Abstract
Congenital heart disease (CHD) in pregnancy is associated with an increased risk of adverse maternal, obstetric, and neonatal outcomes, plausibly through mechanisms involving abnormal placental development and function. This retrospective study aims to elucidate how maternal CHD influences placental health. Demographic and clinical information were collected via electronic medical record review, and placentas underwent histopathological evaluation. Fifty-three singleton pregnancies were included: 35 participants (66%) were classified as lower cardiovascular risk (modified World Health Organization Classification (mWHO) I, II, II-III), and 18 (34%) were classified as higher cardiovascular risk (mWHO III, IV). 12 participants (23%) had a fetus with small for gestational age (SGA). Maternal vascular malperfusion (53%) and placental abruption (11.6%) were common in this cohort, with prevalence above baseline risk. Participants at higher cardiovascular risk had higher rates of SGA (p = 0.04), subchorionic hematomas (p = 0.01) and birth weight:placental weight < 10th percentile (p = 0.04), but did not differ in rates of maternal vascular malperfusion (p = 0.15) compared to those at lower cardiovascular risk. In pregnancies with maternal CHD, SGA and histologic evidence of maternal vascular malperfusion and placental abruption were common, though patients at higher cardiovascular risk did not show evidence of worsened placental health compared to those at lower risk.
Similar content being viewed by others
Introduction
With advances in surveillance and medical and surgical intervention for congenital heart disease (CHD), there has been a substantial increase in reproductive-aged adults with CHD1. In pregnancy, alterations in normal cardiovascular changes of pregnancy put birthing persons with CHD at high risk for heart failure, arrhythmias, and thrombotic events due to increased hemodynamic stress1,2,3. Additionally, maternal CHD is associated with adverse pregnancy and neonatal outcomes, such as higher rates of preterm birth, small for gestational age (SGA) birth weights, and neonatal mortality2,3. Although it remains unclear what mediates the relationship between maternal CHD and these adverse pregnancy and neonatal outcomes, it is biologically plausible that altered placental development and function, as a conduit of the feto-maternal system, drive these outcomes4.
The development of the placenta, a structure imperative for transporting oxygen and nutrients to fetus, is heavily influenced by both maternal and fetal health5. Maternal cardiovascular risk factors such as obesity, diabetes, and hypertension have been identified to negatively affect placental development and function. Maternal obesity increases inflammation of placental tissues, alters expression and activity of the lipid transporters in the placenta, and creates excess production of reactive oxygen species6,7. These changes in placental function seen in maternal obesity, can result in poor fetal growth and even fetal demise8. However, while it is clear that maternal cardiovascular health influences placental health, there are very few studies investigating the effects of maternal CHD on placental function. Maternal CHD is thought to restrict cardiac output and decreas blood flow to the placenta, resulting in maternal vascular malperfusion4,8. Evidence of maternal vascular malperfusion on histopathological evaluation has been shown in pregnancies with maternal CHD4.
Maternal cardiovascular health appears to influence placental vascular development and the adverse birthing outcomes seen in pregnancies complicated by maternal CHD may be placentally driven. In this study, we aim to investigate the relationship between maternal CHD cardiovascular risk and placental health. We hypothesize that pregnancies with higher cardiovascular risk will have greater evidence of maternal vascular malperfusion on histopathologic placenta examination.
Results
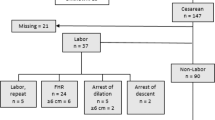
For initial analysis, 134 pregnancies with maternal CHD that received care with our facility’s cardio-obstetrics team were identified. A total of 81 pregnancies were excluded due to not having placental pathology data available. The remaining 53 participants were included in analyses. The average maternal age of participants was 29.8 years (range: 18–41). 27 (50.9%) participants had vaginal births, 16 (30.2%) had cesarean births, 6 (11.3%) had forceps-assisted vaginal births, and 4 (7.6%) had vacuum-assist births. 12 participants (22.6%) had fetuses with SGA. Additional demographic and clinical characteristics can be found in Table 1.
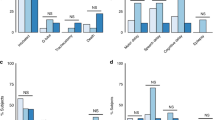
There was a wide range of congenital cardiac defects seen in our population (Fig. 1). The most common diagnoses included aortic valvular disease (17.0%), Tetralogy of Fallot or pulmonary valve disease (17.0%), intracardiac shunt lesion (13.2%), and transposition of the great arteries (11.3%). Participants were classified using the modified World Health Organization (mWHO) classification: mWHO I: 8 (15.1%) participants, mWHO II: 12 (22.6%) participants, mWHO II-III: 15 (28.3%) participants, mWHO III: 17 (32.1%) participants, mWHO IV: 1 (1.9%) participant. 35 participants (66.0%) were classified as being at lower cardiovascular risk (mWHO I, II, II-III) and 18 (34.0%) were classified as higher cardiovascular risk (mWHO III, IV). Further details regarding participant congenital cardiac diagnosis, and associated mWHO classification are listed in Table 2.
Participants at higher cardiovascular risk (mWHO III, IV) were more likely to have chronic hypertension (p = 0.01), be delivered at earlier gestational ages (p = 0.02), have lower birth weights (p = 0.002), higher prevalence of SGA (p = 0.04), and greater rates of maternal intensive care unit (ICU) admission (p = 0.01) compared to those at lower cardiovascular risk (mWHO I, II, II-III). There were no differences in mode of delivery (vaginal, cesarean, forceps-assisted, or vacuum assisted) between the mWHO I, II, II-III and mWHO III, IV cohorts (p = 0.40). All other demographic, clinical comorbidities, and pregnancy and neonatal outcomes were similar between the mWHO I, II, II-III and mWHO III, IV cohorts (Table 1, Fig. 1).
53 placentas were reviewed by pathology after birth and the frequencies of placental histopathologic findings are shown in Table 3. 28 placentas (52.8%) showed histopathologic evidence of maternal vascular malperfusion. The most common findings of maternal vascular malperfusion were infarction (17%), subchorionic hematoma (15.1%), and placental abruption (11.6%). 11 (20.8%) placentas had chorioamnionitis and 4 (7.6%) had deciduitis.
Participants showed few differences in placental histopathologic findings based on modified WHO classification risk (Table 4). Both mWHO I, II, II-III and mWHO III, IV cohorts had similar rates of birth weight:placental weight < 3rd percentile (p = 0.11), evidence of thrombosis (p = 0.32), infarction (p = 0.47), chorangiosis (p = 1.00), hypomature villus (p = 1.00), placental abruption (p = 0.40), chorioamnionitis (p = 0.37) and deciduitis (p = 1.00), and cord abnormalities (p’s > 0.34). Further, there was no difference in evidence of maternal vascular malperfusion (p = 0.15) between these cohorts. Notably, participants at higher cardiovascular risk did have higher rates of birth weight:placental weight < 10th percentile (p = 0.04) and subchorionic hematomas (p = 0.01) compared to participants at lower cardiovascular risk.
Similar findings were seen between cohorts when defining “low cardiovascular risk” as mWHO I, II and “high cardiovascular risk” as mWHO II-III, III, IV (Supplemental Table 3). No differences were seen in placental histopathologic findings between mWHO I, II and mWHO II-III, III, IV cohorts.
Discussion
Placentas of 53 birthing persons with congenital heart disease underwent histopathological evaluation. The most common findings were maternal vascular malperfusion (53%) and histologic evidence of infection (26%). Interestingly, there were high rates of infarction (17%) and placental abruption (11%) above baseline risk. SGA (23%) was also a common finding in this cohort of pregnancies complicated by maternal CHD. When comparing placental findings by mWHO classification of cardiovascular risk, there were few differences in histopathological findings between those at higher cardiovascular risk compared to lower cardiovascular risk, though higher cardiovascular risk was associated with higher prevalence of SGA. Importantly, higher cardiovascular risk (mWHO III, IV) was not associated with evidence of maternal vascular malperfusion.
Proper placental development is heavily influenced by maternal cardiovascular factors9. During pregnancy, alterations in the maternal vascular system initiates remodeling of maternal spiral arteries to increase oxygen and nutrient flow to the developing fetus10. Biological plausibility about placental development in maternal cardiovascular development could result from aberrant angiogenesis signaling, response, and subsequent differential vascular perfusion with neonatal sequalae. Howell et al. showed that maternal obesity results in downregulation of placental VEGF/Flt signaling resulting in decreased placental angiogenesis, placental hypoperfusion, and increased risk for pregnancy complications like SGA and pre-eclampsia9. Additionally, Wu et al. found that placentas from pregnancies complicated by maternal CHD, arrhythmia, cardiomyopathy, connective tissue disease, acquired valvular disease, ischemic heart disease, or vascular disease commonly showed evidence of vascular abnormalities4. Within the maternal CHD cohort, Wu et al. found no differences in anatomic, infectious, inflammatory, or vascular pathology based on anatomic or physiologic classification of CHD4. Our study similarly found high rates of SGA in pregnancies affected by maternal CHD, particularly in participants at higher cardiovascular risk. Additionally, maternal vascular malperfusion was common in our cohort, though few differences were seen in rates of histopathologic evidence of maternal vascular malperfusion based on cardiovascular risk. This finding was surprising, as we hypothesized that individuals with higher risk CHD (mWHO III, IV) have decreased blood flow and oxygenation to the placenta, resulting in greater evidence of maternal vascular malperfusion on histology and subsequent poorer fetal growth.
Our results may be limited by the wide variety of congenital cardiac defects seen in our cohort including: intracardiac shunts, aortic valve disease, mitral stenosis, mitral regurgitation and/or prolapse, Tetralogy of Fallot, pulmonary valve disease, transposition of the great arteries, single ventricle physiology, double outlet right ventricle, pulmonary hypertension, Marfan syndrome, Ebstein anomaly, and other cardiac defects. Although we estimated the cardiovascular risk of maternal CHD using the mWHO classification, the heterogeneity in our cohort possibly confounded our results as various cardiac defects may impact placental development in different ways. Instead, a more appropriate way to assess maternal CHD severity is not through anatomical classification, but rather looking at more functional variables such as maternal hypoxia which more directly relate to cardiac function in pregnancy. Additionally, our results may be confounded by selection bias given that other high-risk co-morbidities may have led to placental pathology collection in our patients. Our study institution currently utilizes a standardized protocol to collect placentas from all pregnancies impacted by maternal CHD, however placentas obtained prior to 2017 were collected based on the discretion of the delivering physician. In patients delivered prior to 2017, other health factors besides CHD may have led to placental collection. As such, our patient population may be skewed towards a “higher-risk” population in which other co-morbidities may have impacted placental development and function. Further, although evidence of maternal vascular malperfusion was common in our cohort, we may not have seen differences in maternal vascular malperfusion by CHD cardiovascular risk as our study examined placental pathology at birth and not placental function during pregnancy. It is difficult to assess when placental pathology arose during pregnancy and how that pathology influenced placental function though out pregnancy. In future prospective studies should assess placental function and development during gestation and pregnancy by interrogating growth, vascular parameters, and Doppler indices in this population with CHD.
The prevalence of placental abruption in our cohort is strikingly above a baseline rate of 0.6–1%11. Placental abruption, or a premature separation of a previously normally implanted placenta, occurs through unknown mechanisms outside of the coup-countercoup phenomena in trauma and with hypertensive diseases of pregnancy. However, it is well documented that an obstetrical history of placental abruption history has been correlated to future coronary heart disease risk, including both hemorrhagic and ischemic stroke12,13. These findings raise the consideration for a common abnormal vascular etiology related to both the biology underlying the abruption and the subsequent vascular event.
In summary, our study indicates that in pregnancies with maternal CHD, placental abruption, and histologic evidence of maternal vascular malperfusion are common. Notably, greater cardiovascular risk did not show evidence of poorer placental health compared to those with lower cardiovascular risk.
Methods
In this retrospective study, pregnant people with known congenital heart disease delivered at the University of California, Los Angeles Ronald Reagan Hospital between April 23rd, 2016 and November 1st, 2021 were eligible, including participants with surgical correction of congenital heart disease. Participants who had acquired heart disease, terminated their pregnancy, did not deliver at our institution, or those without placental specimen collection were excluded. The study was approved by the Institutional Review Board (IRB), University of California, Los Angeles ((UCLA) IRB#17-000778) and informed consent was waived given the retrospective nature of the study. All study experiments were performed in accordance with the Institutional Review Board guidelines.
Demographic and clinical data
Baseline demographic and clinical data were collected via electronic medical record review. Maternal health data included age, BMI, race/ethnicity, tobacco use, diabetes, chronic hypertension, asthma, thyroid, and autoimmune disorders. Classification of cardiovascular risk was estimated using the modified WHO (mWHO) classification system3. Lower risk CHD included mWHO I, mWHO II, and mWHO II-III. Higher risk CHD included mWHO III and mWHO IV. Birthing outcomes included gestational age at delivery, mode of delivery, neonatal birthweight, and SGA. SGA infants were defined by birthweight percentiles using the WHO growth standards14.
Placental collection and processing
Per standardized clinical practice at the study institution initiated in 2017, placentas from pregnancies affected by maternal congenital heart disease were collected and sent to pathology for gross and microscopic histopathologic examination at birth. Prior to 2017, indication for placental pathology was up to the discretion of the delivering physician. The placental size was measured, and trimmed weight was recorded. All placentas were fixed in 10% buffered formalin. Sections submitted included two sections of umbilical cord, two sections of membrane, three full thickness sections of grossly normal appearing placenta from the chorionic plate to the basal plate and additional submitted sections of any grossly abnormal placenta. Sections underwent routine processing, were paraffin-embedded, sectioned at 3 to 5 µm and stained with hematoxylin and eosin. The pathologists categorized the histopathologic lesions according to the Amsterdam criteria15. Placenta histopathologic findings of interest included placental weight and evidence of thrombosis, infarction, chorangiosis, hypomature villi, subchorionic hematoma, placental abruption, chorioamnionitis, and deciduitis. Placentas were classified as having “maternal vascular malperfusion” if there was evidence of any of the following: thrombosis, infarction, chorangiosis, hypomature villi, subchorionic hematoma, and/or placental abruption. Cord abnormalities included hypocoiled cords, hypercoiled cords, or presence of a single umbilical artery. Cord insertion abnormalities included marginal or velamentous insertion. Of note, some pathological terms were collapsed within thrombosis, hypomature villus, and subchorionic hematoma (see Supplemental Table 1).
Statistical analysis
A total of 53 participants were included in analyses. Chi square analysis, Fisher’s exact test, and Student’s T-test were used to determine statistical differences in demographic and clinical characteristics between low-risk CHD participants and high-risk CHD. Chi-square analysis and Fisher’s exact test were used to detect differences in placental pathology findings between participants low-risk CHD and high-risk CHD. Data were presented with means with standard deviations, medians with interquartile ranges, and counts with percentages, as appropriate. Statistical analyses were performed using STATA, and statistical significance was set at a p-value of < 0.05.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ramlakhan, K. P., Ahmed, I., Johnson, M. R. & Roos-Hesselink, J. W. Congenital heart disease and family planning: Preconception care, reproduction, contraception and maternal health. Int. J. Cardiol. Congenit. Hear. Dis. 1, 100049 (2020).
Ramage, K., Grabowska, K., Silversides, C., Quan, H. & Metcalfe, A. Association of adult congenital heart disease with pregnancy, maternal, and neonatal outcomes. JAMA Netw. Open 2, e193667–e193667 (2019).
Suwanrath, C., Thongphanang, P., Pinjaroen, S. & Suwanugsorn, S. Validation of modified World Health Organization classification for pregnant women with heart disease in a tertiary care center in southern Thailand. Int. J. Womens. Health 10, 47–53 (2018).
Wu, F. M. et al. Placental findings in pregnancies complicated by maternal cardiovascular disease. JACC Adv. 1, 100008 (2022).
Godfrey, K. M. The role of the placenta in fetal programming—a review. Placenta 23, S20–S27 (2002).
Easton, Z. J. W. & Regnault, T. R. H. The impact of maternal body composition and dietary fat consumption upon placental lipid processing and offspring metabolic health. Nutrients 12, 3031 (2020).
Mele, J., Muralimanoharan, S., Maloyan, A. & Myatt, L. Impaired mitochondrial function in human placenta with increased maternal adiposity. Am. J. Physiol. Endocrinol. Metab. 307, E419–E425 (2014).
Uebing, A., Steer, P. J., Yentis, S. M. & Gatzoulis, M. A. Pregnancy and congenital heart disease. BMJ 332, 401–406 (2006).
Howell, K. R. & Powell, T. L. Effects of maternal obesity on placental function and fetal development. Reproduction 153, R97–R108 (2017).
Parks, W. T. & Catov, J. M. The placenta as a window to maternal vascular health. Obstet. Gynecol. Clin. North Am. 47, 17–28 (2020).
Tikkanen, M. Placental abruption: Epidemiology, risk factors and consequences. Acta Obstet. Gynecol. Scand. 90, 140–149 (2011).
Rich-Edwards, J. W., Fraser, A., Lawlor, D. A. & Catov, J. M. Pregnancy characteristics and women’s future cardiovascular health: An underused opportunity to improve women’s health?. Epidemiol. Rev. 36, 57–70 (2014).
Ananth, C. V. et al. Maternal cardiovascular and cerebrovascular health after placental abruption: A systematic review and meta-analysis (CHAP-SR). Am. J. Epidemiol. 190, 2718–2729 (2021).
WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. Suppl. 450, 76–85 (2006).
Khong, T. Y. et al. Sampling and definitions of placental lesions: Amsterdam placental workshop group consensus statement. Arch. Pathol. Lab. Med. 140, 698–713 (2016).
Acknowledgements
All members of the UCLA cardio-obstetrics clinical team in the care of our complex patients including Leigh Reardon, MD, Gentian Lluri, MD, PhD, Jamil Aboulhosn, MD, Prashanth Venkatesh, MD, Michael Richley, MD, and Jenny Mei, MD.
Funding
Funding by Carolyn L. Kuckein Student Research Fellowship (M.A.) and National Institute of Health K12 HD000849 (Y.A.) by the Eunice Kennedy Shriver National Institute of Child Health & Human Development and American College of Obstetricians and Gynecologists, as part of the Reproductive Scientist Development Program.
Author information
Authors and Affiliations
Contributions
M.A. and T.M. assisted with data collection. T.M. and Y.A. advised on study concept and design. M.A. performed statistical analysis and Y.A. oversaw statistical analysis. M.A., T.M., and Y.A. contributed to interpretation of data. M.A., E.A. T.M. J.G. J.L, and Y.A. significantly contributed to drafting and revision of manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Altendahl, M., Mok, T., Adimkpayah, E. et al. Vascular malperfusion and abruption are prevalent in placentas from pregnancies with congenital heart disease and not associated with cardiovascular risk. Sci Rep 13, 1439 (2023). https://doi.org/10.1038/s41598-023-28011-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-28011-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.