Abstract
In this paper, Nostoc commune crude polysaccharide was extracted by heating and Ultrasonic-assisted methods separately, homogeneous polysaccharide HNCP3 and UNCP4 were obtained after purified by DEAE-52 cellulose column chromatography and Sephacryl G-100 gel column chromatography. The structures of HNCP3 and UNCP4 were characterized by molecular weight determination, infrared spectroscopy, DSC detection, sodium periodate oxidation, smith degradation reaction and methylation analysis. The conformation of the solution was studied by SEM and AFM. The results showed that the Ultrasonic-assisted extraction had effects on the molecular weight, monosaccharide composition, molar ratio and configuration of Nostoc commune. The main chain of HNCP3 and UNCP4 was → 6)-D-Glcp(1→ and → 2, 6)-D-Glcp, but UNCP4 contained 1, 2, 6-galactose and 2, 3-Me2-D-Ara branches, while HNCP3 did not. The results of the monosaccharides composition of indicated that mannose was presented in both HNCP3 and UNCP4. SEM and AFM showed that the structure of UNCP4 was helical, and the solution conformations of HNCP3 and UNCP4 were different in different solution environments. Studies on DPPH radicals, superoxide anions, and hydroxyl radicals scavenging abilities showed that UNCP4 had higher antioxidant activity, while studies on the antidiabetic activities showed that the hypoglycemic effect of UNCP4 was stronger than that of HNCP3. Therefore, Ultrasonic-assisted extraction (UAE) increases the bioactivity of Nostoc commune polysaccharide (NCP) as well as the extraction rate.
Similar content being viewed by others
Introduction
Nostoc commune is a species of lower heterocystous blue-green alga capable of forming an extended jelly layer consisted of polysaccharides, which grows vigorously in dry and cold barren mountainous regions1,2. Nostoc commune is rich in protein, polysaccharide, amino acids, lipids and a variety of vitamins and metal elements, with rich nutritional value. Many reports have proved that different species of Nostoc contain a variety of unique medicinal compounds (amino acids, fatty acids, polysaccharides, antimicrobial substances) that exhibit good bioactivities, such as nitrogen-fixing3, reducing the risk of coronary heart4, antioxidation5, anti-microbico6, suppressing the human T-lymphoid Jurkat cell growth7, anti-tumor8, etc. Until now, N. commune also has been revealed to be rich in proteins, sugars, and lipids, as well as trace elements and vitamins9, and has beneficial physiological functions such as anti-infection10, decreasing blood lipid levels11, activating autophagy by down regulating PI3K/AKT/mTOR pathway12, antimicrobial and anti-inflammatory13, etc. Hence, N. commune, commonly referred to as ‘‘ishikurage’’, has previously been used as food ingredient or folk medicine for prevention and cure illnesses in Southeast Asia14.
It has been pointed out that polysaccharides of N. commune play an important role in resisting extreme desiccation, readily restoring metabolic activity and having novel bioactivities upon rehydration, and these functions exhibit structure–activity relationship15,16,17,18,19,20. It is well known that the extraction method has a significant effect on the yield, structural characteristics and biological activities of polysaccharides21,22. The conventional heating reflux extraction (HRE) is the most commonly used method for extracting polysaccharides. Generally, the yield of this process is highly dependent on the extraction time and temperature15,23. However, the higher extraction temperature and longer extraction time may result in the degradation of polysaccharides and reduction of pharmacological activities24. Therefore, several new techniques have been employed to extract polysaccharides for increasing extraction rate25, such as ultrasonic-assisted extraction (UAE)26,27,28 and microwave-assisted extraction (MAE). However, there is few research has been conducted on the comparation of physical properties, structural features and bioactivities between the polysaccharides obtained by HRE and UAE. The ultrasound-assisted extraction technology has been used to obtain the crude polysaccharides from Nostoc commune in previous study20. In this study, the conventional heated reflux extraction, ultrasonic-assisted extraction and alcohol precipitation technology were used to prepare the crude polysaccharides (NCP) of N. commune which collected from cold-humid area at an altitude of about 2000 m (Weiyuan County, Gansu Province, China), and then the physicochemical properties, structural features and bioactivities of the purified segments purified by the chromatography were studied for evaluating the structure–activity relationships.
Results and discussion
Purification of HNCP3 and UNCP4 by chromatography technique
10 mg/mL of HNCP3 and UNCP4 were loaded into the DEAE-52 chromatographic column respectively, and eluted with NaCl solution in the range of 0–1.0 mol/L. The elution with 0.3 mol/L of NaCl was collected and traced by the phenol sulfuric acid method, two peaks were obtained with the recoveries of 29.34% and 34.28%. After dialysis and lyophilization, the sample was further purified by Sephadex G 100 chromatography column, two single narrow symmetrical peaks named HNCP3 and UNCP4 were obtained (Fig. 1A,B) with the recovery rates of 80.9% and 86.4%, which indicated HNCP3 and UNCP4 might be homogeneous components polysaccharide29. Therefore, UAE was a more efficient technique to obtain higher polysaccharides yield from N. commune than HRE, and has more molecules with similar physical and chemical properties due to the cavitation, mechanical and heat effects synthetically30.
Elution profiles and molecular weight distributions of HNCP3 and UNCP4 purified by DEAE-52 cellulose column and Sephadex G-100 column. (A): The elution curve of HNCP3 purified by Sephadex G100; (B): The elution curve of UNCP4 purified by Sephadex G100; (C): Molecular weight distributions of HNCP3 by high-performance liquid chromatography coupled with multi-angle laser light scattering determination; (D): Molecular weight distributions of UNCP4 by high-performance liquid chromatography coupled with multi-angle laser light scattering determination.
Molecular weights of HNCP3 and UNCP4
The average molecular weights of HNCP3 and UNCP4 were evaluated by HPSEC-MALLS-RID technique as shown in Table 1 and Fig. 1C,D. All HNCP3 and UNCP4 samples exhibited the similar composition structures with different molecular weight distributions, indicating that HNCP3 and UNCP4 were heteropolysaccharides. The highest Mw of HNCP3 and UNCP4 were 48.6 kDa and 14.54 kDa, respectively, while the lowest Mn were 22.72 kDa and 44.32 kDa, respectively. As shown in Table 1, the averages molecular weight of UNCP4 was lower than that of HNCP3, and the low-molecular weight distribution of UNCP4 (68.57%) was greater than that of HNCP3 (46.18%), suggesting that the UAE method could produce a larger amount of the low Mw fractions than the traditional HRE method31. This might be attributed to the polysaccharides were degraded by ultrasonic to some extent, and a portion of the high-Mw fractions were converted into the low-Mw fractions, thereby increasing the amounts of the low Mw fractions. The same phenomenon was also observed in previous report29.
Monosaccharide compositions analysis
The monosaccharide compositions of samples were determined by GC–MS and the results were presented in Fig. 2. HNCP3 and UNCP4 were analyzed by matching their retention times with those of the standard monosaccharides (Fig. 2A), indicating the two polysaccharides had the different monosaccharide compositions. HNCP3 was composed of rhamnose, mannose, glucose, galactose in a molar ratio of 0.65: 0.55: 2.91: 2.73 and UNCP4 was composed of mannose, arabinose, glucose in a molar ratio of 0.30: 6.84: 2.81. The results illustrated that both HNCP3 and UNCP4 were enriched in glucose, indicating that glucose was the predominant monosaccharide. S. Jensen reported that the monosaccharide composition of Nc-5-s purified from an alkali extract of N. commune was Glc, GlcA, Xyl, Man, Ara, Rib, Gal in the ratio of 24: 24: 15: 13: 13: 7: 4. While the monosaccharide of a water soluble polysaccharide (NSKP) purified by precipitating hot-water extract from Nostoc with 40% (v/v) ethanol hot-water was composed of mannose, glucose, xylose, galactose, and glucuronic acid with a molar ratio of 1.00: 1.92: 0.97: 1.02: 0.9132. These results demonstrated that the difference monosaccharide composition was caused by the different processing methods and material sources33.
GC–MS spectra of the reference sample. (A) HNCP3; (B) UNCP4; (C) UNCP; (D) The total ion chromatogram from methylation analysis of HNCP by GC-MS; (E) The total ion chromatogram from methylation analysis of UNCP by GC-MS; (F) The total ion chromatogram of the methylated product of HNCP3; (G) The total ion chromatogram of the methylated product of UNCP4.
Linkage analysis of HNCP3 and UNCP4
According to the results of periodate oxidation reaction, the consumptions of sodium periodate of HNCP3 and UNCP4 were 0.61 mol and 0.93 mol, and the total amounts of formic acid produced were calculated to be 0.43 mol and 0.65 mol. The molar ratios of periodate acid consumption to formic acid production were 1.42 and 1.43, respectively, indicating that HNCP3 and UNCP4 might contain glycosidic bonds of 1 → 2, 1 → 2, 6, 1 → 3, 1 → 3, 6, etc34. The GC of smith degradation of HNCP3 was presented in Fig. 2D. The detection of glycerol suggested that HNCP3 may contain 1→ , 1→ 2, 1 → 6 and 1 → 2,6 glycosidic bond. The detection of erythritol demonstrated that the bond type of the formation of erythritol existed, namely 1 → 4 and 1 → 4, 6-hexose. Meanwhile, the detection of rhamnose, mannose, glucose and galactose revealed that the polymers maybe linked by four kinds of monosaccharides with 1 → 3, 1 → 2, 3, 1 → 3, 4, 1 → 3, 6 and 1 → 2, 3, 4 glycosidic bonds. The GC of smith degradation of UNCP4 was presented in Fig. 2E, the erythritol formation indicated the possible presence of 1 → 4 and 1 → 4, 6 bonds hexoses. The detection of mannose and glucose and the increasing of glucose content comparing with the samples without periodate oxidation exhibited that most of UNCP4 were not oxidized by periodate, and the polymer mainly linked with 1 → 3 or 1 → 2,3 or 1 → 2, 4 or 1 → 3, 4 or 1 → 3, 6 or 1 → 2, 3, 6 or 1 → 2,4,6 or 1 → 3, 4, 6 glycosidic bonds. Methylation analysis was carried out to determine the inter monosaccharides linkages in HNCP3 and UNCP4 by GC/MS (Fig. 2F,G), and the linkage details were shown in Table 2. The results demonstrated that the methylated products of HNCP3 were total six liberations of 2, 4, 6-Me3-Glcp [peak 1: → 3)-Glcp-(1 →], 2, 3, 6-Me3-Glcp [peak 2: → 4)-Glcp-(1 →], 3, 4, 6-Me3-Galp [peak 3: → 2-Galp-(1 →], 2, 4-Me2-Galp [peak 4: → 2, 6)-Galp-(1 →], 2, 3, 4-Me3-Glcp [peak 5: → 6)-Glcp-(1 →], 2, 3, 4-Me3-Galp [peak 6: → 6)-Galp-(1 →] in a relative molar ratio of 1: 1.34: 1.28: 1.98: 3.32: 3.12 (Table 2). No rhamnose was detected due to the lower molar ratio in this polysaccharide. The following repeating unit of HNCP3 was deduced as below:

The methylated product of UNCP4 was composed of the 2, 4-Me2-Rhap [peak 1: → 3)-Rhap-(1→], 2, 3-Met-Glcp [peak 2: → 4, 6)-Glcp-(1 →], 2, 3, 4-Me3-Glcp [peak 3: → 6)-Glcp-(1 →], 2, 3, 4, 6-Me4-Glcp [peak 4: → 4)-Glcp-(1 →], , 3, 6-Men-Glcp [peak 5: → 3)-Glcp-(1 →], 2, 4-Me2-Glcp [peak 6: → 3, 6)-Glcp-(1 →] in a relative molar ratio of 1: 24.63: 36.13: 41.32: 9.87: 17.78 (Table 2). The results revealed that the presence of the following repeating unit in the UNCP4.

At present, a variety of plant polysaccharides have been reported to have antioxidant activities, including green algae, brown algae and red algae and other algal polysaccharides35,36,37. It has been reported that most polysaccharides with outstanding biological activities are linked by (1 → 3) glycosidic bonds, which have β–(1 → 3)-D-glucan backbone structure with some side chains38,39. However, the structure and stereo configuration of plant polysaccharides are very complex, and their structure–function relationship remains to be further studied. An edible mushroom (Calocybe indica) has been reported that possesses immunostimulatory activity and the primary structural backbone has the same α-D-Glcp-(1 → 4) glycoside unit as HNCP3 and UNCP440. Toona sinensis leaves polysaccharide has good antioxidant and other protective effects against acute liver injury in mice41. Wherein, the Toona sinensis leaves polysaccharide TSP-1 main chain has the same α-D-Manp-(1 → 6) glycosidic unit as HNCP3 and the same α-D-Glcp-(1 → 6) glycosidic unit as UNCP4. While the branched chain has the same α-D-Glcp-(1 →) glycosidic unit as HNCP3 and the same α-D-Manp-(1 → 3) glycosidic unit as UNCP4. The detection of the primary structure of Nostoc commune polysaccharide proved that its polysaccharide main chain has α-D-Glcp-(1 → 4), α-D-Manp-(1 → 6) or α-D-Glcp-(1 → 6) glycoside, while the side chain has α-D-Glcp-(1 →) or α-D-Manp-(1 → 3) glycoside, so it was speculated to have potential antioxidant activity. In a word, these methylate results were consistent with the observations of periodate oxidation and smith degradation of HNCP3 and UNCP4.
FT-IR spectrum and TGA/DSC detection
No visible differences could be observed between the spectra of the HNCP3 and UNCP4 as displayed in Fig. 3A,B, indicating that the HNCP3 and UNCP4 extracted by the different methods had similar functional groups. In details, the absorptions were very obvious at 3400 cm−1 and 1100 cm−1, that were caused by the stretching and angular vibration of O–H linkage42. The absorption at 3423.085 cm−1 was attributed to the C-H stretching vibration. An asymmetrical stretching peak at 1637 cm−1 and an absorption peak approximately at 1412 cm−1 were the indicators of the presence of carbonyl and carboxyl groups stretching vibration43,44. The two absorption peaks at 1064.531 cm−1 and 1062.603 cm−1 corresponded to the stretching vibrations of C–O–C or C–O–H of a pyranose ring, confirming that HNCP3 and UNCP4 contained pyranose sugar45. The mid-peak at 590 cm−1 and the weak peak at 586 cm−1 were associated with the presence of α and β-glycosidic linkages, respectively.
The thermogravimetric (TG) spectrum was used to determine the weight loss ofHNCP3 and UNCP4 on heating from 25 to 300 °C. As shown in Fig. 3C,D, the results revealed a one stage weight loss corresponding to the loss of water around 25–248 °C. The curve showed that UNCP4 did not decompose before 248 °C with a relatively smoothly weight loss of 10.97% at 71.97 °C, while HNCP4 began to decompose at 216 °C with a faster weight loss of 13.35% at 90.09 °C. It has been reported46 that water was formed by intra- and intermolecular condensation of polymer hydroxyls, and the decomposition of polymer occurred at temperatures below 300 °C. The weight loss rate of UNCP4 was lower than that of HNCP3, indicating that UNCP4 might has good thermal stability or has the rumbly textures and coarse unconsolidated surfaces47.
Differential scanning calorimetry (DSC) was applied to determine the exothermal or endothermal changes with the temperature increasing of HNCP3 and UNCP4 (Fig. 3C,D). In which, the three key temperature points involved in the energy change were marked as T0 (initial temperature), Tp (intermediate temperature), and Tc (final temperature). Both polysaccharides exhibited amorphous portions. The glass transition temperatures of HNCP3, UNCP4 occurred at 61.2 °C and 62.8 °C without melting peaks. The continuous (broad) endothermic transitions were observed as an indicative of moisture loss in HNCP3 and UNCP4. Figure 3C displayed the enthalpy change value of HNCP3 was − 211.6 J/g and the exothermic peak was relatively gentle with the temperature of exceeding 249.9 °C, and the pyrolysis reaction proceeded relatively smoothly. Nevertheless, the enthalpy change value of HNCP3 was − 226.1 J/g and a steep exothermic peak occurred at the temperature over 248.4 °C and the pyrolysis reaction proceeded more vigorously (Fig. 3D). In a word, there was a little difference between both polysaccharides by the DSC curves analysis.
SEM and AFM observations of HNCP3 and UNCP4
Different extraction methods could affect the microstructures of polysaccharides48,49, the morphology and structures of HNCP3 and UNCP4 were observed by SEM and AFM (Fig. 4) in this paper. At the resolution of 100 μm, the surface of HNCP3 was smooth and irregularly jagged with a filamentous and sheet-like connection (Fig. 4A), and UNCP4 exhibited the higher smooth and uniform surface with the pronounced honeycomb-like and helical voids structure (Fig. 4B), which indicated that the changes between the two polysaccharides might be due to the long heating time, high temperature, and the cavitation effect caused by ultrasonic vibration. These results were similar to previously reported results that UAE had a more drastic effect on the microstructure of polysaccharides50.
To further observe the fine structures of HNCP3 and UNCP4, the AFM was used for the morphological observation. As shown in Fig. 4C, D, HNCP3 and UNCP4 molecules were ellipsoidal. UNCP4 molecules were loosely packed and uniform in size and shape, and the horizontal length of the molecules was 226.56 nm with the vertical height of 7.126 nm. Compared to UNCP4, HNCP3 molecules showed different sizes and shapes with large number of spherical micelles and a small amount of dispersion, and the horizontal length of the molecules was 101.56 nm with the vertical height of 13.961 nm. According to above results, we could speculate that molecular aggregation existed in UNCP4, and its structural units might branch and entangle with each other. The SEM and AFM analysis provided strong evidences for the improved efficiency of polysaccharide extraction by UAE.
Antioxidant activities of HNCP3 and UNCP4
Various mechanisms of anti-oxidation have been reported that including suppression of chain initiation, chelating metal ions, peroxides decomposition and radical scavenging51,52. However, the mechanism of action of the antioxidant activities of HNCP3 and UNCP4 remained unclear. The antioxidant activities of HNCP3 and UNCP4 were estimated and presented in Fig. 5, that were the free radical cleaning rates gradually increased with increasing concentrations of the samples. Figure 5A showed the scavenging activities of HNCP3 and UNCP4 on the DPPH· radical compared with Vc. Among all the samples, the positive control Vc solution had the highest clearance rate, reaching the maximum of 95.41% at the concentration of 0.2 mg/mL. UNCP4 also exhibited relatively stronger scavenging capacity (52.72%) than HNCP3 (40.21%) at the concentration of 1.0 mg/mL. The results of the removal of superoxide anion radicals by HNCP3 and UNCP4 were shown in Fig. 5B. The scavenging ability increased significantly with the rise of the concentration from 0.2 to 1.0 mg/mL, and the maximum clearance rates of HNCP3 and UNCP4 at 1.0 mg/mL were 30.12% and 26.02% respectively. Figure 5C exhibited the effects of HNCP3 and UNCP4 on scavenge ·OH, with the highest clearance rates reaching 22.71% and 26.70% respectively, that were significantly lower than the scavenging effect of Vc on hydroxyl radicals. Previous studies have reported that the scavenging effect of crude polysaccharide (HRSA) of N. commune on HO·could reach at 92.71% with the concentration of 10 mg/mL53. The mechanisms of the antioxidant action of HNCP3 and UNCP4 might be the molecule’s ability to supply hydrogen atoms to free radicals, thus terminating free radical chain reactions and converting free radicals to unharmful products15,54. In this paper, HNCP3 and UNCP4 showed different antioxidant activities, that were attributed to their monosaccharide composition ratios and side-chain linkages. In addition, ultrasonic wave might appropriately degrade the high-molecular-weight polysaccharides and change their antioxidant activities55.
Antioxidant activities of HNCP3, UNCP4 and vitamin C (Vc). Note: (A) Effect of different concentration of samples on DPPH radical scavenging activities; (B) Effect of different concentration of samples on removal of hydroxyl radicals; (C) Effect of different concentration of samples on removal of superoxide anion.
It has been reported that physical properties such as molecular weight, solubility and viscosity of polysaccharides, as well as the extraction method of polysaccharides could affect the antioxidant effect of plant polysaccharides56. Yang et al. found that hot water extraction and ultrasound-assisted extraction of Pleurotus citrinopileatus polysaccharides had stronger free radical scavenging ability compared to alkali extraction57. Moreover, Ni et al. studied the monosaccharide composition of eight plant polysaccharides and their DPPH scavenging activity. The results showed that the DPPH radical scavenging ability of the eight polysaccharides was not related to the polysaccharide content but to the microstructure, and the specific way and intensity of the effect of monosaccharide composition on the antioxidant activity of the polysaccharides need to be further investigated58. In this study, when the concentration of polysaccharides extracted by two kinds of extract methods was 1 mg/mL, the antioxidant effect of UNCP4 was slightly stronger than that of HNCP3, but not significantly.
Inhibition rates of α-amylase and α-glucosidase
HNCP3 and UNCP4 could inhibit the activities of α-amylase and α-glucosidase, which were involved in glucose metabolism by reducing the cleavage of carbohydrate glycosidic bonds and releasing glucose. As shown in Fig. 6A, the inhibition rate of acarbose on α-glucosidase reached the maximum of 86.42 ± 1.59% when the concentration of samples was 3.0 mg/ml, and the highest inhibition rates of HNCP3 and UNCP4 were also obtained with the value of 60.03 ± 1.58% and 79.01 ± 1.41%, respectively. Meanwhile, the inhibitory activities of HNCP3 and UNCP4 on α-glucosidase were showed in Fig. 6B, where the inhibition ratio presented the dose-dependent relationship. The highest inhibition rates of HNCP3 and UNCP4 on α-amylase were 57.76 ± 1.88% and 77.72 ± 2.03%, respectively. The results showed that HNCP3 and UNCP4 could prevent the metabolism of carbohydrates in food and effectively reduce postprandial blood glucose levels in the body59.
In fact, it has been found that many extracts from plants and fruits possessed inhibitory effects on α-glucosidase with minimal side effects60. Although HNCP3 and UNCP4 had lower inhibitory effects than the drug acarbose, the results indicated they could be the potential inhibitors of α-glucosidase. In this study, the difference in the inhibitory activities of HNCP3 and UNCP4 might be due to the fact that ultrasound-assisted extraction could extract and/or preserved more bioactive compounds with anti-hyperglycemia activity in a short time under medium temperature conditions61.
Nostoc commune is rich in protein, calcium, phosphorus, iron and other nutrients, and has been used as an ingredient for a long time for human consumption, with the effect of lowering fat, clearing heat and brightening eyes, etc. The biological activity of polysaccharide, the important active ingredient in Nostoc commune, is not clear. In recent years, with the vigorous development of antioxidants, it has gradually developed from simple synthetic antioxidant to natural, green, efficient, low-toxicity free radical scavenger in vivo obtained from natural products. Diabetes is one of the three most persistent diseases threatening human health, with a mortality rate second only to cardiovascular diseases and cancer. The development of therapeutic drugs for diabetes has been a hot research topic. Many types of polysaccharides have been found to have certain antidiabetic activity in natural product components. Therefore, in this study, Nostoc commune polysaccharides extracted by hot water extraction and ultrasonic-assisted extraction were purified and structurally characterized. Their antioxidant and anti-diabetic activities were also investigated exploratively. The results showed that HNCP3 and UNCP4 have certain activities of scavenging free radicals.
α-amylase and α-glucosidase are important enzymes in the digestion of carbohydrates in food. α-amylase inhibitors and α-glucosidase inhibitors can inhibit the activity of α-amylase and α-glucosidase, slow down the conversion and absorption of glucose, reduce the postprandial blood glucose peak, and adjust the blood glucose level, thus reducing the stimulation of blood glucose to the pancreas, improving the sensitivity of insulin, protecting the function of the pancreas, and effectively preventing and improving the occurrence and development of diabetes. In this study, the in vitro inhibitory activities of polysaccharides extracted by both methods against α-amylase and α-glucosidase were examined, and it was clear that both HNCP3 and UNCP4 have certain antidiabetic effects.
Conclusions
In the study, the average molecular weight of HNCP3 was greater than the average molecular weight of UNCP4. Infrared spectroscopy indicated the conformational characteristics of UNCP4 and HNCP3, that is, the two polysaccharides had similar characteristic absorption peaks (O–H stretching vibration, C–H stretching vibration, C–H variable angular vibration and C–O bond) and different peak transmittances. AFM and SEM images showed molecular aggregation and polysaccharide conformation. HNCP3 was loose and presented a soft fibrous texture, while UNCP4 was dry and had a few branches on the surface. The monosaccharide component assay showed that the monosaccharide composition of HNCP3 and UNCP4 differed only in the molar ratio. The comparison of antioxidant activities of the two polysaccharides indicated that UNCP4 had strong antioxidant and hypoglycemic activities. In summary, Ultrasonic-assisted extraction had a certain effect on the structure and solution conformation of NCP, especially the effect on solution properties and chain conformation could affect the biological activity of polysaccharides. Therefore, it is of great significance to systematically study the effect of ultrasound on the advanced structure of traditional Chinese medicine polysaccharides.
Materials and methods
Materials
Nostoc commune is a plant belonging to the genus Nostocaceae, Cyanobacteria, Nostocaceae. Morphologically, it is initially gelatinous and spherical, and later expands into lamellae, up to 10 cm, like a gelatinous cortex, dark olive or tea-brown, dark brown or black after drying. The algal filaments are curled, and only at the perimeter of the group there are obvious gelatinous sheaths, yellowish brown, thick and laminar, and constricted at the transverse septum. The air-dried fruiting bodies of N. commune were obtained from Gansu Kangxinyuan Ecological Agriculture Technology Development Co., Ltd (Lanzhou, Gansu Province, China. The production of raw materials shall refer to the food safety enterprise standard Q/SXZN0001S-2019 Ground Soft. Plants (either cultivated or wild), including the collection of plant material, complied with relevant institutional, national, and international guidelines and legislation.), powdered into fine powder of 0.178 mm by a high-speed pulverizer (FZ102, Beijing Zhongxing Weiye Instrument Co., Ltd., Beijing, China), and stored at room temperature until used. Monosaccharide standards (i.e., rhamnose, arabinose, galactose, glucose, xylose, mannose, fucose, glucuronic acid) α-amylase were purchased from Solarbio Science & Technology Co., Ltd. (Beijing, China), 1,1-diphenyl-2-picrylhydrazyl (DPPH), Acarbose solution, p-nitrophenol glucopyranoside (PNPG) and α-glucosidase were purchased from Sigma-Aldrich (USA). All other reagents were of analytical grade.
Heated reflux extraction
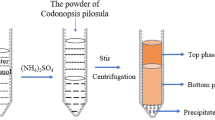
HRE was performed based on the previously reported method with some modifications62. The powder of N. commune (5 g) was reflux extracted with deionized water (250 mL) in a flat-bottom flask at 85 °C for 190 min, and the extract was treated three times by Savage method (Chloroform: butyl alcohol in 4: 1 ratio) to remove protein. The deproteinizing solution was precipitated (4 °C for 12 h) by the addition of anhydrous ethanol to a final concentration of 80% (V/V), and the precipitate was lyophilized by vacuum freeze dryer (SCIENIZ-18 N, Ningbo Biotechnology Co., Ltd., Ningbo, China) to obtain crude polysaccharide of N. commune (HNCP).
Ultrasonic-assisted extraction
The detailed UAE procedure of crude polysaccharides (UNCP) was carried out as our described previously63. UNCP was extracted under the optimal extraction condition at solid–liquid ratio of 1: 50, solvent of anhydrous ethanol, extraction temperature of 353.15 K, ultrasonic power of 540 W, extraction time of 25 min, and then were treated according to the description in “Monosaccharide compositions analysis”section.
Purification of crude polysaccharide and determination of average molecular weights
200 mg of the HNCP and UNCP were prepared into a 10 mg/mL solution, and loaded into DEAE-52 anion-exchange chromatography column (2.6 × 45 cm), respectively, and eluted with 0–1.0 mol/L stepwise NaCl gradient at the flow rate of 1 mL/min. The HNCP3 and UNCP4 fractions were collected with 0.3 mol/L NaCl solution, dialysed (MWCO: 8–14 kDa) and lyophilized with the yield of 24.5 mg. The freeze-dried sample were further purified by Sephadex G100 with the deionized water at the flow rate of 1 mL/min for further study. The molecular weight of HNCP3 and UNCP4 were determined by high-performance liquid chromatography coupled with multi-angle laser light scattering as we previously described.
Modification of chemical and physical properties of periodate-oxidized polysaccharides
As described in previous report, the monosaccharide compositions of HNCP3 and UNCP4 were achieved by gas chromatography (GC)63, the detailed procedure was according to the reported methods with some modification. 30 mg of HNCP3 and UNCP4 was mixed with the 30 mL of sodium periodate solution (15 mmol/L), and the mixtures were sufficiently dissolved with a magnetic stirrer in a dark room at 4 °C. 1.0 mL of mixed solution was pipetted into the ampoule, placed into 250 mL volumetric flask and diluted to volume with deionized water. The absorbance of the solution at 223 nm was measured by ultraviolet spectrophotometer at intervals of 6 h until the absorbance value remained constant. The consumption of periodate was calculated with the sodium periodate calibration. 2.0 mL of the reaction solution was added 0.5 mL of ethylene glycol to terminate the reaction and was titrated by NaOH standard solution (0.01 mol/L) with the indicator of phenolphthalein. The volumes of titrant were recorded and the amount of formic acid was calculated according to the following formula 1.
Note y is formic acid production, x is accurate concentration of NaOH, n is volume for titration, v is reaction volume.
The GC chromatographic conditions were as follows: ThermoITQ1100 Gas Chromatograph; FID detector; HP-5 flexible quartz capillary column (0.32 mm × 0.25 μm × 30 m); N2 as carrier gas; 1 mL/min as flow rate; 1: 10 as split ratio; 220 °C as inlet temperature; 250 °C as detector temperature; programmed ramp-up (160 °C as the starting temperature and ramped up to 240 °C at 10 °C/min).
The HPLC chromatographic conditions were as follows: the column was TSK-GELG6000PWXL; the mobile phase was 0.2% sodium iterate aqueous solution, the flow rate was 1 mL/min, the column temperature was 30 °C, the detector was a differential refractive index detector, and the injection volume was 20 μL.
Smith degradation and methylation analysis of HNCP3 and UNCP4
The above periodic acid oxidation reaction solution terminated by ethylene glycol was transferred to a dialysis bag (3500 Da) and dialyzed with the distilled water for 48 h. 100 mg of sodium borohydride was added and mixed in the dark for 24 h, then the solution was further neutralized with 50% acetic acid until the excess sodium borohydride was decomposed. The neutralized solution was dialyzed for 48 h, and dried by freeze drying. The dried sample (10 mg) was hydrolyzed with 2 mL of trifluoroacetic acid (2 mol/L) at 120 °C for 3 h, and repetitive evaporated with methanol to remove the excess trifluoroacetic acid. The residue was treated with the mixture of 0.5 mL of pyridine and 10 mg of hydroxylamine hydrochloride at 95 °C for 30 min, and then acetylated with 0.5 mL of acetic anhydride at 95 °C for 35 min. The acetylate was blow-dried with nitrogen, dissolve with 1 mL of chloroform, washed twice with distilled water. 1 mL of chloroform layer solution was used to GC determination under the analytical conditions we establised64. 5 mg of the monosaccharide standard, glycerin and erythritol were taken respectively and performed GC analysis according to the method described above.
Methylations of HNCP3 and UNCP4 were conducted to analyze the linkage patterns of polysaccharide
In detail, 10 mg of HNCP3 and UNCP4 were dissolved in 1.5 mL of DMSO (1.5 mL), then 1.5 mL of NaOH (50%)-DMSO solution (V:V = 1:1) was added and reacted at 30 °C for 3 h. Methyliodide (0.5 mL) was added and stirred at 30 °C for 2.5 h in dark place under N2 protection, and the reaction was terminated with 1 mL of deionized water. The above operation step was repeated three times until the absorption peak of OH group disappeared substantially. The methylated HNCP3 and UNCP4 were further hydrolyzed with H2SO4 (2 mol/L) at 120 °C for 2 h and dried by rotary evaporation under the protection of nitrogen. The rotary-dried sample was dissolved with NaOH solution, and shaken with the adding of 25 mg of sodium borohydride at 25 °C for 2 h, the pH was adjusted to 5.5–7.0 with acetic acid for removing excess sodium borohydride.
Subsequently, the reduced samples were acetylated by mixing with 0.7 mL of pyridine and 1 mL of acetic anhydride at 90 °C for 30 min. The dried reaction product was dissolved in ethyl acetate and then analyzed by a GLC-MS system (THERMO 1310 GC-ISQ LT MS, USA) apparatus equipped with a TG-200MS capillary column (30 mm × 0.25 mm, 0.25 µm). The specific program was as follows: from 160 to 210 °C, the temperature was raised at a rate of 2 °C/min, then increased to 240 °C at a rate of 5 °C/min, and finally kept for 20 min. MS scan range was set at m/z 35–4000.
FT-IR spectroscopy analysis and TGA/DSC measurement
FT-IR spectrophotometer (Nicolet iS5, Thermo Fisher Scientific, USA) was used to determine the organic functional groups of HNCP3 and UNCP4 in the scanning range of 4000–400 cm−1 with a resolution of 4 cm−165. HNCP3 and UNCP4 were placed in Al2O3 crucible, and the temperature was raised to 350 °C at a rate of 10 °C /min with the shielding gas of nitrogen on TGA/DSC simultaneous thermal analyzer (STA449C, Netzsch, Germany)66. The denaturation temperature was calculated using the Origin 9.0 data analysis software.
Molecular surface morphology observation of HNCP3 and UNCP4
In order to investigate the effect of different extraction methods on the microstructure of polysaccharides, HNCP3 and UNCP4 were fixed on gold-coated silicon wafers for and observed by scanning electron microscope (SEM, JSM-5600LV, American Kevex Company, America). Meanwhile, 50 µL of HNCP3 and UNCP4 (1.0 µg/mL) were dropped to mica substrate and dried at room temperature overnight. Then the conformational transformations of HNCP3 and UNCP4 macromolecules were studied by AFM (MultiMode-HR, Brooke, USA)67.
In vitro antioxidant activities determination
According to our previously described method65,68,69, HNCP3, UNCP4 and Vc solutions with different concentrations (0.2 mg/mL, 0.4 mg/mL, 0.6 mg/mL, 0.8 mg/mL, 1.0 mg/mL) were prepared to evaluate their antioxidant activities in terms of DPPH radical scavenging ability, superoxide anion scavenging ability and hydroxyl radical scavenging ability. To be specific, 1 mL of crude polysaccharides of HNCP3 and UNCP4 with concentrations of 0.2 mg/mL, 0.4 mg/mL, 0.6 mg/mL, 0.8 mg/mL and 1.0 mg/mL were added into the test tubes. 5 mL of 5 mmol/L DPPH solution was then added, shaken well, and placed in the dark for 30 min. The mixture of 5 mL absolute ethanol and 3 mL distilled water was used as the reference, and the absorbance value at 517 nm was measured. The DPPH scavenging activity was calculated by formula 2.
Note A0 is the absorbance of the blank control solution with distilled water instead of sample solution; Ai is absorbance of sample solution; Aj is Absorbance of sample solution with distilled water instead of chromogenic agent.
1 mL of crude polysaccharides of HNCP3 and UNCP4 with concentrations of 0.2 mg/mL, 0.4 mg/mL, 0.6 mg/mL, 0.8 mg/mL, 1.0 mg/mL and 3 mL of Tris–HCl buffer with a pH of 8.2 were added to the test tubes. The reaction was carried out in a water bath at 25 °C for 20 min. After adding 0.3 mL of 7 mmol/L pyrogallol and reacting for 4 min, 1 mL of 10 mol/L HCl was added, and the absorbance was measured at 420 nm. The superoxide anion scavenging ability was calculated by formula 2.
2 mL of crude polysaccharides of HNCP3 and UNCP4 with concentrations of 0.2 mg/mL, 0.4 mg/mL, 0.6 mg/mL, 0.8 mg/mL, 1.0 mg/mL, 1 mL of 9 mmol/L FeSO4 and 2 mL of 9 mmol/L salicylic acid–ethanol solution were added to the test tubes. Then 2 mL of 8.8 mmol/L H2O2 was added and reacted at room temperature for 1 h. The absorbance value of the samples was measured at 510 nm by zeroing with distilled water. The hydroxyl radical scavenging ability was calculated by formula 2.
In vitro antidiabetic activity
The in vitro antidiabetic ability of HNCP3, UNCP4 was assessed by their inhibitory potential against α-amylase and α-glucosidase that could metabolize carbohydrate67. The sample concentration (IC50) for the ability to inhibit the enzyme by 50% was also calculated. Specifically, HNCP3 and UNCP4 were dissolved in phosphate buffer (0.2 mo1/L, pH 6.6) to concentrations of 1 mg/mL, 2 mg/mL, 3 mg/mL, 4 mg/mL, 5 mg/mL, 6 mg/mL, respectively. 0.2 mL of α-amylase solution (6 U/mL) was added into the sample solutions of different concentrations, followed by 0.4 mL of starch solution (1%), and the reaction was performed at 37 °C for 10 min. 2 mL of DNS reagents were sequentially added and reacted in boiling water batch for 10 min, the absorbance was measured at 520 nm as described in earlier reports70. The inhibition rate of α-amylase was calculated according to Formula 3.
Note A0 is the blank control absorbance, A1 is the absorbance of the polysaccharide sample, and A2 is the absorbance of the base tube.
The α-glucosidase inhibitory activity was determined by measuring the release of p-nitrophenol at 405 nm in a 96-well plate. The reaction mixture containing 20 µl of different concentrations of samples, 10 µl of α-glucosidase (2 U/ml), 50 µl of phosphate buffer (pH 6.8, 100 mM) was incubated at 37 °C for 15 min. Then, 20 μl of p-nitrophenol glucopyranoside (PNPG) (5 mM) was added and incubated at the same temperature for 20 min. The reaction was stopped by adding of 150 μl of sodium carbonate (0.1 M). Acarbose was used as standard. The inhibitory rate was calculated according to formula 4.
A0 is the blank absorbance, A1 is the absorbance of the polysaccharide sample, A2 is the blank absorbance of the sample, and A3 is the absorbance of the control.
Statistical analyses
The data were expressed as mean ± SD and examined for their statistical significance of difference with variance (ANOVA) and T tests (and nonparametric tests) by GraphPadPrism5. P values less than 0.05 were considered to be statistically significant.
Data availability
The original contributions presented in the study are included in the article and Supplementary Material, further inquiries can be directed to the corresponding author.
Abbreviations
- ANOVA:
-
Analysis of variance
- NCP:
-
Nostoc commune polysaccharide
- HRE:
-
Heating reflux extraction
- MAE:
-
Microwave-assisted extraction
- UAE:
-
Ultrasonic-assisted extraction
- TG:
-
Thermogravimetric spectrum
- DSC:
-
Differential scanning calorimetry
- Vc:
-
Vitamin C
- HNCP3:
-
Nostoc commune polysaccharide 3 obtained by heated reflux extraction
- UNCP4:
-
Nostoc commune polysaccharide 4 obtained by ultrasonic-assisted extraction
- DPPH:
-
1,1-Diphenyl-2-picrylhydrazyl
- PNPG:
-
P-nitrophenol glucopyranoside
- GC:
-
Gas chromatography
- FT-IR:
-
Fourier transform infrared spectroscopy
- IC50 :
-
Half maximal inhibitory concentration
- DMSO:
-
Dimethyl sulfoxide
- GC-MS:
-
Gas liquid chromatography mass spectrometry
- TGA:
-
Thermogravimetric analyzer
- MWCO:
-
Molecular weight cut-off
- DEAE-52:
-
Diethylaminoethyl-52
- SEM:
-
Scanning electron microscope
- AFM:
-
Atomic force microscope
- HRSA:
-
Hydroxyl radicals scavenging activity
References
Wright, D., Prickett, T., Helm, R. F. & Potts, M. Form species Nostoc commune (Cyanobacteria). Int. J. Syst. Evol. Microbiol. 51(5), 1839–1852 (2001).
Krizova, L., Maixnerova, M. & Sedo, O. International journal of systematic and evolutionary microbiology. Soc. Gen. Microbiol. 51, 1839–1852 (2015).
Ambrosio, R. et al. 5-Promises and challenges for expanding the use of N2-fixing cyanobacteria as a fertilizer for sustainable agriculture. Cyanobacterial Lifestyle Appl. Biotechnol. 2022, 99–158. https://doi.org/10.1016/B978-0-323-90634-0.00002-0 (2022).
Rasmussen, H. E. et al. Lipid Extract of Nostoc commune var. sphaeroides Kutzing, a Blue-Green Alga, inhibits the activation of sterol regulatory element binding proteins in HepG2 cells. J. Nutr. 138(3), 476–481 (2008).
Masayuki, N. I., Hitoshi, S. A., Yuji, Y. A., Hiroyuki, T. A. & Mamoru, K. O. Antioxidative activity and chemical constituents of edible terrestrial alga Nostoc commune Vauch. Biosci. Biotech. Biochem. 75(11), 2175–2177 (2011).
Gómez-Espinoza, O., Núñez-Montero, K., Díaz, L. B. in The Pharmacological Potential of Cyanobacteria (ed. Lopes, D., Silva, M., Vasconcelos, V.) 145–172. https://doi.org/10.1016/B978-0-12-821491-6.00006-5 (2022).
Itoh, T. et al. Reduced scytonemin isolated from Nostoc commune induces autophagic cell death in human T-lymphoid cell line Jurkat cells. Food Chem. Toxicol. 60, 76–82 (2013).
Sousa, M. L., Preto, M., Vasconcelos, V., Linder, S. & Urbatzka, R. Antiproliferative effects of the natural oxadiazine Nocuolin A are associated with impairment of mitochondrial oxidative phosphorylation. Front. Oncol. 9, 224. https://doi.org/10.3389/fonc.2019.00224 (2019).
Kaewmaneesuk, J., Ariyadet, C., Thirabunyanon, M., Jaturonglumlert, S. & Daengprok, W. Influence of led red-light intensity on phycocyanin accumulation in the cyanobacterium Nostoc Commune Vaucher. J. Fundam. Appl. Sci. 10(3S), 457–467. https://doi.org/10.4314/jfas.v10i3s.39 (2018).
Yamaguchi, Y., Naito, M., Nishio, E., Tomita, Y. & Takenaka, H. The anti-infectious activity of edible blue-green algae, Nostoc flagelliforme, Nostoc commune, and Aphanotece sacrum on Listeria monocytogenes in mice. Algal Resour. (Japanese) 1, 61–62 (2009).
Li, Z. Y. & Guo, M. Healthy efficacy of Nostoc commune Vaucher. Oncotarget 9(18), 14669–14679. https://doi.org/10.18632/oncotarget.23620 (2018).
Guo, M. et al. Migration suppression of small cell lung cancer by polysaccharides from Nostoc commune Vauch. J. Agric. Food Chem. 64(32), 6277 (2016).
Itoh, T. et al. Antimicrobial and anti-inflammatory properties of nostocionone isolated from Nostoc commune Vauch and its derivatives against Propionibacterium acnes. Anaerobe 27, 56–63 (2014).
Mishra, A., Tandon, R., Kesarwani, S., Singh, R. & Tiwari, G. L. Emerging applications of cyanobacterial ultraviolet protecting compound scytonemin. J. Appl. Phycol. 27(3), 1045–1051 (2015).
Wang, H. B., Wu, S. J. & Liu, D. Preparation of polysaccharides from cyanobacteria Nostoc commune and their antioxidant activities. Carbohydr. Polym. 99, 553–555 (2014).
Jensen, S. et al. Structural characterisation of a complex heteroglycan from the cyanobacterium Nostoc commune. Carbohydr. Polym. 91(1), 370–376 (2013).
Jaki, B. et al. Novel extracellular diterpenoids with biological activity from the cyanobacterium Nostoc commune. J. Nat. Prod. 63(3), 339–343 (2000).
Mohamed, M. F., Kuzuha, S., Takani, Y. & Sakamoto, T. Novel thermostable glycosidases in the extracellular matrix of the terrestrial cyanobacterium Nostoc commune. J. Gen. Appl. Microbiol. 54(5), 243–252 (2008).
Helm, R. F. et al. Structural characterization of the released polysaccharide of desiccation-tolerant Nostoc commune DRH-1. J. Bacteriol. 182(4), 974–982 (2000).
Tamaru, Y., Takani, Y., Yoshida, T. & Sakamoto, T. Crucial role of extracellular polysaccharides in desiccation and freezing tolerance in the terrestrial cyanobacterium Nostoc commune. Appl. Environ. Microb. 71(11), 7327–7333 (2005).
Yang, R. F., Zhao, C., Chen, X., Chan, S. W. & Wu, J. Y. Chemical properties and bioactivities of Goji (Lycium barbarum) polysaccharides extracted by different methods. J. Funct. Foods 17, 903–909 (2015).
Yan, Y. J. et al. Effect of extraction methods on property and bioactivity of water-soluble polysaccharides from Amomum villosum. Carbohydr. Polym. 117, 632–635 (2015).
Quan, Y. et al. Optimization for the extraction of polysaccharides from Nostoc commune and its antioxidant and antibacterial activities. J. Taiwan Inst. Chem. Eng. 52, 14–21 (2015).
Ebringerová, A. & Hromádková, Z. An overview on the application of ultrasound in extraction, separation and purification of plant polysaccharides. Cent. Eur. J. Chem. 8(2), 243–257 (2010).
Zhong, K., Lin, W., Wang, Q. & Zhou, S. Extraction and radicals scavenging activity of polysaccharides with microwave extraction from mung bean hulls. Int. J. Biol. Macromol. 51(4), 612–617 (2012).
Afshari, K., Samavati, V. & Shahidi, S. Ultrasonic-assisted extraction and in-vitro antioxidant activity of polysaccharide from Hibiscus leaf. Int. J. Biol. Macromol. 74, 558–567 (2015).
Chen, R. Z., Li, S. Z., Liu, C. M., Yang, S. M. & Li, X. L. Ultrasound complex enzymes assisted extraction and biochemical activities of polysaccharides from Epimedium leaves. Process Biochem. 47(12), 2040–2050 (2012).
Cheung, C. Y., Siu, K. C., Liu, Y. S. & Wu, J. Y. Molecular properties and antioxidant activities of polysaccharide protein complexes from selected mushrooms by ultrasound-assisted extraction. Process Biochem. 47(5), 892–895 (2012).
Chipiti, T., Ibrahim, M. A., Singh, M. & Islam, Md. S. In vitro α-amylase and α-glucosidase Inhibitory and cytotoxic activities of extracts from Cissus cornifolia planch parts. Pharmacogn. Mag. 13(Suppl 2), S329–S333 (2017).
Briones-Nagata, M. P., Martinez-Goss, M. R. & Hori, K. A comparison of the morpho-cytology and chemical composition of the two forms of the cyanobacterium, Nostoc commune Vauch. from the Philippines and Japan. J. Appl. Phycol. 19(6), 675–683 (2007).
Ogutu, F. O. Ultrasonic modification of selected polysaccharides-review. J. Food Process. Technol. 06(05) (2015).
Athanasios, C. K. et al. Comparison of distillation and ultrasound-assisted extraction methods for the isolation of sensitive aroma compounds from garlic (Allium sativum). Ultrason. Sonochem. 13(1), 54–60 (2006).
Liu, Y. F. et al. Structural characterization of a bioactive water-soluble heteropolysaccharide from Nostoc sphaeroids kütz. Carbohydr. Polym. 15(11), 552–559 (2018).
Tian, Y., Zheng, B., Chen, C. Ultrasound-assisted extraction, preliminary characterization, and antioxidant activity of a novel water-soluble polysaccharide from Lotus (Nelumbo Nucifera Gaertn.) Seeds. Sep Sci Technol. 47(16) (2012).
Ren, B. B. et al. Optimization of microwave-assisted extraction of Sargassum thunbergii polysaccharides and its antioxidant and hypoglycemic activities. Carbohydr. Polym. 173, 192–201. https://doi.org/10.1016/j.carbpol.2017.05.094 (2017).
Wang, X., Lan, L., Yuan, J. Y., Shi, W. Y. & Chu, W. H. Preliminary studies on antioxidant and anti-aging activity of three seaweed polysaccharides. Pharm. Biotechnol. 27(1), 29–32. https://doi.org/10.19526/j.cnki.1005-8915.20200106 (2020).
Wang, F. et al. Purification, structural characterization, and biological activities of degraded polysaccharides from Porphyrayezoensis. J. Food Biochem. 45(4), e13661. https://doi.org/10.1111/jfbc.13661 (2021).
Rout, D., Mondal, S., Chakraborty, I., Pramanik, M. & Islam, S. S. Chemical analysis of a new (1→3)-, (1→6)-branched glucan from an edible mushroom Pleurotus florida. Carbohydr. Res. 340(16), 2533–2539. https://doi.org/10.1016/j.carres.2005.08.006 (2005).
Wu, M., Wu, Y., Zhou, J. & Pan, Y. Structural characterisation of a water-soluble polysaccharide with high branches from the leaves of Taxus chinensis var. mairei. Food Chem. 113, 1020–1024. https://doi.org/10.1016/j.foodchem.2008.08.055 (2009).
Mandal, E. K. et al. Chemical analysis of an immunostimulating (1→4)-, (1→6)-branched glucan from an edible mushroom, Calocybe indica. Carbohydr. Res. 347, 172–177. https://doi.org/10.1016/j.carres.2011.10.040 (2012).
Cao, J. J. Structural characterization, physicochemical properties and hepatoprotective activity of polysaccharides from Toona sinensis leaves. Hefei University of Technology. https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD202001&filename=1019185726.nh (2019).
Nie, S. P. & Xie, M. Y. A review on the isolation and structure of tea polysaccharides and their bioactivities. Food Hydrocoll. 25(2), 144–149 (2011).
Wang, Z. M. et al. Structural characterisation and immunomodulatory property of anacidic polysaccharide from mycelial culture of Cordyceps sinensis fungus Cs-HK1. Food Chem. 125, 637–643 (2011).
Li, S., Hao, L. & Kang, Q. Purification, characterization and biological activities of a polysaccharide from Lepidium meyenii leaves. Int. J. Biol. Macromol. 2017, 103 (2017).
Guerrero, P., Kerry, J. P. & Caba, K. D. L. FTIR characterization of protein-polysaccharide interactions in extruded blends. Carbohydr. Polym. 111, 598–605 (2014).
Zhu, Z. Y., Liu, N. & Si, C. L. Structure and antitumor activity of a high-molecular weight polysaccharide from cultured mycelium of Cordyceps gunnii. Carbohydr. Polym. 88, 1072–1076 (2012).
Sardari, R. R., Kulcinskaja, E. & Ron, E. Y. Evaluation of the production of exopolysaccharides by two strains of the thermophilic bacterium Rhodothermus marinus. Carbohydr. Polym. 156, 1–8 (2017).
Ahmed, Z., Wang, Y. & Anjum, N. Characterization of new exopolysaccharides produced by coculturing of L. kefiranofaciens with yoghurt strains. Int. J. Biol. Macromol. 59, 377–383 (2013).
Yin, X., You, Q. & Jiang, Z. Optimization for ultrasonic-microwave synergistic extraction of polysaccharides from Cornus officinalis and characterization of polysaccharides. Int. J. Biol. Macromol. 83, 226–232 (2016).
Zhu, Z. Y., Pang, W. & Li, Y. Y. Effect of ultrasonic treatment on structure and antitumor activity of mycelial polysaccharides from Cordyceps gunnii. Carbohydr. Polym. 114, 12–20 (2014).
Wang, J. & Nie, S. Application of atomic force microscopy in microscopic analysis of polysaccharide. Trends Food Sci. Tech. 2, 02–05 (2018).
Zou, C. et al. Preparation of lacquer polysaccharide sulfates and their antioxidant activity in vitro. Carbohydr. Polym. 73, 322–331 (2008).
Meng, L., Sun, S. & Li, R. Antioxidant activity of polysaccharides produced by Hirsutella sp. and relation with their chemical characteristics. Carbohydr. Polym. 117, 452–457 (2015).
Hu, C., Zhang, Y. & Kitts, D. D. Evaluation of antioxidant and prooxidant activities of bamboo phyllostachys nigra Var. Henonis leaf extract in vitro. J. Agric. Food Chem. 48(8), 3170–3176 (2000).
Zhang, W., Huang, J. & Wang, W. Extraction, purification, characterization and antioxidant activities of polysaccharides from Cistanche tubulosa. Int. J. Biol. Macromol. 93, 448–458 (2016).
Song, C. G. Advances in antioxidant activity of plant polysaccharides. Chin. Fruit Veg. 4, 25–33. https://doi.org/10.19590/j.cnki.1008-1038.2022.04.005 (2022).
Yang, J. T. et al. Structure-activity relationship of polysaccharides from Pleurotus citrinopileatus based on different extraction methods. J. Fungal Res. 20(03), 190–197. https://doi.org/10.13341/j.jfr.2021.1433 (2022).
Ni, L. J., Wang, Y. Y., He, W. Y. & Zhang, L. G. Monosaccharide composition, activity and their correlation analysis in eight polysaccharides. J. Tianjin Univ. 47(4), 326–330. https://doi.org/10.11784/tdxbz201207047 (2014).
Zheng, Q., Ren, D. Y., Yang, N. N. & Yang, X. B. Optimization for ultrasound-assisted extraction of polysaccharides with chemical composition and antioxidant activity from the Artemisia sphaerocephala Krasch seeds. Int. J. Biol. Macromol. 91, 856–866 (2016).
McCue, P., Kwon, Y. I. & Shetty, K. Anti-diabetic and anti-hypertensive potential of sprouted and solid-state bioprocessed soybean. Asia Pacifific J. Clin. Nutr. 14(2), 145–152 (2005).
Liu, C. W., Wang, Y. C., Lu, H. C. & Chiang, W. D. Optimization of ultrasound-assisted extraction conditions for total phenols with anti-hyperglycemic activity from Psidium guajava leaves. Process Biochem. 49(10), 1601–1605 (2014).
Dong, H. M. et al. Effect of extraction methods on the properties and antioxidant activities of chuanminshen violaceum polysaccharides. Int. J. Biol. Macromol. 93, 179–185 (2016).
Wang, Y. G. et al. Extraction, purification, and bioactivities analyses of polysaccharides from Glycyrrhiza uralensis. Int. J. Biol. Macromol. 15(7), 172–183 (2019).
Wang, Y. G. et al. Kinetic modeling of the ultrasonic-assisted extraction of polysaccharide from Nostoc commune and physicochemical properties analysis. Int. J. Biol. Macromol. 128(1), 421–428 (2019).
Lu, Y. et al. Structural characteristics and anticancer/antioxidant activities of a novel polysaccharide from Trichoderma kanganensis. Carbohydr. Polym. 205, 63–71 (2018).
Emeje, M. et al. Extraction and physicochemical characterization of a new polysaccharide obtained from the fresh fruits of Abelmoschus Esculentus. Iran J. Pharm. Res. 10(2), 237–246 (2011).
Liu, W. et al. Preparation, characterization, and α-glycosidase inhibition activity of a carboxymethylated polysaccharide from the residue of Sarcandra glabra (Thunb.) Nakai. Int. J. Biol. Macromol. 99, 454–464 (2017).
Xie, Y. et al. Two phenolic antioxidants in Suoyang enhance viability of ·OH-damaged mesenchymal stem cells: Comparison and mechanistic chemistry. Chem. Cent. J. 25, 84. https://doi.org/10.1186/s13065-017-0313-1 (2017).
Ye, D. Y. et al. Optimized extraction of polysaccharides from Grateloupia livida (Harv.) yamada and biological activities. Molecules 20, 16817–16832. https://doi.org/10.3390/molecules200916817 (2015).
Wongsa, P., Chaiwarit, J. & Anis, Z. In vitro screening of phenolic compounds, potential inhibition against α-amylase and α-glucosidase of culinary herbs in Thailand. Food Chem. 131(3), 964–971 (2012).
Acknowledgements
This work was financially supported by Research on prevention and control technology of important epidemic diseases of yaks—Study on new preparations of Tibetan veterinary drugs (No. XZ202101ZD0002N-05), Agricultural Science and Technology Innovation Program of Chinese Academy of Agricultural Sciences (ASTIP, CAAS-LMY-03), and Gansu Longyuan Youth Innovation and Entrepreneurship Talent Team Project (No. 2022lLQTD38). We are grateful to the Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences for their technical support to determine the structure of NCP.
Author information
Authors and Affiliations
Contributions
X.W., B.H. and S.W. conceived and designed the study. X.W., Z.Y. and B.H. drafted the manuscript. Z.Y., X.W., C.L. and C.W. preformed the antioxidant and antidiabetic activity in vitro; Y.L., H.Z. and R.S. helped to analyze the structure of NCP; B.H. and S.W. are the corresponding authors of this article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, X., Yang, Z., Liu, Y. et al. Structural characteristic of polysaccharide isolated from Nostoc commune, and their potential as radical scavenging and antidiabetic activities. Sci Rep 12, 22155 (2022). https://doi.org/10.1038/s41598-022-26802-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-26802-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.