Abstract
The valorization of new polymer sources from underutilized plants as structuring, encapsulating, and texturizing agents for food and nutraceutical applications is gaining attention. This provides an opportunity where inexpensive plant-sourced biopolymers can play an impactful role, on both ecological and economic aspects performing equivalently effectual yet cost-effective substitutes to synthetic polymers. With this aim, we explored the use of mucilage from Althea rosea and reveal its physicochemical, in vitro antidiabetic and antihypertensive activity. Besides, structural, micrometric, crystallization, and anti-microbial properties was also seen. We determined the probable structure of the extracted mucilage by FTIR which confirmed the residues of saccharides as galactose and uronic acid with α and β configurations. It consists of 78.26% carbohydrates, 3.51% ashes, and 3.72% proteins. Here, we show that the mucilage offered protection to DNA against the oxidative damage caused by (-OH) radicals and the morphology of the mucilage particles displayed a fibrillary material settled in a net-like, tangled structure. Our results demonstrate that the reconstituted mucilage powder exhibited good water holding capacity (2.89 g water/g mucilage), solubility (27.33%), and oil holding capacity (1.79 g oil/g mucilage). Moreover, high emulsifying property (95.83%) and foaming capacity (17.04%) was noted. Our results indicate that A.rosea mucilage can potentially serve as economical and eco-friendly hydrocolloid substitute for the food and nutraceutical industry owing to its functional, hypo-lipidemic, anti-hyperglycemic, antioxidant, and anti-bacterial properties.
Similar content being viewed by others
Nowadays, due to the hazardous effect of synthetic polymers on human health, people showed major interest in sustainable, eco-green, polymeric carbohydrates of inherent technological (texturizing, structuring, stabilizing) and functional potential is in the progress. Moreover, the hydrocolloid market remains a highly active industry domain experiencing a progressive demand for technologically resilient, novel, and sustainably produced ingredients1. Amongst the entire carbohydrate polymers, mucilage has been comprehensively exploited in the food and nutraceutical industries as a result of its diverse health (angiotensin-converting enzyme inhibition, immunity stimulation, anticancer activities) and food properties2. All therapeutic areas including diabetes, osteoporosis, blood pressure, cholesterol, sleeping disorders, and anti-arthritic have been covered by nutraceuticals3. Structurally, plant mucilage mostly comprises carbohydrates with the monomer units of galacturonic acid, L-arabinose, L-rhamnose, D-galactose, and D-xylose4. Besides, these contain abounding bioactive compounds (alkaloids, phenolics, steroids, tannins) and glycoproteins5. It exhibits higher antioxidant capacities as compared to its polysaccharide equivalents including pectin and xanthan gum6. Moreover, several researchers7,8,9,10 revealed the applications of mucilage in enhancing the shelf-life of baked products, food emulsions, and powders. Similarly, Jannasari et al.11 improved the thermal stability of vitamin A and vitamin D by using mucilage from cress seed as the encapsulating material. Additionally, these possess antimicrobial properties and thus can be used in food preservation applications. These developments in mucilage research have revealed that there is a requisite for more food-grade mucilages, and thus, offers an opportunity where the valorization of new biopolymer from underutilized plants can play an impactful role as structuring, encapsulating, and texturizing agents for food and nutraceutical applications, serving as equally efficacious yet cost-effective alternatives to synthetic polymers1.
Several natural polymers have been investigated in the literature as a potential source of plant mucilage8. Generally, former studies have focused widely on chia seed, psyllium, and flaxseed mucilage9. By contrast, the mucilage derived from the medicinal plant, Althea rosea has not been studied in any detail till now for the reason that the consumers do not have copious scientific data regarding its health-promoting effects. It is a perennial garden plant, locally named “saze posh” with various biological activities including cytotoxic, anti-urolithiatic, immunomodulatory, anticancer, anti-inflammatory, and, antiulcer activities10. Almost all parts of the plant contain water-soluble acidic bioactive polysaccharides11. We have explored the detailed information about the plant so that it will assist us in better understanding its techno-functional properties together with its nutraceutical potential. In addition to its low cost, the use of A.rosea mucilage entails numerous practical benefits over the synthetic excipients; nonirritant nature, biodegradable, and biocompatible12. It can be effortlessly eluted from the plant via a simple extraction procedure, requiring no harmful chemicals or solvents for its synthesis13. Below, we report the experimental characterization of the extraction process, functional, structural, morphological, antimicrobial, antioxidant, and nutraceutical properties of mucilage extracted from A.rosea. In this study, we hypothesized that the mucilage will act as a new source of hydrocolloids making it an exciting constituent in applications such as bioactive compound carrier, coating agents, film‑forming, gelling agent, and a safe functional food ingredient. Moreover, finding novel uses of A.rosea mucilage has the potential to add value to the plant and help us expand the utilization of this underutilized plant.
Results and discussions
Polysaccharide extraction, characterization, and yield analysis of extracted mucilage
We extracted the water-soluble polysaccharide from A.rosea by soaking the plant powder in the solution of sodium hydroxide (pH ~ 11.7) at 75 °C (25 min). The average yield of dried and powdered A.rosea mucilage obtained after extraction was 17 ± 0.1% (Table 1). Consistent results with this finding have been reported14,15. A substantial increase in the yield of mucilage was observed with an increase in pH due to the separation of the acidic groups such as uronic acids, and due to the attraction between the negatively charged ones increasing the solubility of the mucilage. Moreover, alkaline conditions could increase yield by hydrolyzing insoluble constituents into soluble ones16. Moreover, there is an increase in cell membrane permeability with the increase in temperature, leading to the release of the mucilage from the mucilage canal cells. Besides, a decrease in viscosity of the mucilage takes place at the higher temperature, making the mucilage less sticky and thus released efficiently5.
The principal constituent of the A.rosea, mucilage amounting to about 60% by weight, is considered to be arabinoxylan, of high molar mass (≥ 106 g/mol) and the rest essentially consists of an acidic fraction having several negatively charged rhamnogalacturonans17. The extracted mucilage is discreetly viscoelastic owing to these dispersed long-chain polysaccharides. The amalgam obtained after filtration consists of dissolved constituents, with the gel-like fractions and low molar mass solutes below (≤ 150 kDa).
Identification and chemical analysis of the isolated mucilage
The identification of isolated mucilage was confirmed by reaction with ruthenium red and Molisch’s reagent (Table 2).
The total proteins and ash content of the isolated A.rosea mucilage were found to be 3.72 ± 0.2% and 3.51 ± 0.1% respectively. While the total carbohydrate content of the mucilage was 78.26 ± 0.3% (w/v). These values are consistent with previous studies conducted on other mucilages 14. The ash content of A.rosea mucilage was found to be higher than commercially available gums including xanthan gum (1.5%) and Arabic gum (1.2%)18. The pH of A.rosea mucilage was found proximate to neutral (pH = 6.65) at 27 °C, implying that it is less likely to irritate the gastrointestinal and the mucous membrane once orally ingested, therefore it is suitable for various formulations that preferably remain stable at this pH. The result is in agreement with the reports of Busia19 conducted on Althea mucilage, exploited as a medicine for cough, as it is well-known for treating inflammation and irritation of mucous membranes.
The values for acidic and neutral fractions of the A.rosea mucilage expressed as mg of D-galacturonic acid and D-xylose/mg of the mucilage respectively were found to be 0.58 g/g for the acidic fraction and 0.87 g/g for the neutral fraction. These outcomes corroborate with the previous studies20.
Phytochemical investigation
Qualitative confirmation tests were employed for the phytochemical investigation of the isolated A.rosea mucilage (Table 2). The mucilage was found to be a rich source of glycosides, tannins, saponins, and steroids. Tannins act as antioxidants. These are also reported to have antibacterial properties21. Glycosides possess cardiac activities and are used in treating cardiac arrhythmia, and congestive heart failure22. Saponins are likely to demonstrate antibacterial, antidiabetic, and anticancer activities. The presence of all these phytochemicals might contribute to the therapeutic potential of A.rosea mucilage.
Micrometric characterization
The micrometric characterization consists of flow properties including density (bulk density, tapped density), Hausner’s ratio, and Carr’s index or % compressibility index (Table 3). Powders having Carr’s index within the range of 5 to 15% are considered to have good compressibility properties23. The powder having less Carr’s index determines the lower inter-particle interaction (lower cohesiveness) thereby reflecting good flow properties. Likewise, Hausner’s ratio < 1.25 depict better flow properties24. Similar results have been reported by Husain et al.25 in the Althea mucilage obtained from roots. As all the calculated values are within the acceptable ranges, it can be stated that A.rosea mucilage has a notable flow property and could have a potential application in the food and nutraceutical industry.
Color and water activity
A.rosea mucilage exhibited a darker yellow-brown color that may perhaps be related to the polyphenols and natural pigments present in A.rosea (Table 3) (Fig. 1a). Browning is proposed to be due to oxidative stress following high-temperature exposure. A.rosea possess high total phenolic content26, and oxidation of phenolic compounds possibly will result in a deep brown color of the mucilage. Furthermore, extraction temperature-induced browning in the color of the polysaccharide/mucilage. At high temperatures, the reducing sugar concentration increases, and the Maillard reaction is activated, which may cause cell membrane oxidative stress accompanied by browning. Moreover, there is substantiation that once greenly plants are heated; there is a loss of magnesium along with the loss of the phytol side chain of chlorophyllide, resulting in the formation of the deep brown colored product27. Other reasons for the dark color might be due to the browning reaction that occurred during oven drying and may be due to the breakage of cellular membranes which would increase enzyme–substrate contact with the consequent increase in tissue browning. Furthermore, cellulose and pigments remained in the mucilage solution even after centrifugation which led to the darkening of mucilage. Moreover, the precipitation process with ethanol dissolves part of the chlorophyll present in the plant, allowing producing a depigmented mucilage part of the chlorophyll present in the plant28.
The water activity (aw) value of the obtained mucilage powder was 0.438 ± 0.1, which is below the critical aw (0.6) to prevent microbial growth in various food systems29 thereby signifying the relative stability and less vulnerability to spoilage.
Particle size
The particle size is the significant parameter that influences the hydration rate, sedimentation, emulsifying, and dissolution features in the hydrocolloids30. Smaller particles lead to the larger scattering ability of the light thereby resulting in better gloss. The particle size of polysaccharides is important regarding dissolution, hydration, and emulsifying ability31. In the present study, aqueous mucilage dispersions (1% w/v) presented particles with an average diameter between about 90 to 160 nm (Fig. 1b). These values were consistent with those reported by Kaewmanee32 while Vignesh14 observed substantial particle size in the mucilage particles. The variances in the size of the particles among the solutions of the polysaccharide could be linked through the various processes including grinding and drying methods applied for obtaining the material.
X-ray diffraction (XRD) characterization
XRD of the sample was performed to study the semi-crystalline, crystalline or amorphous structure of the isolated mucilage. From the spectra obtained through XRD, it was deduced that the mucilage powder showed the presence of weak peaks depicting the crystalline form which is characteristic of calcium oxalates (calcium salts), usually found in flowering plants mucilage of Malvaceae family33 (Fig. 1c). Regarding the XRD, our results concord with the findings of Tomás Jesús34. This outcome is quite appealing as A.rosea mucilage shows the presence of minerals such as Ca+2, which would have bioavailability for the human body. Moreover, Ilango35 identified numerous halos with a weak peak in the polysaccharide mucilage, confirming the crystalline nature.
Analysis of functional groups
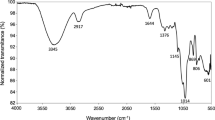
FTIR analysis determines the functional groups of various compounds based on the peak value. The band of functional groups found in the mucilage of A.rosea is characteristic of proteins and polysaccharides (Fig. 2). The mucilage showed characteristic and sharp peaks at 3420 (O–H stretching vibrations), 2931(C–H stretching vibrations), and 1623 cm−1 (–COO− asymmetric vibrations) which corresponds to the molecules of carbohydrate and uronic acid. It was reported by Yekta36 that bands at 3400–3100 cm−1 correspond to the carbohydrate (O–H) bonds and peaks at 1621, 1597, and 1421 cm−1 to deprotonated carboxylic acid groups (–COO−) in the flower gum of A.rosea. Comparable bands are perceived at 1623, 1582, and 1431 cm−1 in our spectrum. The bands present at 1431–1264 cm−1 indicate deformation vibrations of O–H and stretching of C–O. Peaks at 1085 and 1045 cm−1 specify the existence of monosaccharides including glucose and mannose37. The band at 2917.1 cm−1 appears to be C–H stretching of the methyl linked with the aromatic ring system. The thick band at 3267.3 cm−1 indicates hydroxyl groups (intra- and inter-molecular), constituting the gross polymeric structure. In addition, the presence of different peaks at 1417 to 1733 cm−1 (stretching of C=C and C=O), 1068 to 1256 cm−1 (stretching of –C–O and –C–O–C) can be ascribed to the occurrence of carboxylic compounds, flavonoid and phenolic, as reported previously by38.
Scanning electron microscopy
The morphological analysis of the A.rosea mucilage demonstrated a wide distribution of particle size with irregular morphology (Fig. 3). The mucilage showed characteristically loose, tangled, and densely-ordered fibers with a thick ‘scab ‘-like structure. The visible component consists of the thin, extended fibrils interconnected in a tangled, and irregular manner. Moreover, shorter, linear, and/or branched chains were spread between them providing the net-like arrangement of the components. According to Kreitschitza39, the chains covering their surface and cross-linking them may be considered hemicelluloses (Fig. 3a). However, in some places, it was irregularly distributed so that the surface was visible. Besides, at low magnification, the images of the mucilage revealed that the surface of particles was rough aggregates of irregular shape, and size. Moreover, it is likely to detect that large particles aggregate with the smaller particles, which might be attributable to the electrostatic charge among the particles (Fig. 3b). Our study was comparable to the literature data34,39.
Functional properties
Solubility
Solubility acts as an essential factor in the analysis of the performance of the hydrocolloids in the dispersion systems as other functional properties are affected by this property40. Water solubility determines the strength of hydrogen bond interactions amid the water and polysaccharide driven by various hydrophilic groups of the polymer41. Table 4 displays the solubility of mucilage particles. Results show that solubility of the mucilage increased with increased temperature which might be due to the reduction of particle aggregation on heating, thereby leading to diffusion of water to the bulk of the granules42.
Water holding capacity (WHC) and oil holding capacity (OHC)
WHC is an essential parameter in foods in terms of yield, stability, sensory evaluation, and texture42. Table 4 exhibits the WHC and OHC of mucilage. WHC of the mucilage increased with increased temperature. On increasing the temperature, the mobility of the molecules increases which promotes water absorption, thus enhancing the amount of water grasped by the sample40. Similar results have been reported previously43.
One of the important functional properties of a hydrocolloid is the oil holding capacity44. It represents the oil absorption from nonpolar positions inside the molecules44. The OHC of the mucilage increased with increased temperature. This may be explained by the fact that on increasing the temperature, the accessibility of the nonpolar chains rises leading to increased mobility of molecules thereby binding the hydrocarbon components of oil42. Moreover, the size of the pore also influences the OHC. The size of the pore increases on increasing the temperature, thus permitting more entrapment of fat45. OHC of the mucilage was similar to those of xanthan and guar gum with the value of 2–7 g oil/g44. Thus, the results show that A.rosea mucilage might play a significant part in food processing, as fat acts on flavor retainers in addition to the surge in the mouth feel of foods40.
Swelling index (SI)
The swelling index denotes the degree of granular hydration. An increase in the swelling index suggests the feeble binding forces between the granules. Values for the swelling index of mucilage at various temperature conditions (20, 35, and 50 °C) and pH (2, 6, and 8) are presented in Table 4. Results indicate that swelling capacity accelerates on an increase in temperature and pH. An increase in the temperature results in weaker binding interactions between the mucilage molecules resulting in the enlargement of mucilage chains thus allowing higher entrapment of water. Moreover, the increase in swelling index with the increase of pH can be explained by the electrostatic repulsion of the functional groups thereby causing intensification in the available area for the water retention46. The results obtained are similar to that of Italian flax (SI value from 8.0 to 21.0 g/g) and psyllium polysaccharide (SI value from 4.0 to 19.0 g/g)32.
Foaming capacity and foam stability
Different factors are responsible for the foaming capacity and the stability of the foam including molecular weight, protein, structure, and carbohydrate content in the hydrocolloid47. Due to the flexible structure of the mucilage, there is a reduction in the surface tension that leads to better foaming properties. As seen in Table 5, the foaming capacity of mucilage is concentration-dependent, being significantly greater at 1.2% solution (foaming capacity of 17.04%) than at 0.3% solution (foaming capacity of 11.77%). Moreover, the increase in the stability of the foam also improved. The outcome is probably attributed to the increased amount of mucilage that is transported to the interface, forming the viscoelastic films thereby improving the formation of foam. Results were similar to those reported previously48. Furthermore, Rezaei49 also reported a similar trend in the foaming capacity and foam stability of the almond gum.
Emulsifying ability (EA) and stability (ES)
To obtain the preferred characteristics in food products, the formation of a stable emulsion by a hydrocolloid is essential. According to Table 5, with the increase in mucilage/oil ratio, an increment in the emulsifying ability of the mucilage is seen. Similar values have also been stated in other mucilages50. Emulsifying properties of polysaccharide mucilage are owing to their surface activity. The presence of various hydrophobic components including acetyl and methyl groups together with the uronic acid and proteinaceous portion contributes to the emulsifying properties of these polysaccharides. These surface actives adsorb the fine droplets, thereby reducing the tension at the interface, and inhibiting the recoalescence of the newly formed droplets. Moreover, increasing the fraction of mucilage leads to a subsequent surge in the branched chains amid the surface actives to hold the molecules of oil thereby lowering the surface tension. On the other hand, the results from the table demonstrated that the emulsion stability decreased on increasing the mass of the mucilage powder which might be ascribed to the diminution of the surface tension in the dried mucilage. However, the values of emulsion stability are higher than the results of Jouki51 and comparable to the results of Jindal52, signifying that A.rosea mucilage exhibit good emulsifying property.
Nutraceutical properties
Antioxidant activity against oxidative DNA damage
A.rosea mucilage was assessed for its DNA damage protective ability where the DNA damaging reagent consists of the Fenton reagent, which is responsible for oxidative stress and DNA damage. The isolated mucilage from A.rosea protects DNA against oxidative damage induced by the Fenton reagent (Fig. 4). Hydroxyl radicals are generated during the Fenton’s reaction inducing the DNA damage by splitting the deoxyribose, consequently unwinding DNA and making it run fast. The DNA band occurs clearly in Lane-1. However, it disappears in Lane-2. In Lane-2, H2O2 led to the cleavage of the DNA strand in the presence of ferric nitrite and ascorbic acid. Meanwhile, the DNA band began to reappear upon treatment with A.rosea mucilage (10, 20, 40 & 80 mg/ml) in Lane 3–6, with a pronounced appearance in Lane 6, which contains the highest concentration (80 mg/mL). It is proposed that the chelating ability of mucilages could be owing to various functional groups (–COOH, –OH, –C=O, and –SH)53. Thus, the antioxidant ability of the mucilage could be owing to the presence of –OH groups in the chain of polysaccharides. Moreover, A.rosea mucilage may inhibit the reaction of Fe2+ with hydrogen peroxide in the Fenton reagent, or it may donate the electron or hydrogen atom to inhibit the hydroxyl radicals54.
Antioxidant activity against oxidative damage to DNA formucilage, Lane 1: Calfthymus DNA. Lane 2: (DNA + Fenton’s reaction), Lane3: (DNA + Fenton’s reaction + 20µL of extract). Lane 4: (DNA + Fenton’s reaction + 30µL of extract). Lane 5: (DNA + Fenton’s reaction + 50µL of extract). Lane6: (DNA + Fenton’s reaction + 100µL of extract). Lane 7:(DNA + H2O2). (Fentons reaction = ferricnitrate + H2O2 + ascorbic acid).
In vitro anti-diabetic activity
The therapeutic approach for controlling postprandial hyperglycemia involves the inhibition of α-amylase and α-glucosidase55. The present finding shows that A.rosea mucilage effectively inhibits both α-amylase and α-glucosidase enzymes (Fig. 5a, b). The inhibitory activity of the A.rosea mucilage at a concentration of 40, 80, 120, 160, and 200 µg/ml was carried out with respect to the standard drug, acarbose. The mucilage showed α-amylase inhibition activity of 75 ± 1.4% and α-glucosidase inhibition of 81 ± 1.2% at the highest concentration of 200 µg /ml. However, at the lowest concentration (40 µg/ml), α-amylase and α-glucosidase inhibition of 35 ± 1.2% and 44 ± 1.5% was observed respectively. An earlier report showed that mucilage showed a dose-dependent inhibitory effect on α-amylase and α-glucosidase activity56. As compared to crude mucilage, acarbose exhibited high α-amylase inhibitory activity, but then low α-glucosidase inhibitory action. Our results were in agreement with the earlier studies that have shown that phenolic phytochemicals result in lower α-amylase inhibitory action, whereas α-glucosidase possesses stronger inhibitory potential57. The mucilage controls the postprandial serum glucose level generally by three mechanisms: (1) A.rosea mucilage has good swelling and water holding capacity, therefore it leads to viscosity intensification of the small intestine content thereby hindering the glucose diffusion. (2) the availability of glucose in the small intestine is decreased due to the binding of glucose with the mucilage. (3) A.rosea mucilage delays the release of glucose owing to the presence of fiber-associated total polyphenols. Fibers enable a slower absorption of glucose in the gastrointestinal tract58 and the fermentation products of dietary fiber (propionate, acetate, and butyrate) are also responsible for the amelioration of diabetic status. Moreover, literature studies have indicated that phytochemicals including phenols, terpenes, flavonoids, and alkaloids, are also responsible for antidiabetic activity. Interestingly, it has been shown that Althea rosea is rich in phytochemicals which are responsible for its potential antidiabetic activity26. The presumed mechanism of action could be owing to an insulin-mimetic effect on the peripheral tissues either by stimulation of the regeneration process or release of pancreatic secretion of insulin from existing β-cells59. Thus A.rosea mucilage acts as a natural enzyme inhibitor anticipated to provide an attractive therapeutic approach for the treatment of postprandial hyperglycemia mitigating the side effects of synthetic drugs (miglitol, metformin, and acarbose).
In vitro antihypertensive activity
Angiotensin-Converting Enzyme (ACE) acts as an important target clinically and nutritionally in the treatment of hypertension. The inhibition of ACE for the management of hypertension by dietary anti-hypertensive agents is a promising strategy. However, adverse side effects of synthetic ACE inhibitors are known, and plant molecules may offer a natural and cost-effective alternative for ACE inhibitors. The findings of the ACE inhibitory activity of A.rosea mucilage are illustrated in Fig. 6. The results signified that the activity of the mucilage was concentration-dependent, with values increasing with the increase in concentrations with the highest ACE inhibitory activity (68 ± 1.5%) observed at 1.2 µg/mL of mucilage. Similar results have been reported previously45,60. Plant mucilages exhibit the ability to lower blood pressure by improving endothelial dysfunction, and reducing inflammation in the vasculature61.The results are in agreement with Narasimhacharya61. The authors suggested that the inhibitory activity is owing to the presence of polyphenols and flavonoids linked with the mucilage. The foremost mechanism consists of inhibition of the synthesis of hepatic-3-hydroxy-3-methylglutaryl-Coenzyme-A which regulates the cholesterol synthesis62. Thus, the results are evidence of the potential of the natural product as an inexpensive and safe approach for cardioprotection/hypertension.
In-vitro antimicrobial activity
Mucilage showed activity against both the gram-positive and gram-negative bacteria studied with the stronger anti-microbial activity against E. coli (inhibition zone, around 12 mm) than for B.subtilis (inhibition zone, 7 mm) (Fig. 7). Even though slightly active, the mucilage can be used as a natural antimicrobial agent in foods, obstructing the proliferation and growth of microorganisms. The exhibited antibacterial property of the mucilage can be attributed to the presence of bioactive compounds such as tannins, saponins, alkaloids, phenols, and flavonoids63,64 together with the presence of antimicrobial components such as uronic acids65 and monosaccharides (glucose, arabinose, xylose or mannose)66. Moreover, mucilage can attach to the bacterial cell wall through several interactions in the cell membrane; thereby reducing the viability of the bacterial cell by inhibiting DNA replication.
Summary and outlook
Today, there is much interest in the use of natural ingredients in the food industry. In this study, we have provided a more complete understanding of the composition and morphology of mucilage extract from A.rosea (saze posh) which grows in the Himalayan region of India and stands out for its health benefits and functional characteristics. The study outlines useful information regarding functional, structural, antioxidant, antimicrobial, and nutraceutical characteristics of the extracted mucilage. We have shown that the isolated bio polysaccharide can be exploited as an alternative hydrocolloid for food and nutraceutical industry applications. Our results show that the isolated mucilage has potential as a functional food ingredient owing to its anti-hypertensive, anti-diabetic, antioxidant, and anti-bacterial effects. We hypothesize that A.rosea mucilage is a very promising system for the natural antimicrobial packaging and this property of the mucilage can make it an important ingredient in many food formulations not only for their ability to enhance the bioavailability of the bioactive compounds but also for their thickening, gelling, stabilizing, and film-forming properties. Moreover, finding novel uses of A.rosea mucilage has the potential to add value to the plant and help us expand the utilization of this underutilized plant.
With further advances in this direction, we believe that by profiling the functional and nutraceutical content of the mucilage polysaccharide, several benefits could be achieved: (1) It could amend the food systems and increase their quality rheologically (2) fermentable fibers together with other phytonutrients derived from the plant will possibly amend a varied range of inflammatory- microbiome besides oxidative stress-associated disorders.
Materials and methods
Plant material
Althea rosea plant used in this study was collected from the University of Kashmir, Srinagar, India. Experimental research and field studies on the plant, including the collection of plant material complied with the institutional guidelines. Moreover, for the collection of our plant, a license was not required, as it is abundantly found in the region. The specimen was identified and authenticated by Akhter H. Malik, curator, Centre for Biodiversity and Taxonomy, University of Kashmir and voucher specimen was deposited in the herbarium (Voucher specimen no. 3736- KASH). The plant was dried in the dark without humidity for about 7 days. After complete drying, the obtained material was powdered using a commercial blender (John Morris Scientific, Chatswood, NSW, Australia).
Polysaccharide extraction, characterization, and yield analysis of extracted mucilage
The solution of aqueous mucilage was prepared by macerating plant powder with distilled water (75 °C), at the ratio of 1:10 w/v (plant powder: water), subsequently, 0.1 M sodium hydroxide (pH ~ 11.7) was added followed by stirring with 19 kHz ultrasonic probe (QSONICA/Q700, USA) for 25 min. The extraction method was selected as per the method reported by Naji-Tabasi67. Sequentially, 80% ethanol was added to the supernatant and kept at 5 °C (2 h). The clear solution of mucilage was separated from the slurry by filtering through a cotton mesh cloth, (grade 90). The gravimetric evaluations of mucilage components were achieved by weighing the dry solid after lyophilization and the gel-like brown fibrous fraction was isolated by centrifuging (Hitachi, CR 21GIII, Japan) the aqueous mucilage extract at RCF = 9500 × g for 25 min. The high molar mass polysaccharide fraction was separated from the supernatant using a 150 kDa MWCO filter.
Yield (%) was determined following Archana, et al.68
In Eq. (1), w1 is the weight of mucilage obtained and w2 is the weight of powdered plant taken.
Identification and chemical analysis
The presence of mucilage in the extracted material was tested by performing Molisch's and ruthenium red test69. Proximate analysis of mucilage including total proteins, total polysaccharide, ash content, and pH was evaluated based on standard methods of AOAC: 981.10, 985.29, 923.03, respectively70. For the estimation of neutral sugar, the method reported by Monsigny71 was used. In brief, 1% (w/v) solution of mucilage was treated with 4 mg/ml of resorcinol together with 70% H2SO4. The suspension was heated (85 °C) with vigorous shaking (25 min) and consequently placed under dark conditions for 20 min in the cold-water bath. The absorbance was detected at 480 nm and compared to a standard solution of D-xylose. The results were expressed as mg of D-xylose /mg mucilage. The acidic sugar present in the mucilage powder was determined following the method of Melton and Smith72. The extracted mucilage (1%) was hydrolyzed in H2SO4 (0.5 ml). After centrifugation (1500 g, 15 min), potassium sulfamate (4 M, pH = 1.6) and sodium tetraborate (75 mM) were added to the supernatant and incubated in a shaking water bath (80 °C, 25 min) under dark conditions for 15 min. Subsequently, a solution of m-hydroxydiphenyl was added, the contents were vortex-mixed and the absorbance was measured at 525 nm, using D-galacturonic acid as standard. The results were expressed as mg of D-galacturonic acid/mg mucilage powder.
Phytochemical investigation
The mucilage extracts were subjected to phytochemical qualitative reactions for usual plant secondary metabolites as described by the method reported by Deore73. The extract was tested with specific reagents for the presence of starch, alkaloids, saponins, tannins, steroids, and glycosides. The formation of precipitate or color intensity was used as an analytical response to these tests.
Test for Starch (Iodine test). Add a few drops of iodine solution to the mucilage. The appearance of purple color indicates the presence of starch.
Test for Tannins (Ferric chloride test). Approximately 8 drops of FeCl3 were mixed with 3 ml of the mucilage extract. The presence of tannins was indicated by the appearance of bluish or greenish-black residue.
Test for Alkaloids (Wagner’s test and Mayer’s test). Mucilage extract was stirred with HCl and 2 drops of Mayer’s reagent. (Potassium mercuric iodide solution).The formation of red residue indicated alkaloids in the sample.
Test for Steroid (Libermann-Burchard’s test). Chloroform (2 ml), acetic anhydride (3 ml) together with H2SO4 was stirred with 2 ml of mucilage extract. The appearance of red, followed by the blue color of the solution indicated steroids in the extract.
Test for Glycoside (Legal’s and Borntrager’s test). Mucilage extract was treated with 1 ml of 5% H2SO4. The mixture was boiled in a water bath and then filtered. The filtrate was then shaken with an equal volume of chloroform. Then a lower layer of chloroform was shaken with dilute ammonia. The presence of rose pink to red color indicates glycosides.
Test for Saponins (Froth test). Mucilage extract was boiled in water for about 5 min followed by filtration. The obtained filtrate was shaken briskly. The presence of steady froth for about 60 min specifies the existence of saponins.
Micrometric characterization
The powder flowability of mucilage powder including bulk density, tapped density, Hausner’s ratio, and Carr’s or compressibility index was analyzed as previously described74.
Bulk Density (BD) Mucilage powder (15 g) was poured into a graduated measuring glass cylinder and the volume of the powder was recorded. BD was calculated as the ratio of the weight of the mucilage powder (g) to the volume occupied by the powder (ml).
Tapped Density (TD) Mucilage powder (15 ml) was transferred into a graduated measuring cylinder and tapped. The volume of the mucilage powder was noted, and TD was calculated as the ratio of the mass of the mucilage powder (g) to the tapped volume of mucilage powder (ml).
Hausner’s ratio and Carr’s index were calculated as:
where ρoT = Tapped density; ρoB = Bulk density.
Color and Water Activity The color was evaluated through a colorimeter (Minolta CR-400, Japan) following the CIE (Commission Internationale de l'Eclairage) Hunter Colour Lab (Mini Scan XE Plus, 45/0-L, Reston, USA). It expresses L*, a*, and b* values as color’s lightness, red/green intensity, and yellow/blue intensity respectively.
Water activity (aw) of A.rosea mucilage powder was estimated at 25 °C ± 0.3 °C using a water activity meter (Rotronic, HygroPalm AW1, Switzerland).
Particle size The powdered mucilage (1% w/v) was dispersed in distilled water and the distribution of particle size was estimated using a particle analyzer (Malvern Instruments).
X-ray diffraction (XRD) A diffractometer (Siemens model D5000, Germany) was used for recording the XRD patterns of the sample at an angular range of 10–90° (2θ) employing a Bragg Brentano geometry besides the monochromatic Cu Kα radiation (λ = 1.4529 Å), at 25 kV, 2 s/0.004°, 25 rpm, and 35 mA75.
Analysis of functional groups The functional groups of A.rosea mucilage were recorded using FTIR (Shimadzu FTIR 8400) characterized in the transmittance range of 4000–500 cm−1.
Scanning electron microscopy The morphology of the mucilage particle was attained by using a scanning electron microscope (Hitachi Ion Sputter E-1010, HITACHI) at a voltage of 15 kV. The sample was mounted on the aluminum stubs and coated with 40 nm gold.
Functional properties
Solubility The dried mucilage powder of A.rosea (1.5 g) was immersed in water (20 mL) at altered temperatures (20, 35, and 50 °C) for 45 min with constant stirring at 650 rpm and centrifuged (Hettich Universal 32, Germany) for 20 min at 600 × g. The resultant supernatant was dried in a convection oven at 80 °C for 12 h42. The solubility was expressed as:
where, wD = weight of dried supernatant, wS = weight of the sample.
Water holding capacity and oil holding capacity Water holding capacity (WHC) and oil holding capacity (OHC) were recorded following Ghribi et al.45 under different temperature conditions (20, 35, and 50 °C). The mucilage powder (1 g) was stirred for about 40 min at the prescribed temperature (20, 35, and 50 °C) using an agitation water bath (Techne 12/TE-10 D, UK). The suspensions were then centrifuged at 600 × g for 20 min. Supernatants were decanted, and swollen samples were reweighed. The WHC was expressed as:
1% mucilage powder with corn oil (w/v) was vortexed for 40 min at 20, 35, and 50 °C and centrifuged at 2000 rpm for 20 min. The swollen granules were weighed after removing the supernatant. OHC was expressed as:
Swelling index (SI) The modified method of Archana et al.68 was used for the determination of the swelling index of mucilage at varying temperatures (20, 35, and 50 °C), and pH of 2, 6, and 8. About 70 mg of the mucilage powder was immersed in 100 ml of water. After 24 h, the swollen mass was removed, slightly dried, and reweighed. The swelling index (SI) was calculated as follows:
where, ws is the weight of swollen sample and wd is the weight of the dried sample.
Foaming capacity and foam stability
The procedure of Rezaei et al.49 with minor modifications was used to estimate the Foaming capacity (FC) and foam stability (FS). Dispersion of mucilage sample at various concentrations was made (0.3, 0.6, 0.9, and 1.2% (w/v) followed by whipping using a homogenizer (Ultra Turrax, USA) at 5000 rpm for 10 min. Closely after (~ 25 s) of whipping, foaming capacity was calculated as:
FS was calculated after 20 min as the volume of the foam as:
Emulsifying ability and Emulsifying stability
The emulsion ability (EA) of the mucilage was adapted from Jindal52 with some modifications. The emulsion of the mucilage suspensions at various concentrations (0.3, 0.6, 0.9, 1.2%) with oil (15 ml) was prepared by homogenizing (Ultra Turrax, USA) the suspensions for 5 min followed by centrifugation (Hettich Universal 32, Germany) of the emulsion at 900 r.p.m for 15 min.
A method similar to EA was used to determine the Emulsion Stability (ES) of the mucilage powder. After homogenizing, the emulsions were heated at 80 °C for 30 min and subsequently centrifuged at 600 r.p.m for 20 min and the emulsified layer was noted. The EA and ES were expressed as:
Nutraceutical properties
Antioxidant activity of A.rosea mucilage against oxidative damage to DNA using submarine electrophoresis
Hydroxyl radicals generated by the Fenton reaction were used to induce oxidative damage to DNA was determined as described by Shah et al.76 with some modifications. The reaction mixture consists of DNA of calf thymus in phosphate buffer saline (pH = 7.4). The aliquots of the sample were taken and incubated with DNA for 15 min at room temperature. The oxidation of DNA was initiated with ferric nitrate (15 m M), H2O2 (2.8 mM), and ascorbic acid (50 mM). After incubating the reaction mixture (37 °C, 1 h), the reaction was terminated by adding the loading buffer consisting of 30% glycerol together with 0.25% bromophenol blue followed by subjecting the reaction mixture to gel electrophoresis (1% agarose/TAE buffer) at 100 V. DNA was then visualized by Gel Doc (THE LIFE SCIENCES, Vikas Marg, Delhi, India).
In-vitro anti-diabetic activity
α-amylase inhibition activity
The inhibition assay for α-amylase was determined by the method of Akkarachiyasit77 with some modifications. α-amylase was premixed with the extracts at various concentrations (40- 200 µg/ml) and starch as a substrate was added (0.5%) to start the reaction at 37 °C for 5 min. Furthermore, 20 μl of DNS (3,5-dinitrosalicylic acid) reagent was added to terminate the reaction. The reaction mixture was incubated for 15 min at 100 °C and diluted with 10 ml of distilled water. The absorbance was detected at 540 nm and the results were expressed in mg of AE (Acarbose equivalent) per gram of sample.
where, Ac = absorbance of the control (sample was replaced by sodium phosphate buffer).
As = absorbance of sample.
α-glucosidase inhibition activity
α-glucosidase (50 μl) was pre-incubated with a sample (40–200 µg/ml). at 37 °C for 5 min. pNPG (p-Nitrophenyl- β-D-Glucopyranoside) was used as a substrate and the reaction mixture was incubated for 30 min (37 °C) and stopped by adding Na2CO3. The formation of p-nitrophenol from pNPG was detected at 405 nm. The hydrolysis of pNPG without an inhibitor was used as a negative control (Girish et al.78). Results were expressed in mg of AE (Acarbose equivalent) per gram of sample. The inhibition was calculated as follows (%):
where Ag is the absorbance with α-glucosidase and sample, and As is the absorbance without α-glucosidase but with the sample, Ah is the absorbance with α-glucosidase but without sample, Ao is the absorbance without α-glucosidase and sample.
Antihypertensive activity
Antihypertensive activity was measured by Angiotensin-converting enzyme (ACE) inhibitory activity60. Briefly, a volume of sample solution (70 μl) containing different concentrations (0.3–1.2 mg/ml) of mucilage was mixed with 150 μl of Hippuryl-histidyl-leucine (HHL, 5 mM) and pre-incubated at 37 °C for 10 min). Consequently, the reaction was initiated by adding 15 μL of ACE (0.1 U/ml). After incubation for 30 min at 37 ℃, the enzymatic reaction was ceased by the addition of 200 μL HCl (1 M). The formed hippuric acid (HA) was subjected to evaporation in the rotatory evaporator at 80 ℃ for 15 min. The residue was diluted in 3 ml of distilled water and the absorbance was measured at 228 nm. The amount of inhibition was calculated as:
where B = absorbance of HA in the presence of ACE inhibitor, C = absorbance of HA without ACE inhibitors (0.1 M borate buffer pH 8.3 was used instead of mucilage), D = absorbance of HA without ACE (corresponding to HHL autolysis in the course of the enzymatic assay).
In-vitro antimicrobial activity
The antimicrobial activity of the mucilage powder was estimated by the agar disc diffusion method79. Microorganisms with the expansion potential in food were selected: Gram-positive (Bacillus subtilis, ATCC 25,923) and the other being Gram-negative (Escherichia coli, ATCC 25,922), attained from the Fiocruz, (Oswaldo Cruz Foundation) Rio de Janeiro. Briefly, each culture (0.2 ml) with a cell density of 105–107 CFU/ml was inoculated on the nutrient agar. Sterile discs of 10 mm were kept on agar plates and permeated with 40 μl of the sample. As a positive control, gentamycin discs were used. The plates were incubated for 24 h (37 °C) and the extent of microbial inhibition was expressed as the zone of inhibition (diameter in mm).
Statistical analysis
Data were analyzed for mean ± standard deviation, two-way ANOVA through a statistical software package (SPSS10.1, USA). To find the significant differences between means, the obtained data were compared using Duncan’s multiple range tests at p < 0.05 significance level.
Human and animal rights
No animal/human samples are used for the present study.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Freitas, T. K. F. S. et al. Optimization of coagulation-flocculation process for treatment of industrial textile wastewater using okra (A. esculentus) mucilage as natural coagulant. Ind. Crop. Prod. 76, 538–544 (2015).
Ameri, A. et al. Medicinal plants contain mucilage used in traditional Persian medicine (TPM). Pharm. Biol. 53, 615–623 (2015).
Ahmad, F. et al. Nutraceutical is the need of hour. World J. Pharm. Pharm. Sci. 2, 2516–2525 (2013).
Beikzadeh, S., Khezerlou, A., Jafari, S. M., Pilevar, Z. & Mortazavian, A. M. Seed Mucilages as the functional ingredients for biodegradable films and edible coatings in the food industry. Adv. Colloid Interface Sci. 280, 102164 (2020).
Gebresamuel, N. & Gebre-Mariam, T. Comparative physico-chemical characterization of the mucilages of two cactus pears (Opuntia Spp.) obtained from Mekelle, Northern Ethiopia. J. Biomater. Nanobiotechnol. 3, 79–86 (2012).
Wu, Y., Hui, D., Eskin, N. A. M. & Cui, S. W. Water-soluble yellow mustard mucilage: A novel ingredient with potent antioxidant properties. Int. J. Biol. Macromol. 91, 710–715. https://doi.org/10.1016/j.ijbiomac.2016.05.088 (2016).
Basiri, S., Haidary, N., Shekarforoush, S. S. & Niakousari, M. Flaxseed mucilage: A natural stabilizer in stirred yogurt. Carbohyd. Polym. 187, 59–65 (2018).
Salgado-Cruz, M. D. L. P., Ramírez-Miranda, M., Díaz-Ramírez, M., Alamilla-Beltran, L. & Calderón-Domínguez, G. Microstructural characterisation and glycemic index evaluation of pita bread enriched with chia mucilage. Food Hydrocoll. 69, 141–149 (2017).
Felisberto, M. H. F. et al. Use of chia (Salvia hispanica L.) mucilage gel to reduce fat in pound cakes. LWT Food Sci. Technol. 63(2), 1049–1055 (2015).
Campos, B. E., Ruivo, T. D., da Silva Scapim, M. R., Madrona, G. S. & Bergamasco, R. D. Optimization of the mucilage extraction process from chia seeds and application in ice cream as a stabilizer and emulsifier. LWT-Food Sci. Technol. 1(5), 874–883 (2016).
Jannasari, N., Fathi, M., Moshtaghian, S. J. & Abbaspourrad, A. Microencapsulation of vitamin D using gelatin and cress seed mucilage: Production, characterization and in vivo study. Int. J. Biol. Macromol. 129, 972–979. https://doi.org/10.1016/j.ijbiomac.2019.02.096 (2019).
Chawla, P. et al. Gum arabic capped copper nanoparticles: Synthesis, characterization, and applications. Int. J. Biol. Macromol. 146, 232–242 (2020).
Pakravan, M., Abedinzadeh, H. & Safaeepur, J. Comparative studies of mucilage cells in different organs in some species of Malva, Althaea and Alcea. Pak. J. Biol. Sci. 10(15), 2603–2605. https://doi.org/10.3923/pjbs.2007.2603.2605 (2007) (PMID: 19070140).
Vignesh, R. M. & Nair, B. R. Extraction and characterization of mucilage from the leaves of hibiscus rosa-sinensis Linn (MALVACEAE). Int. J. Pharm. Sci. Res. 9(7), 2883–2890 (2018).
Prabakaran, L., Murthy, V. S. & Karpakavalli, M. Extraction and characterization of Hibiscus rosa-sinensis leaves mucilage for Pharmaceutical applications. RGUHS J. Pharm. Sci. 1(3), 221–225 (2011).
Karazhiyan, H., Razavi, S. Y. M. & Philips, G. O. Extraction optimization of a hydrocolloid extract from cress seed (Lepidium sativum) using response surface methodology. Food Hydrocoll. 25, 915–920. https://doi.org/10.1016/j.foodhyd.2010.08.022 (2010).
Atkhamova, S. K. et al. Rhamnoglucouronan from Alcea rosea stems. Chem. Nat. Compd. 37, 203-207. https://doi.org/10.1023/A:1012559102398 (2001).
Samavati, V., Lorestani, M. & Joolazadeh, S. Identification and characterization of hydrocolloid from Cordia myxa leaf. Int. J. Biol. Macromol. 65, 215–221 (2014).
Busia, K. Fundamentals of herbal medicine: Major plant families, analytical methods, materia medica 1st edn. (Xlibris Corporation, 2016).
Qian, K. Y., Cui, S. W., Wu, Y. & Goff, H. D. Flaxseed gum from flaxseed hulls: Extraction, fractionation, and characterization. Food Hydrocoll. 28(2), 275–283 (2012).
Atanassova, M., Georgieva, S. & Ivancheva, K. Total phenolic and total flavonoids contents, antioxidant capacity and biological contaminants in medicinal herbs. J. Univ. Chem. Technol. Metal. 46, 81–88 (2011).
Doss, A., Parivuguna, V., Vijayasanthi, M. & Surendran, S. Antibacterial evaluation and phytochemical analysis of Medicago sativa against some microbial pathogens. Indian J. Sci. Technol. 4, 550–552 (2011).
Javed, S., Kohli, K. & Ahsan, W. Incorporation of METHOCEL E6 PLV by a novel foam granulation technique for dissolution augmentation of the poor soluble silymarin. J. Pharm. Res. 10(2), 79–89 (2016).
Sinko, P. J. (ed.) Martin’s physical pharmacy and pharmaceutical sciences: Physical, chemical and biopharmaceutical principles in the pharmaceutical sciences 406–429 (Lippincort Williams and Wilkins, 2011).
Husain, M., Wadud, A., Sofi, G., Perveen, S. & Hafeez, K. A. Physicochemical standardization of mucilage obtained from Althaea officinalis Linn – Root. Phcog. Mag. 15, S155-61 (2019).
Dar, P. A., Ali, F., Sheikh, I. A., Ganie, S. A. & Dar, T. A. Amelioration of hyperglycaemia and modulation of antioxidant status by Alcea rosea seeds in alloxan-induced diabetic rats. Pharm. Biol. 55(1), 1849–1855. https://doi.org/10.1080/13880209.2017.1333127 (2017).
Hong, N. T., & Ibrahim, N. H. Extraction and characterization of mucilage from leaves of Pereskia bleo (ROSE CACTUS). Jurnal Teknologi dan Industri Pangan 23(2), 210–216 (2013). Doi:https://doi.org/10.6066/jtip.2012.23.2.210.
Farahnaky, A. et al. Ultrasound-assisted isolation of mucilaginous hydrocolloids from Salvia macrosiphon seeds and studying their functional properties. Innov. Food Sci. Emerg. Technol. 20, 182–190 (2013).
Reid, D. S. Water Activity: Fundamentals and Relationships. In Water act. foods fundam. appl 1st edn (eds Barbosa-Cánovas, G. V. et al.) 15–28 (Blackwell Publishing, Iowa, 2007).
Hubbe, M. & Gill, R. Fillers for papermaking: A review of their properties, usage practices, and their mechanistic role. Mater. Sci. https://doi.org/10.15376/BIORES.11.1.2886-2963 (2016).
Mathur, N. K. Industrial Galactomannan Polysaccharides Vol. 3 (CRC Press, 2012).
Kaewmanee, T. et al. Characterisation of mucilages extracted from seven Italian cultivars of flax. Food Chem. 148, 60–69. https://doi.org/10.1016/j.foodchem.2013.10.022 (2014).
Siener, R., Seidler, A. & Hönow, R. Oxalate-rich foods. Food Sci. Technol. https://doi.org/10.1590/fst.10620 (2020).
Madera-Santana, T. J. et al. Mucilage from cladodes of Opuntiaspinulifera Salm-Dyck: Chemical, morphological, structural and thermal characterization. CyTA- J. Food 16(1), 650–657 (2018).
Ilango, K. B. et al. Mucilage of Coccinia grandis as an efficient natural polymer-based pharmaceutical excipient. Polymers 14(1), 215. https://doi.org/10.3390/polym14010215 (2022).
Yekta, R., Mirmoghtadaie, L., Hosseini, H. & Norouzbeigi, S. Development and characterization of a novel edible film based on Althaea rosea flower gum: investigating the reinforcing effects of bacterial nanocrystalline cellulose. Int. J. Biol. Macromol. 158, 327–337 (2020).
Nejatzadeh-Barandozi, F. & Enferadi, S. T. FT-IR study of the polysaccharides isolated from the skin juice, gel juice, and flower of Aloe vera tissues affected by fertilizer treatment. Org. Med. Chem. Lett. 2(1), 33 (2012).
Tian, S., Shi, Y., Yu, Q. & Upur, H. Determination of oleanolic acid and ursolic acid contents in Ziziphora clinopodioides Lam by HPLC method. Pharmacogn. Mag. 6, 116–119. https://doi.org/10.4103/0973-1296.62898 (2010).
Kreitschitza, A. & Gorb, S. N. How does the cell wall ‘stick’ in the mucilage? A detailed microstructural analysis of the seed coat mucilaginous cell wall. Flora 229, 9–22 (2017).
Alpizar-Reyes, E. et al. Functional properties and physicochemical characteristics of tamarind (Tamarindus indica L.) seed mucilage powder as a novel hydrocolloid. J. Food Eng. 209, 68–75. https://doi.org/10.1016/j.jfoodeng.2017.04.021 (2017).
Doublier, J.-L. & Cuvelier, G. Gum and Hydrocolloids: Functional Aspects. In Carbohydrates food 2nd edn (ed. Eliasson, A.-C.) 233–272 (CRC Press, 2006).
Amid, B. T. & Mirhosseini, H. Optimisation of aqueous extraction of gum from durian (Durio zibethinus) seed: A potential, low-cost source of hydrocolloid. Food Chem. 132(3), 1258–1268. https://doi.org/10.1016/j.foodchem.2011.11.099 (2012).
Dhull, S. B. et al. Functional, thermal and rheological behavior of fenugreek (Trigonella foenum–graecum L.) gums from different cultivars: A comparative study. Int. J. Biol. Macromol. 159, 406–414 (2020).
Segura-Campos, M. R., Ciau-Solís, N., Rosado-Rubio, G., Chel-Guerrero, L. & Betancur- Ancona, D. Chemical and functional properties of Chia seed (Salvia hispanica L.) gum. Int. J. Food Sci. https://doi.org/10.1155/2014/241053 (2014).
Ghribi, A. M. et al. Effect of drying methods on physico-chemical and functional properties of chickpea protein concentrate. J. Food Eng. 165, 179–188. https://doi.org/10.1016/j.jfoodeng.2015.06.021 (2015).
Rao, M. R. P., Warrier, D. U., Gaikwad, S. R. & Shevate, P. M. Phosphorylation of psyllium seed polysaccharide and its characterization. Int. J. Biol. Macromol. 85, 317–326. https://doi.org/10.1016/j.ijbiomac.2015.12.043 (2016).
Mahfoudhi, N. et al. Assessment of emulsifying ability of almond gum in comparison with gum. Arabic using response surface methodology. Food Hydrocoll. 37, 49–59. https://doi.org/10.1016/j.foodhyd.2013.10.009 (2014).
Sciarini, L. S., Maldonado, F., Ribotta, P. D., Perez, G. T. & Leon, A. E. Chemical composition and functional properties of Gleditsia triacanthos gum. Food Hydrocoll. 23(2), 306–313. https://doi.org/10.1016/j.foodhyd.2008.02.011 (2009).
Rezaei, A., Nasirpour, A. & Tavanai, H. Fractionation and some physicochemical properties of almond gum (Amygdalus communis L.) exudates. Food Hydrocoll. 60, 461–469. https://doi.org/10.1016/j.foodhyd.2016.04.027 (2016).
Timilsena, Y. P., Adhikari, R., Kasapis, S. & Adhikari, B. Molecular and functional characteristics of purified gum from Australian chia seeds. Carbohydr. Polym. 136, 128–136. https://doi.org/10.1016/j.carbpol.2015.09.035 (2016).
Jouki, M., Mortazavi, S. A., Yazdi, F. T. & Koocheki, A. Characterization of antioxidant-antibacterial quince seed mucilage films containing thyme essential oil. Carbohydr. Polym. 99, 537–546 (2014).
Jindal, M., Kumar, V., Rana, V. & Tiwary, A. K. Exploring potential new gum source Aegle marmelos for food and pharmaceuticals: Physical, chemical and functional performance. Ind. Crops Prod. 45, 312–318. https://doi.org/10.1016/j.indcrop.2012.12.037 (2013).
Zou, Y., Jiang, A. & Tian, M. Extraction optimization of antioxidant polysaccharides from Auricularia auricula fruiting bodies. Food Sci. Technol. 35, 428–433 (2020).
Liu, F., Liu, W. & Tian, S. Artificial neural network optimization of Althaea rosea seeds polysaccharides and its antioxidant activity. Int. J. Biol. Macromol. 70, 100–107 (2014).
Kwon, Y. I., Apostolidis, E. & Kim, Y. C. Health benefits of traditionalcorn, beans and pumpkin: in vitro studies for hyperglycemia and hyperten-sion management. J. Med. Food 10, 266–275 (2007).
Uddin Zim, A., Khatun, J., Khan, M. F., Hossain, M. A. & Haque, M. M. Evaluation of in vitro antioxidant activity of okra mucilage and its antidiabetic and antihyperlipidemic effect in alloxan-induced diabetic mice. Food Sci. Nutr. 9(12), 6854–6865. https://doi.org/10.1002/fsn3.2641 (2021).
Apostolidis, E. & Lee, C. In vitro potential of Ascophyllum nodosum phenolicantioxidant-mediated α-glucosidase and α-amylase inhibition. J. Food Sci. 75, 97–102 (2010).
Suresh Kumar, G., Shetty, A. K., Sambaiah, K. & Salimath, P. V. Antidiabetic property of fenugreek seed mucilage and spent turmeric in streptozotocin-induced diabetic rats. Nutr. Res. 25, 1021–1028 (2005).
Muhtadi, M., Primarianti, A. U., Sujono, T. A. Antidiabetic activity of durian (Durio zibethinus Murr.) and rambutan (Nephelium lappaceum L.) fruit peels in alloxan diabetic rats. Proced. Food Sci. 3, 255–261 (2015).
Tan, H.-F. & Gan, C.-Y. Polysaccharide with antioxidant, α-amylase inhibitory and ACE inhibitory activities from Momordica Charantia. Int J Biol Macromol 85, 487–496 (2016).
Sila, A. et al. Water-soluble polysaccharides from agro-industrial by-products: Functional and biological properties. Int. J. Biol. Macromol. 69, 236–243 (2014).
Narasimhacharya, A. V. R. L., Vasant, R. A. & Prajapati, P. C. Angiotensin-Converting Enzyme inhibition by certain fruits: An In vitro study. Curr. Trends Biotechnol. Pharm. 4(3), 801–808 (2010).
Elaloui, M. et al. Quantification of total phenols, flavonoides and tannins from Ziziphus jujuba (mill.) and Ziziphus lotus (l.) (Desf). Leaf extracts and their effects on antioxidant and antibacterial activities. Int. J. Second. Met. 4, 18–26. https://doi.org/10.21448/ijsm.275886 (2017).
Yahia, Y., Benabderrahim, M. A., Tlili, N., Bagues, M. & Nagaz, K. Bioactive compounds, antioxidant and antimicrobial activities of extracts from different plant parts of two Ziziphus Mill. species. PLoS One 15(5), e0232599. https://doi.org/10.1371/journal.pone.0232599 (2020).
Phadtare, S., Yamanaka, K., Kato, I. & Inouye, M. Antibacterial activity of 4, 5-dihydroxy-2-cyclopentan-1- one (DHCP) and cloning of a gene conferring DHCP resistance in Escherichia coli. J. Mol. Microbiol. Biotechnol. 3, 461–465 (2001).
Al-Baarri, A. N., Hayashi, M., Ogawa, M. & Hayakawa, S. Effects of mono- and disaccharides on the antimicrobial activity of bovine lactoperoxidase system. J Food Prot. 74(1), 134–139. https://doi.org/10.4315/0362-028X.JFP-10-184 (2011) (PMID: 21219776).
Naji-Tabasi, S., Razavi, S. M. A., Mohebbi, M. & Malaekeh-Nikouei, B. New studies on basil (Ocimum bacilicum L.) seed gum: Part I-Fractionation, physicochemical and surface activity characterization. Food Hydrocoll. 52, 350–358 (2016).
Archana, G. et al. Preparation and characterization of mucilage polysaccharide for biomedical applications. Carbohydr. Polym. 98(1), 89–94. https://doi.org/10.1016/j.carbpol.2013.04.062 (2013).
Sarkar, G. et al. Taro corms mucilage/HPMC based transdermal patch: an efficient device for delivery of diltiazem hydrochloride. Int. J. Biol. Macromol. 31(66), 158–0036 (2014).
AOAC, 2006. Official Methods of Analysis of AOAC International In: Association of official analytical chemists, 18th ed., vol. 534. (Washington, DC, USA. Retrieved from http://www.eoma.aoac.org/).
Monsigny, M., Petit, C. & Roche, A.-C. Colorimetric determination of neutral sugar by a resorcinol sulfuric acid micromethod. Anal. Biochem. 175, 525–530 (1988).
Melton, L. D., & Smith, B. G. Determination of the uronic acid content of plant cell walls using a colorimetric assay. in Current Protocols in Food Analytical Chemistry (eds. Wrolstad, R. E. et al.) 1-6 (Wiley and Sons, 2001).
Deore, U. V., Mahajan, H. S., Surana, S. J. & Wagh, R. D. Thiolated and carboxymethylated Cassia obtusifolia seed mucilage as novel excipient for drug delivery: Development and characterisation. Mater. Technol. 5, 1–11. https://doi.org/10.1080/10667857.2020.1800307 (2020).
Kalegowda, P., Chauhan, A. S. & Nanjaraj Urs, S. M. Opuntia dillenii (Ker-Gawl) Haw cladode mucilage: Physico-chemical, rheological and functional behavior. Carbohydr. Polym. 157, 1057–1064 (2017).
Rodríguez-Núñez, J. R., Madera-Santana, T. J., Sánchez-Machado, D. I., López-Cervantes, J. & Soto Valdez, H. Chitosan/hydrophilic plasticizer-based films: Preparation, physicochemical and antimicrobial properties. J. Polym. Environ. 22, 41–51 (2014).
Shah, A. et al. Effect of γ-irradiation on structure and nutraceutical potential of β-glucan from barley (Hordeum vulgare). Int. J. Biol. Macromol. 72, 1168–1175 (2015).
Akkarachiyasit, S., Yibchok-Anun, S., Wacharasindhu, S. & Adisakwattana, S. In vitro inhibitory effects of cyandin-3-rutinoside on pancreatic α-amylase and its combined effect with acarbose. Molecules 16(3), 2075–2083. https://doi.org/10.3390/molecules16032075 (2011).
Girish, T. K., Vasudevaraju, P. & Prasada Rao, U. J. S. Protection of DNA and erythrocytes from free radical induced oxidative damage by black gram (Vigna mungo L.) husk extract. Food Chem. Toxicol. 50(5), 1690–1696. https://doi.org/10.1016/j.fct.2012.01.043 (2012).
Yahia, Y., Benabderrahim, M. A., Tlili, N., Bagues, M. & Nagaz, K. Bioactive compounds, antioxidant and antimicrobial activities of extracts from different plant parts of two Ziziphus Mill. species. PLoS One 15(5), 0232599. https://doi.org/10.1371/journal.pone.0232599 (2020).
Acknowledgements
Dr. Adil Gani is thankful to Indian Council of Medical Research (ICMR), Government of India for the award of Senior Research Fellow (SRF) in favor of Ms. Ifra Hassan (3/1/2/173/2020-Nut).
Author information
Authors and Affiliations
Contributions
I.H.: Methodology, Writing- Original draft preparation. A.G.: Conceptualization, Visualization, Investigation, Supervision. M.A.: Revised the manuscript. J.B.: Formal analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hassan, I., Gani, A., Ahmad, M. et al. Extraction of polysaccharide from Althea rosea and its physicochemical, anti-diabetic, anti-hypertensive and antioxidant properties. Sci Rep 12, 17116 (2022). https://doi.org/10.1038/s41598-022-20134-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-20134-6
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.