Abstract
There is a growing amount of plastic waste that needs to be properly disposed of in order to protect the environment from the negative effects of increasing reliance on plastic products. Recent interest has focused on chemical recycling as a means of reducing plastic's negative environmental effects. Converting waste plastics into basic petrochemicals allows them to serve as hydrocarbon feedstock or fuel oil through pyrolysis operations. Scientists have taken a keen interest in the production of bioethanol from renewable feedstocks due to its potential as a source of energy and alternative fuel. Due to its beneficial effects on the environment, ethanol has emerged as a promising biofuel. In this paper, energy recovered from low-density polyethylene and high-density polyethylene waste was converted into an alternative plastic fuel and evaluated for its environmental impact with the blending of ethanol in a diesel engine. Ternary fuel blends with 20%, 30%, and 40% waste plastic fuel and 10%, 15%, and 20% ethanol with standard diesel were tested. The study found that blending 10% ethanol with 20% plastic fuel decreased fuel consumption by around 7.9% compared to base diesel. Carbon monoxide emissions are reduced by about 10.2%, and hydrocarbon emissions are reduced by about 13.43% when using the same ternary blend. The optimum values of fuel consumption and emissions were obtained by full factorial design for a ternary fuel blend of 10% ethanol and 20% plastic fuel at the full load condition.
Similar content being viewed by others
Introduction
Since the invention of the first synthetic plastic, both its production and use around the world have skyrocketed. The problem is that plastics last a long time and do not break down easily, so they end up as waste that sticks around for a while. Waste management issues, improper recycling, and littering contribute to plastic pollution, which has far-reaching negative effects on the natural world. Reusing and recycling only account for a small percentage of plastic trash at this time. Plastic recycling has yet to reach its full potential due to technical difficulties and low revenues, especially for mixed plastics1. The average lifespan of a plastic product is only one day to three years. However, the time required for these goods to decompose is still excessively long; for instance, it takes plastic products an average of 300–500 years to deteriorate2. Many plastic pieces are contaminated with metals, paper, other kinds of polymers, and/or other fillers, preventing mechanical recycling of the entire plastic collection. Approximately 70% of plastic garbage is burnt or disposed of in landfills around the world. Landfilling and incineration with minimal energy recovery are not viable long-term solutions for plastics because they are derived from crude oil and natural gas products. For polluted and mixed chemicals, recycling is a viable option3. In addition to solar and wind power, the burning of a plastic garbage can be used to recover energy. It is possible that these hydrocarbon polymers can be used to replace fossil fuels, hence reducing emissions of carbon dioxide. This is because the energy produced by incinerating polyethylene is about equivalent to that used in its production and because polyethylene has a calorific value similar to that of fuel oil4. Recycling plastics is a good way to reduce plastic pollution. Mechanical reprocessing is the primary technique of plastic recycling; however, it only works with high-purity, selectively selected garbage. Thermal or catalytic recycling creates hydrocarbon, which is appealing. Compared to other procedures, pyrolysis is cost-effective. Pyrolysis thermally destroys the plastic component to create oil and gas5.
Plastic oil is the byproduct of processing polyethylene, polypropylene, and polystyrene. During the experiments, researchers manipulate fuel injection time by adding shims to the engine, which retards timing, and removing shims, which advances the timing. Timing fuel injection enhances performance and minimizes emissions. By modifying fuel injection timing in advance, they reduce Break fuel usage, Nitrogen Oxides (NOx) emissions, and smoke emissions. Retard's fuel injection timing has been lowered to reduce NOx emissions, improving engine performance6. Waste plastic fuels produce more NOx than diesel. Due to the engine's inability to properly ignite the pyrolysis fuels at the outset of combustion, carbon emissions increase. The smoke index is decreased via carbon-chain-link length reduction. But the brake thermal efficiency and fuel consumption were benefited from higher heating value and cetane index7.
Ethanol's volumetric efficiency is enhanced by its many positive qualities, including its low viscosity, high oxygen content, high hydrogen/carbon ratio, low sulphur content, and strong evaporative cooling. However, diesel engines may utilize ethanol when combined with diesel fuel. Ethanol's reduced viscosity compared to diesel enables improved atomization of the fuel supplied into the cylinders and enhanced mixing with air. Due to its high latent heat of evaporation, ethanol can be used to improve volume efficiency in a diesel engine when blended with biodiesel fuel. This is accomplished through evaporative cooling of ethanol during engine operations8. Typically, ethanol can make up to 20% of the total volume of diesel fuel when combined with diesel. The blend's stability, cetane number, and lubricity are all affected by the substance employed to stabilize it. Increased alcohol content improves engine performance, reduces emissions, and lowers density and kinematic viscosity9.
In all engine loads, Waste Plastic Oil (WPO) burns similarly to diesel fuel; however, castor oil slows combustion compared to palm biodiesel. Both biodiesel blends were shown to increase efficiency and decrease smoke emissions in comparison to diesel. The WPO in biodiesel helped cut down on harmful emissions of hydrocarbons and nitrogen oxides. Both carbon monoxide and smoke emissions rose when biodiesel was blended with WPO10. When the n-butanol concentration in the blends was increased, a researcher observed improved thermal efficiency. A 10% butanol blend in plastic fuel and diesel was found to decrease NOx and smoke pollutants while increasing engine performance11. According to the tests conducted, the most effective mixture was one containing 50% diesel, 30% waste plastic oil, and 20% n-hexanol by volume. At full load, this mixture improves brake thermal efficiency by about 1.3% and 14% compared to pure diesel and waste plastic oil while also reducing oxides of nitrogen pollution by about 4% and reducing smoke emission by about 30% and 40%, respectively12.
The ethoxy ethyl acetate and ethanol as oxygenated additives were blended with fuel produced from the high-density polyethylene by the pyrolysis process. Due to the high oxygen content, low viscosity, and high carbon/hydrogen ratio of ethanol, quaternary fuels offer greater thermal efficiency, reduced fuel usage, and reduced harmful emissions13. Ethanol is a renewable biofuel that serves as an oxygenated fuel in diesel engines, reducing emissions. Three percentages of polyethylene plastic pyrolysis oil were blended with ethanol to form ternary fuel. W20E10's fuel usage drops 7.5% with diesel. W20E10 had lower carbon monoxide emissions than diesel and hydrocarbon at varied loads14.
Increasing the load and compression ratio, as well as adding waste plastic oil and ethanol to diesel, boosts thermal efficiency and emission quality. Using ANOVA and multivariate analysis, the appropriate engine load, compression ratio, and fuel blend were identified, improving performance and reducing emissions. Maximum braking thermal efficiency and reduced emissions were observed with a compression ratio of 18.1, and 20% WPO and 20% ethanol blended diesel15. As opposed to full factorial designs, the calibration procedure can be completed in a fraction of the time using DoE approaches for the reduction of experimental plans to determine the optimal values for the injection parameters. It was also mentioned that the optimization process might be heavily impacted by how the goal function is formulated16. In order to reduce NOx and smoke emissions while maintaining optimal fuel efficiency, a response surface methodology-based optimization using a three-factor, a three-level full factorial experimental design was implemented. Statistically, significant multiple surface regression models were found for NOx, smoke density, and fuel consumption. Injection timing and EGR were compared for all fuel blends11.
It was found in the literature that a variety of pyrolysis reactors were utilized to convert individual plastics into liquid products17. But there is not much data on how to turn various types of plastics into useful recovery. In this paper, energy recovered from low-density polyethylene (LDPE) and high-density polyethylene (HDPE) waste into an alternative plastic fuel was evaluated for its environmental impact with the blending of ethanol in a diesel engine. Ternary fuel blends with 20%, 30%, and 40% waste plastic fuel and 10%, 15%, and 20% ethanol with standard diesel were tested. The performance and emission characteristics of a diesel engine are studied and assessed. The fuel consumption and emissions were studied through a full factorial design on the influence of engine load, ethanol, and plastic fuel blend ratios.
Material and methods
Plastics have proved to be "one of the greatest innovations of the millennium." Plastic is lightweight, does not rust or rot, is cheap, reusable, and saves natural resources; hence it is popular18,19. India produces 10 million tons of plastic annually. Three million metric tons of plastic waste are dumped daily, but 70% is recycled. Most nations recycle and burn plastic waste to reduce its impact. Pyrolysis is the most efficient way to recycle plastic since the energy may be used directly. Pyrolysis reduces waste volume by 90%, produces solid, liquid, and gaseous fuels, and has the least environmental impact of any thermal waste degradation process20.
Researchers studied the influence of polystyrene (PS), polyethylene (PE), polypropylene (PP), and polyethylene terephthalate (PET) on pyrolysis oil yield and quality. PS plastic trash produced the most liquid oil (80.8%) and the least fumes (13%), char (6.2%). All liquid plastics contain aromatic chemicals, alkanes, and alkenes21. Hydrocracking at 350 °C with 20 mL/min of hydrogen gas stream took one hour. LDPE plastic waste converted over Co-Mo/Z at 350 °C yielded 71.49% fuel selectivity. Catalysts lower liquid and raise gaseous fractions. Based on GCMS data, liquid yield comprised of C6-C19 hydrocarbon compounds, such as paraffin, olefins, and naphthenes22. Commercial and natural Y-zeolite catalysts were studied. The feedstock types affect liquid and solid product yields and quality. HDPE waste produces more fuel yield23.
Table 1 lists the characteristics of the various plastics like polyethylene, polypropylene, polyethylene terephthalate, and polyvinyl chloride found in plastic garbage. Shredding the plastic into pieces between 2 and 5 mm was a necessary step in the recycling process. All of the plastics were broken down using the catalytic pyrolysis technique, and their individual characteristics were analyzed and compared25. The pyrolysis process is used to recover energy, with liquid fuel as the major product. The pyrolysis procedure was carried out using a reactor-furnace combination equipped with a PID controller. The reactor was filled with a ZSM (zeolite) to plastics, and the ratio was a 1:4 combination. The reactor was heated from 450 to 600 °C in a vacuum-sealed furnace. A container was placed at the base of the reactor in order to collect the condensed liquid26,27.
The results of an exhaustive study suggest that waste plastic fuel could be used in ternary fuel blends with oxygenated ethanol, either with or without a modification to the engine settings. According to the findings of the observations, a number of different attempts are currently being undertaken in the laboratory to use alcohols (Table 2) as oxygenated additions in order to improve the performance characteristics and reduce harmful emissions.
Plastic pyrolysis fuel shares many of the same features as diesel fuel, making it a viable alternative fuel source28,29. From the above literature, it is possible to recover energy through the pyrolysis process of converting waste plastics to liquid fuel. In this investigation, a unique combination of the shredded HDPE and LDPE was taken in the ratio of 50:50 as the source of plastic oil extraction as plastic fuel (LHP)30.
This study looked into the performance and emissions of diesel engines with direct injection, with an investigation of the possibility of using plastic fuel (LHP) extracted from HDPE and LDPE waste and ethanol as a ternary blend. The testing ternary fuel blends were made on a volumetric ratio with standard diesel, plastic fuel (LHP), and ethanol. E10LHP20 ternary blend was created with 70% of diesel, 20% of LHP, and 10% of ethanol. For that, the E15LHP30 blend was made by blending 55% of diesel with 30% of LHP and 15% of ethanol. The E20LHP40 blend was made by blending 40% of diesel with 40% of LHP and 20% of ethanol. The physicochemical properties of ternary blends are listed in Table 3.
Experimental setup
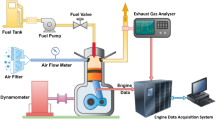
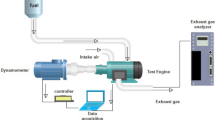
In this experiment, a single-cylinder diesel engine with direct injection, constant speed, and water cooling was used to make 4.4 kW of power. Computer-controlled eddy current dynamometer connected with the engine and engine torque. Eddy current dynamometer controller is used to manage engine speed and load by altering dynamometer excitation current. A piezoelectric pressure transducer fitted in the engine cylinder head recorded combustion pressure. Using an accurate crank angle encoder, the crank angle and piston locations were determined. Thermocouples are used to measure the temperature of the coolant, the exhaust, and the air intake. The emission characteristics and smoke intensity were measured using an AVL exhaust di-gas analyzer and an AVL smoke meter, respectively.
The preliminary investigation required starting the engine on diesel and letting it idle for 15 min before subjecting it to any load. Before starting the engine, the air intake, oil level, and fuel level are all inspected. For the engine to be started, both the fuel cutoff lever and the decompression lever had to be in the "on" position while the cranking motion was performed. The decompression lever was released at engine start, and the speed was increased to 1500 rpm and held constant. The investigation was conducted when the engine was running at a steady state speed with a compression ratio of 18:1. The schematic diagram of the experimental engine setup is represented in Fig. 1, and the test engine specification is tabulated in Table 4.
The feasibility of using LHP in a diesel engine by combining ethanol was studied by analyzing engine performance, combustion, and exhaust characteristics. All of the trial test runs were conducted with 100% diesel fuel and kept as base data for comparison. A single-cylinder diesel engine was tested, with engine power increasing by 1.1 kW up to 4.4 kW. The engine was then made to keep running on three ternary test samples, and readings were taken. The brake thermal efficiency, specific fuel consumption, exhaust gas temperature, and combustion parameters like heat release rate and in-cylinder pressure were examined. Among the exhaust gases, carbon monoxide, hydrocarbons, nitrogen oxides, and smoke were recorded.
Experiment uncertainty may result from instrument selection, maintenance, observation, environmental factors, calibration, test design, and reading. Analysis of uncertainty is desired to show how accurate the experiments were. Depending on operational and environmental conditions, performance and measurement accuracy might change31. As a result, random or fixed errors are the cause of the uncertainty. Table 5 lists the experimentation critical instrumental parameters and their uncertainty.
Results and discussion
Study of brake thermal efficiency
Figure 2 shows the calculated brake thermal efficiency (BTE) for the tested diesel engine performance with a ternary fuel of LHP and ethanol. The efficiency was recorded at 5.4%, 10.7%, and 13.8% reduction of BTE for the E10LHP20, E15LHP30, and E20LHP40 blends compared with base diesel. This could be because LHP blends have fewer calories than diesel. At full load, the temperature and rate of heat release from the exhaust gases of plastic fuel are a little higher than those of diesel10. A higher proportion of plastic fuel in the blend results in a lesser brake thermal efficiency than base diesel would, as the higher aromatic compound content of plastic oil slows down combustion. The reason for this is that plastic fuel has a high aromatic component, which makes it harder to break the chain. Its higher viscosity and lower thermal efficiency compared to the base fuel tested may be due to problems with fuel injection and the quality of liquid fuel spray32.
Study of specific fuel consumption
Figure 3 shows a comparison of the specific fuel consumption (SFC) of three ternary blends with diesel when the various engine load. The specific fuel consumption was reduced by around 2–7.95% when comparing E10LHP20 to diesel as the base fuel at various load levels. Due to better mixing of fuel and air, higher engine speeds lead to better fuel combustion, while higher loads lead to better fuel atomization, better mixing, and a high temperature in the cylinder, all of which help with the combustion process and lead to low specific fuel consumption33. As the recorded amount of SFC rises, so does the amount of LHP in the ternary blend. This is because of LHP's increased viscosity and density, which prevents it from being atomized effectively. For E15LHP30 and E20LHP40, the SFC increase from diesel fuel is 7.9% and 17.9%, respectively. The fact that LHP has a lower heating value magnitude and is thicker can explain why it uses more fuel34.
Study of exhaust gas temperature
Figure 4 demonstrates that an increase in load causes an increase in the Exhaust Gas Temperature (EGT), which is influenced by the temperature at the combustion process. The exhaust gas temperature recorded at LHP and its blends was found to be higher than base diesel. When LHP is used as a fuel, plastic oil has lower volatility and higher viscosity than conventional fuels, leading to incomplete combustion35. As a result, exhaust gas temperature may have increased. EGT was recorded at more than 5.36%, 9.95%, and 15.4% of the base diesel for blends E10LHP20, E15LHP30, and E20LHP40, respectively. It was established that the increased exhaust temperature was brought on by a small quantity of gases traveling through the combustion process at the end of the expansion stroke15. Since ethanol contains more oxygen (about 36% by weight), it burns at a higher temperature than diesel. For this reason, LHP blends produce hotter exhaust than expected32.
Study of carbon monoxide emissions
In the investigation of the E10LHP20 blend under various engine loads, carbon monoxide (CO) emissions were reduced by 3.4–10.2% when compared with base diesel. An LHP blend with higher concentrations of oxygen-rich ethanol burns cleaner and produces fewer carbon dioxide emissions than its binary counterparts13. Appropriate combustion led to lower CO emissions when the load was lower, but as the load was increased, combustion efficiency deteriorated, and CO emissions rose for other LHP blends10. Figure 5 shows that ternary E15LHP30 and E20LHP40 blends produce significantly more carbon monoxide than diesel. The principal reason for the increase in CO concentration is the increased fuel consumption associated with the increased loads. The increased CO emissions have also been linked to the low in-cylinder temperature seen during combustion. The E15LHP30 and E20LHP40 blends produced a maximum of 3.2% and 7.3% more carbon monoxide than base diesel. The increased viscosity and density of plastic fuel causes inefficient atomization of fuel blend, which in turn increases CO emissions32.
Study of hydrocarbon emissions
The amount of unburned hydrocarbon (HC) is reduced when the load is lightened because of the higher charge homogeneity and the greater availability of oxygen. When high-oxygen ethanol blends are incorporated into ternary mixtures, combustion efficiency is increased, leading to a decrease in hydrocarbon emissions36. The hydrocarbon content of ternary blend E10LHP20 is reduced by around 6–13.43% compared to diesel fuel under varying loading conditions, as shown in Fig. 6. The ternary blends E15LHP30 and E20LHP40 generated a maximum of 5.9% and 13.8% more hydrocarbons than base diesel at various engine powers. The fundamental reason for hydrocarbon emissions is the extinction of the flame in cold parts of the combustion chamber and at the cylinder walls. These emissions are also connected to the viscosity and volatility of the fuel. Larger droplet sizes and lower vapour pressure are two undesirable outcomes of increased viscosity. Unsaturated molecules are responsible for an increase in hydrocarbon emissions, a decrease in auto-ignition, and difficulties in the leaner mixture zone inside cylinder37.
Study of nitrogen oxides emissions
Figure 7 demonstrates that when the engine load increases, nitrogen oxide emissions from all three ternary fuel blends, E10LHP20, E15LHP30, and E20LHP40, rise relative to those from base diesel. There is speculation that the presence of oxygenated hydrocarbons in plastic fuel contributes to the higher levels of nitrogen oxides in ternary blends compared to diesel. For all of the blends examined, it was shown that NOx emissions increased with increasing load. Massive amounts of nitrogen oxide are generated due to the high temperature and high pressure experienced in the cylinder when employing blends, as well as the emission of massive amounts of heat during combustion38. E10LHP20, E15LHP30, and E20LHP40 blends have been recorded as having a maximum of 12.4%, 22%, and 46% more nitrogen oxides than base diesel at various load conditions. This is because ethanol produces more NOx emissions due to its higher oxygen content which is available for burning. The longer time between injecting fuel and starting the engine and the late combustion that results in an increase in NOx33.
Study of Smoke Emissions
In Fig. 8 shows the different test fuel conditions lead to noticeably different smoke patterns. The smoke generated by base diesel is lower than that of E15LHP30 and E20LHP40. The high level of aromatics in LHP leads to an unintended fuel blend. Incomplete combustion and high smoke production have an effect on spray formation. At mid-load, E10LHP20 produces 9.4% less smoke than the base diesel. Simultaneously, E15LHP30 and E20LHP40 emit up to 8.33% and 25% more than diesel, respectively. Causes of smoke include poor atomization, high viscosity, and an excess fuel concentration. Since LHP fuel blends take so long to burn and have such a poor rate of flame spread, they emit a great deal of smoke. Due to increased hydrocarbon combustion, smoke production was exacerbated by incomplete combustion7,11.
Study of in-cylinder pressure
The relation between in-cylinder pressure (IP) and crank angle is shown in Fig. 9. For IP measurement, a piezoelectric transducer was used in the combustion chamber. During the first combustion phase, the charge mixer might affect pressure generation. In many cases, the cylinder pressure level is directly related to the charge mixture, fuel viscosity, and cetane number. Engine efficiency and emissions are directly influenced by the combustion parameters, which may be studied extensively through IP analysis. At full load, the highest IP for E10LHP20 was 59.1 bar, whereas the peak IP for diesel was 62.01 bar. Diesel fuels without ethanol had a higher IP than those with ethanol added. This may be because ethanol blends at a cool burning rate and slower oxidation rate than conventional fuels8. Diesel pressure generation was also found to be superior in the investigations. This may be attributable to diesel's high energy density and enhanced flammability, which increase evaporation rate and peak cylinder pressure. When ethanol was added to LHP, the IP peak pressure dropped from 59.1 to 56.8 bar. This occurred because ethanol was added, which caused a delay in the ignition and increased the time required for burning. For ethanol associate LHP blends, E10LHP20, E15LHP30, and E20LHP30 all reached peak cylinder pressures of 59.1 bar, 57.3 bar, and 56.9 bar, respectively. The rate of pressure rise is lowered in ethanol-doped blends because of the pooled impact of the lower cylinder temperature and the lower energy content of ethanol, which restrict the generation of high pressure in cylinder34.
Study of heat release rate
Figure 10 depicts the relationship between heat release rate (HRR) and crank angle (CA) for diesel, E10LHP20, E15LHP30, and E20LHP30. The graph depicts that as ethanol concentration increases, the peak HRR lowers due to the cooling impact and low cylinder temperature, resulting in inadequate combustion. In addition, it was noted that the HRR was greater for conventional fuel operation than for ethanol blend operation. HRR was 67.9 J/°CA for E10LHP20 and 72.34 J/°CA for diesel. Diesel's HRR was greater than E10LHP20, E15LHP30, and E20LHP30 by 4.2%, 11.1%, and 11.9%, respectively. It may be owing to diesel fuel's fast primary combustion, which results in a greater heat release rate. The start of combustion was delayed for ethanol blend combustion than diesel combustion8,34. The ethanol blend LHP demonstrated a delayed peak HRR compared to diesel combustion. It could be due to ethanol's lower cetane rating, resulting in a longer ignition delay period and slower burning characteristics. In addition, blends with lower ethanol contents displayed a greater HRR than other blends due to their moderate heating value and nominal combustion temperature. In addition, inferior oxidation rate and delayed burning rate due to suppressing the cylinder's temperature with ethanol are significant factors in the decrease in HRR26.
Impact of LHP and Ethanol blends on SFC, CO, and HC
The creation of a process often employs the use of experimental design to ensure that all aspects of the problem are addressed. This approach makes use of statistical data collected from individual trials in order to foretell the results of a dynamic, complicated, and unpredictable process. The design of experiments (DoE) is a statistical technique used to collect as much information as feasible for this investigation. The main effects and interactions of a study's variables can both be systematically evaluated with a full factorial design. Full and fractional factorial designs with 2 levels and 3 levels, respectively, are the most popular experimental designs employed by researchers. The use of a factorial design would allow the researcher39 to examine the interaction between multiple processes or design variables and an outcome40. The Minitab 19 software was used for this full factorial design and response optimizer. In this investigation, a full factorial design was adopted for 2 levels and 3 factors with 8 possible combinations. The interaction of engine load, LHP, and ethanol blend has been investigated as the input parameters for the response of fuel consumption, carbon monoxide, and hydrocarbon emissions. Table 6 displays the variables and levels of full factorial design, and the experimental response of a full factorial design is tabulated in Table 7.
Effect of LHP and ethanol blends on SFC
The contour plots in Fig. 11 depict the primary effect elements, which include LHP, ethanol blend ratio, and load variation. In these interactions, engine load is beneficial to specific fuel consumption at its maximum, and an increased ethanol ratio results in the lowest SFC. Increased engine power improves fuel combustion by allowing for a more thorough mixing of the fuel and air and improves fuel atomization and cylinder temperature, all of which contribute to efficient combustion and hence reduce specific fuel consumption. Furthermore, due to the viscosity of plastic blends, the SFC increases with an increasing blend ratio, reducing the blend's burning effect at the initial load conditions.
Effect of LHP and ethanol blends on CO
When the load increases, contour Fig. 12 shows that the carbon monoxide emissions have increased. When the load was low, appropriate combustion resulted in fewer CO emissions; however, when the load was raised, the efficiency of combustion decreased, which resulted in a rise in CO emissions for other LHP and ethanol blends. Blends of ethanol have higher oxygen concentrations, and it promotes complete combustion, which decreases CO concentrations. Incomplete combustion occurs when using LHP blends. Because of their higher content of aromatic components, they burn at a slower rate, and it is harder to break the chain. This leads to higher CO formation at higher ratios.
Effect of LHP and ethanol blends on HC
When the load increases, HC emissions also increase, as shown in the contour Fig. 13. Low vaporization, slow oxidation rates, and high fuel consumption are major contributors to hydrocarbon emission formation. Because of its high oxygen content, ethanol aids in more combustion, reducing HC emissions. Consequently, HC emissions are reduced due to the addition of more oxygen throughout the combustion process on ethanol blend ratios. In contrast, an LHP blend with a larger proportion of aromatic components burns more slowly, and more resistance to breaking the chain is generated, leading to incomplete combustion. At larger ratios, this causes more HC to be produced.
Response optimization of fuel consumption and emissions
Get the best possible result from a single response or a group of responses by using Response Optimizer to find the optimal settings for the input variables. Adjusting the input variable values in this interactive visualization can help with sensitivity studies and may lead to a better solution14. As illustrated in Fig. 14, the optimization plot demonstrates that the optimal ratio of LHP and ethanol yields the lowest SFC, CO, and HC emissions under maximum load conditions with a composite desirability of 0.9196. Increasing the LHP and ethanol blend ratio will increase all the responses. But the effect on SFC was minimal compared to the effect on CO and HC emissions. Therefore, when decreasing the composite's desirability by minimizing the LHP and ethanol blend ratio, the optimal settings of the LHP and ethanol blend were at the minimum levels in the experiment. This result suggests that researchers should consider experimenting with a lower LHP and ethanol blend ratio for better engine performance. Increasing engine load decreases SFC and CO but causes a minimal increase in HC. The optimal settings for SFC and CO are at the maximum load condition in the experiment. This result implies that experimenting with a higher load may improve engine performance.
The optimal parameters and their response values are listed in Table 8. When using 20% LHP and 10% ethanol, the minimum SFC at maximum load was 0.3006 kg/(kW.h), which is the best possible result. The minimum values of HC and CO emissions were obtained for a ternary fuel blend consisting of 20% LHP and 10% ethanol, and their response is 58 ppm and 0.1244 vol%, respectively, at the full load condition. A confirmation test was used to verify the trial outcomes using observable data, hence validating the response optimizer's output. The fuel consumption is 0.2897 kg/(kW.h), the HC emission is 56 ppm, and the CO emission is 0.1256 vol%, according to confirmation testing results. The results are that 3.6% of the SFC and 1% of the CO, and 3.4% of HC findings deviate from the optimal values determined by full factorial design.
Conclusion
Waste plastic, a major disposal issue, can be converted into useful energy. The purpose of this study is to explore the effectiveness of plastic fuel in a diesel engine, which is recovered from the waste of low-density polyethylene and high-density polyethylene. Three ratios of LHP and ethanol were used to generate the ternary fuel blends.
-
In a summary of the ternary fuel blend investigations, specific fuel consumption was reduced by around 2–7.95% when comparing E10LHP20 to diesel as the base fuel at various load levels. Carbon monoxide emissions were reduced by 3.4–10.2% when compared with the base diesel for E10LHP20.
-
Under varying loading conditions, the hydrocarbon content of ternary blend E10LHP20 is reduced by around 6–13.43% compared to diesel fuel. Because the ternary blend contains ethanol that is high in oxygen content, thus the combustion process was improved, which leads to a decrease in emissions.
-
E10LHP20, E15LHP30, and E20LHP40 blends have been recorded as having a maximum of 12.4%, 22%, and 46% more nitrogen oxides than base diesel at various load conditions. This is because ethanol produces more NOx emissions due to its higher oxygen content which is available for burning.
-
LHP has a higher content of aromatic components; they burn at a slower rate, and it is harder to break the chain. This leads to more smoke emissions due to the higher concentration of ternary blends.
-
The minimum values of SFC, CO, and HC emissions were obtained for a ternary fuel blend consisting of 20% LHP and 10% ethanol at the full load condition, and their better results obtained were 0.3006 kg/(kW h), 0.1244 vol%, and 58 ppm, respectively.
On summary, the hydrocarbons found in used plastic represent a valuable alternative source of energy. The most difficult aspect of waste plastic recycling is the possibility of converting the plastic into usable energy. The findings indicate that it might replace fossil fuels in a variety of applications, including diesel locomotives, industrial boilers, and even marine propulsion systems.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on request.
Abbreviations
- ANOVA:
-
Analysis of variance
- BTE:
-
Brake thermal efficiency
- bTDC:
-
Before top dead centre
- C:
-
Carbon
- CA:
-
Crank angle
- CO:
-
Carbon monoxide
- DOE:
-
Design of experiments
- E:
-
Ethanol
- E10LHP20:
-
70% Diesel + 20% LHP + 10% Ethanol
- E15LHP30:
-
55% Diesel + 30% LHP + 15% Ethanol
- E20LHP40:
-
40% Diesel + 40% LHP + 20% Ethanol
- FFD:
-
Full factorial design
- GC–MS:
-
Gas chromatography–mass spectrometry
- H:
-
Hydrogen
- HC:
-
Hydrocarbons
- HDPE:
-
High-density polyethylene
- HRR:
-
Heat release rate
- IP:
-
In-cylinder pressure
- kW:
-
Kilo Watts
- LDPE:
-
Low-density polyethylene
- LHP:
-
Low and high density plastic pyrolysis oil
- NOx:
-
Nitrogen oxides
- O:
-
Oxygen
- PE:
-
Polyethylene
- PET:
-
Polyethylene terephthalate
- PPO or PO:
-
Plastic pyrolysis oil
- PP:
-
Polypropylene
- ppm:
-
Parts per million
- PS:
-
Polystyrene
- SFC:
-
Specific fuel consumption
- W:
-
Waste plastic oil
- WPO:
-
Waste plastic oil
- ZSM-5:
-
Zeolite socony mobil-5
References
Zhao, Y.-B., Lv, X.-D. & Ni, H.-G. Solvent-based separation and recycling of waste plastics: A review. Chemosphere 209, 707–720 (2018).
Matuszewska, A., Hańderek, A., Paczuski, M. & Biernat, K. Hydrocarbon fractions from thermolysis of waste plastics as components of engine fuels. Energies 14, 1–14 (2021).
Kaminsky, W. Chemical recycling of plastics by fluidized bed pyrolysis. Fuel Commun. 8, 100023 (2021).
Cleetus, C., Thomas, S. & Varghese, S. Synthesis of petroleum-based fuel from waste plastics and performance analysis in a CI engine. J. Energy 2013, 608797 (2013).
Senthil Kumar, P., Bharathikumar, M., Prabhakaran, C., Vijayan, S. & Ramakrishnan, K. Conversion of waste plastics into low-emissive hydrocarbon fuels through catalytic depolymerization in a new laboratory scale batch reactor. Int. J. Energy Environ. Eng. 8, 167–173 (2017).
Boopathi, R. & Gopinath, E. Performance analysis of plastic oil blend by changing the fuel injection timing in diesel engine. Mater. Today Proc. 46, 4613–4618 (2021).
Arjharn, W. et al. Distilled waste plastic oil as fuel for a diesel engine: Fuel production, combustion characteristics, and exhaust gas emissions. ACS Omega 7, 9720–9729 (2022).
Kim, H. Y., Ge, J. C. & Choi, N. J. Effects of ethanol–diesel on the combustion and emissions from a diesel engine at a low idle speed. Appl. Sci. 254, 2548. https://doi.org/10.3390/app10124153 (2020).
Niculescu, R., Clenci, A. & Iorga-Siman, V. Review on the use of diesel-biodiesel-alcohol blends in compression ignition engines. Energies 12, 1–41 (2019).
Kaewbuddee, C., Sukjit, E., Srisertpol, J. & Maithomklang, S. Evaluation of waste plastic oil-biodiesel blends as. Energies 13, 1–16 (2020).
Damodharan, D. et al. Effective utilization of waste plastic oil in a direct injection diesel engine using high carbon alcohols as oxygenated additives for cleaner emissions. Energy Convers. Manag. 166, 81–97 (2018).
Venkatesan, S. P. et al. Performance and emission characteristics of diesel engine using waste plastic oil with N-hexanol as an additive. AIP Conf. Proc. 2311, 20025 (2020).
Padmanabhan, S. et al. Energy recovery of waste plastics into diesel fuel with ethanol and ethoxy ethyl acetate additives on circular economy strategy. Sci. Rep. 12, 5330 (2022).
Bhargavi, M., Vinod Kumar, T., Ali Azmath Shaik, R., Kishore Kanna, S. & Padmanabhan, S. Effective utilization and optimization of waste plastic oil with ethanol additive in diesel engine using full factorial design. Mater. Today Proc. https://doi.org/10.1016/j.matpr.2021.10.310 (2021).
Das, A. K., Padhi, M. R., Hansdah, D. & Panda, A. K. Optimization of engine parameters and ethanol fuel additive of a diesel engine fuelled with waste plastic oil blended diesel. Process Integr. Optim. Sustain. 4, 465–479 (2020).
Mallamo, F., Badami, M. & Millo, F. Application of the design of experiments and objective functions for the optimization of multiple injection strategies for low emissions in cr diesel engines. SAE Trans. 113, 208–222 (2004).
Ghodke, P. K. High-quality hydrocarbon fuel production from municipal mixed plastic waste using a locally available low-cost catalyst. Fuel Commun. 8, 100022 (2021).
Patni, N., Shah, P., Agarwal, S. & Singhal, P. Alternate strategies for conversion of waste plastic to fuels. ISRN Renew. Energy 2013, 1–7 (2013).
Mangesh, V. L., Perumal, T., Subramanian, S. & Padmanabhan, S. Clean energy from plastic: Production of hydroprocessed waste polypropylene pyrolysis oil utilizing a Ni–Mo/Laponite catalyst. Energy Fuels 34, 8824–8836 (2020).
Odejobi, O. J., Oladunni, A. A., Sonibare, J. A. & Abegunrin, I. O. Oil yield optimization from co-pyrolysis of low-density polyethylene (LDPE), polystyrene (PS) and polyethylene terephthalate (PET) using simplex lattice mixture design. Fuel Commun. 2–5, 100006 (2020).
Miandad, R. et al. Effect of plastic waste types on pyrolysis liquid oil. Int. Biodeterior. Biodegrad. 119, 239–252 (2017).
Sriningsih, W. et al. Fuel production from LDPE plastic waste over natural zeolite supported Ni, Ni-Mo, Co and Co-Mo metals. Procedia Environ. Sci. 20, 215–224 (2014).
Syamsiro, M. et al. Fuel oil production from municipal plastic wastes in sequential pyrolysis and catalytic reforming reactors. Energy Procedia 47, 180–188 (2014).
Abnisa, F. & Wan Daud, W. M. A. A review on co-pyrolysis of biomass: An optional technique to obtain a high-grade pyrolysis oil. Energy Convers. Manag. 87, 71–85 (2014).
Cortazar, M. et al. Analysis of hydrogen production potential from waste plastics by pyrolysis and in line oxidative steam reforming. Fuel Process. Technol. 225, 107044 (2022).
Mangesh, V. L., Padmanabhan, S., Tamizhdurai, P. & Ramesh, A. Experimental investigation to identify the type of waste plastic pyrolysis oil suitable for conversion to diesel engine fuel. J. Clean. Prod. 246, 119066 (2020).
Sogancioglu, M., Ahmetli, G. & Yel, E. A comparative study on waste plastics pyrolysis liquid products quantity and energy recovery potential. Energy Procedia 118, 221–226 (2017).
Sudershan, B. G., Banapurmath, N. R., Kamoji, M. A., Rampure, P. B. & Khandal, S. V. Experimental investigation of a CRDI engine in terms of performance and emission under the effect of injection strategy using a moderate percentage of plastic pyrolysis oil and its blends with diesel and ethanol. Biofuels 12, 459–473 (2021).
Gadwal, S. B., Banapurmath, N. R., Kamoji, M. A., Rampure, P. B. & Khandal, S. V. Performance and emission characteristic studies on CRDI diesel engine fuelled with plastic pyrolysis oil blended with ethanol and diesel. Int. J. Sustain. Eng. 12, 262–271 (2019).
Dillikannan, D. et al. Effective utilization of waste plastic oil/n-hexanol in an off-road, unmodified DI diesel engine and evaluating its performance, emission, and combustion characteristics. Energy Sources Part A Recover. Util. Environ. Eff. 42, 1375–1390 (2020).
Kusenberg, M. et al. A comprehensive experimental investigation of plastic waste pyrolysis oil quality and its dependence on the plastic waste composition. Fuel Process. Technol. 227, 107090 (2022).
Kalargaris, I., Tian, G. & Gu, S. Experimental characterisation of a diesel engine running on polypropylene oils produced at different pyrolysis temperatures. Fuel 211, 797–803 (2018).
Sambandam, P. et al. Environmental impact of waste plastic pyrolysis oil on insulated piston diesel engine with methoxyethyl acetate additive. Pet. Sci. Technol. https://doi.org/10.1080/10916466.2022.2092498 (2022).
Singh, R. K., Ruj, B., Sadhukhan, A. K., Gupta, P. & Tigga, V. P. Waste plastic to pyrolytic oil and its utilization in CI engine: Performance analysis and combustion characteristics. Fuel 262, 116539 (2020).
Kumar, S., Prakash, R., Murugan, S. & Singh, R. K. Performance and emission analysis of blends of waste plastic oil obtained by catalytic pyrolysis of waste HDPE with diesel in a CI engine. Energy Convers. Manag. 74, 323–331 (2013).
Chintala, V. et al. A comparative assessment of single cylinder diesel engine characteristics with plasto-oils derived from municipal mixed plastic waste. Energy Convers. Manag. 166, 579–589 (2018).
Kaimal, V. K. & Vijayabalan, P. An investigation on the effects of using DEE additive in a DI diesel engine fuelled with waste plastic oil. Fuel 180, 90–96 (2016).
Li, N. et al. Conversion of plastic waste into fuels: A critical review. J. Hazard. Mater. 424, 127460 (2022).
Rezende, M. J. C., Vale, D. L., Aguiar, P. F., Riehl, C. A. S. & Azevedo, D. A. Application of full factorial design to evaluate the effect of different variables on the stability of biodiesel: Diesel blends under storage conditions. J. Braz. Chem. Soc. 28, 1966–1974 (2017).
Antony, J. 6–Full factorial designs. In (ed. Antony, J. B. T.-D. of E. for E. and S. (Second E.) 63–85 https://doi.org/10.1016/B978-0-08-099417-8.00006-7 (Elsevier, 2014).
Author information
Authors and Affiliations
Contributions
Conceptualization, P.S., V.K.T., G.K., S.B., N.N., L.T.J. and K.R.; Data Curation, P.S, V.K.T., G.K., S.B., N.N., L.T.J. and K.R.; Formal analysis, P.S., V.K.T., G.K., S.B., N.N., L.T.J. and K.R.; Investigation, P.S., V.K.T., G.K., S.B., N.N., L.T.J. and K.R.; Methodology, P.S., V.K.T., G.K., S.B., N.N., L.T.J. and K.R.; Project administration, P.S., V.K.T., G.K., S.B., N.N., L.T.J. and K. R., Resources, P.S., V.K.T., G.K., S.B., N.N., L.T.J. and K.R.; Software, P.S., V.K.T., G.K., S.B., N.N., L.T.J. and K.R.; Supervision, P.S., V.K.T., G.K., S.B., L.T.J. and K. R.; Validation, P.S., V.K.T., G.K., S.B., N.N., L.T.J. and K.R.; Visualization, P.S., V.K.T., G.K., S.B., N.N., L.T.J. and K.R.; Writing—original draft, P.S., V.K.T., G.K., S.B., N.N., L.T.J. and K.R.; Data Visualization, Editing and Rewriting, P.S., V.K.T., G.K., S.B., N.N., L.T.J. and K.R.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Padmanabhan, S., Kumar, T.V., Giridharan, K. et al. An analysis of environment effect on ethanol blends with plastic fuel and blend optimization using a full factorial design. Sci Rep 12, 21719 (2022). https://doi.org/10.1038/s41598-022-26046-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-26046-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.