.2012.10.017 (2013).
Osaki, Y. et al. Decrease in alpha-fetoprotein levels predicts reduced incidence of hepatocellular carcinoma in patients with hepatitis C virus infection receiving interferon therapy: A single center study. J. Gastroenterol. 47, 444–451. https://doi.org/10.1007/s00535-011-0505-8 (2012).
Watanabe, S. et al. Cancer preventive effect of pegylated interferon α-2b plus ribavirin in a real-life clinical setting in Japan: PERFECT interim analysis. Hepatol. Res. 41, 955–964. https://doi.org/10.1111/j.1872-034X.2011.00847.x (2011).
Takahashi, H. et al. Post-challenge hyperglycemia is a significant risk factor for the development of hepatocellular carcinoma in patients with chronic hepatitis C. J. Gastroenterol. 46, 790–798. https://doi.org/10.1007/s00535-011-0381-2 (2011).
Asahina, Y. et al. Effect of aging on risk for hepatocellular carcinoma in chronic hepatitis C virus infection. Hepatology 52, 518–527. https://doi.org/10.1002/hep.23691 (2010).
Kurokawa, M. et al. Effect of interferon alpha-2b plus ribavirin therapy on incidence of hepatocellular carcinoma in patients with chronic hepatitis. Hepatol. Res. 39, 432–438. https://doi.org/10.1111/j.1872-034X.2008.00477.x (2009).
Ikeda, K. et al. Antibody to hepatitis B core antigen and risk for hepatitis C-related hepatocellular carcinoma: A prospective study. Ann. Intern. Med. 146, 649–656. https://doi.org/10.7326/0003-4819-146-9-200705010-00008 (2007).
Kobayashi, S. et al. Development of hepatocellular carcinoma in patients with chronic hepatitis C who had a sustained virological response to interferon therapy: A multicenter, retrospective cohort study of 1124 patients. Liver Int. 27, 186–191. https://doi.org/10.1111/j.1478-3231.2006.01406.x (2007).
Soga, K., Shibasaki, K. & Aoyagi, Y. Effect of interferon on incidence of hepatocellular carcinoma in patients with chronic hepatitis C. Hepatogastroenterology 52, 1154–1158 (2005).
Kim, K. I. et al. Prediction of efficacy of interferon treatment of chronic hepatitis C and occurrence of HCC after interferon treatment by a new classification. Intervirology 48, 52–58. https://doi.org/10.1159/000082095 (2005).
Moriyama, M. et al. Long-term outcome, with monitoring of platelet counts, in patients with chronic hepatitis C and liver cirrhosis after interferon therapy. Intervirology 46, 296–307. https://doi.org/10.1159/000073209 (2003).
Ohata, K. et al. Hepatic steatosis is a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C virus infection. Cancer 97, 3036–3043. https://doi.org/10.1002/cncr.11427 (2003).
Tazawa, J. et al. Diabetes mellitus may be associated with hepatocarcinogenesis in patients with chronic hepatitis C. Dig. Dis. Sci. 47, 710–715. https://doi.org/10.1023/a:1014715327729 (2002).
Hirashima, N. et al. Hepatic Fas protein expression might be a predictive factor for hepatocellular carcinoma development in patients with chronic hepatitis C undergoing interferon therapy. J. Clin. Gastroenterol. 34, 263–267. https://doi.org/10.1097/00004836-200203000-00014 (2002).
Yoshida, H., Shiratori, Y., Moriyama, M. et al. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of hepatocarcinogenesis by interferon therapy. Ann. Intern. Med. 131, 174–81. https://doi.org/10.7326/0003-4819-131-3-199908030-00003 (1999).
Horiike, N. et al. The effectiveness of interferon therapy on occurrence of hepatocellular carcinoma in chronic hepatitis C. Oncol. Rep. 5, 1171–1174. https://doi.org/10.3892/or.5.5.1171 (1998).
Miyajima, I. et al. The incidence of hepatocellular carcinoma in patients with chronic hepatitis C after interferon treatment. Oncol. Rep. 5, 201–204 (1998).
Onodera, H., Ukai, K., Suzuki, M. & Minami, Y. Incidence of hepatocellular carcinoma after interferon therapy in patients with chronic hepatitis C. Tohoku J. Exp. Med. 181, 275–283. https://doi.org/10.1620/tjem.181.275 (1997).
Kuwana, K. et al. Risk factors and the effect of interferon therapy in the development of hepatocellular carcinoma: A multivariate analysis in 343 patients. J. Gastroenterol. Hepatol. 12, 149–155. https://doi.org/10.1111/j.1440-1746.1997.tb00398.x (1997).
Acknowledgements
We thank past members of Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan: Hadrien Charvat, Akihisa Hidaka, Mayo Hirabayashi, Motoki Iwasaki, Yuri Kitamura, Nagisa Mori, Michihiro Muto, Chisato Nagata, Mariko Naito, Tomio Nakayama, Yoshikazu Nishino, Atsuko Sadakane, Eiko Saito, Shizuka Sasazuki, Taichi Shimazu, Hiroyuki Shimizu, Kemmyo Sugiyama, Hidekazu Suzuki, Akiko Tamakoshi, Yoshitaka Tsubono, Ichiro Tsuji, Shoichiro Tsugane, Mai Utada, Kenji Wakai, Yoko Yamagiwa, and Taiki Yamaji. This study was supported by the National Cancer Center Research and Development Fund (2021-A-16, 30-A-15, 27-A-4, 24-A-3), Health and Labour Sciences Research Grants for Third Term Comprehensive Control Research for Cancer (H21-3jigan-ippan-003, H18-3jigan-ippan-001, H16-3jigan-010), Japan Health Research Promotion Bureau Research Fund (2019-(1)-1), and JSPS KAKENHI (19K10500).
Author information
Authors and Affiliations
Consortia
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Y.Y. and K.T. The first draft of the manuscript was written by Y.Y. and all authors commented on subsequent versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of the Article contained errors in the Consortia author list of The Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan, where author name, Shiori Tanaka, was duplicated in the main author list.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yamagiwa, Y., Tanaka, K., Matsuo, K. et al. Response to antiviral therapy for chronic hepatitis C and risk of hepatocellular carcinoma occurrence in Japan: a systematic review and meta-analysis of observational studies. Sci Rep 13, 3445 (2023). https://doi.org/10.1038/s41598-023-30467-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30467-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.