Abstract
In the current study, the physicochemical, engine performance, and exhaust emission of different ternary fuel blends containing waste fish oil (WFO) biodiesel, bioethanol, and petro-diesel have been investigated. WFO Biodiesel was prepared from waste fish oil via transesterification method. Different physiochemical properties including the kinematic viscosity, density, flash point, pour point, cloud point, and heat value have been measured for different fuel blends and compared with the neat petro-diesel. The performance and exhaust emission of engine have been also studied using different fuel blends using a single-cylinder diesel engine in full load condition at 1800 rpm. It was found that the engine torque, engine power, and thermal efficiency of the ternary fuel blends was reduced by 2.45%, 9.25%, 2.35% averagely in comparison with the neat petro-diesel, respectively. The average break specific fuel consumption was also increased by 10.44% compared to the neat petro-diesel. The emission of carbon monoxide (CO), carbon dioxide (CO2), unburned hydrocarbons (UHC), and nitrogen oxides (NOx) was also measured. It was also found that the utilization of ternary fuel blends results in a considerable reduction in CO and UHC emission by 50.55% and 43.87% on average compared to the neat petro-diesel, respectively. The emission of NOx was also increased by 28.25% on average compared to the neat petro-diesel. It was also found that the NOx emission can be adjusted by tuning the WFO biodiesel and bioethanol contents of the ternary fuel blends.
Similar content being viewed by others
Introduction
Depleting the fossil fuel resources, the pollution caused by the fossil fuels combustion such as carbon oxides (COx), nitrogen oxides (NOx), unburned hydrocarbons (UHC), particular matter (PM) emissions are the most concerning issues about the fossil fuels utilization1,2. Therefore, the researchers are always looking for appropriate alternatives for the fossil fuels. Solar energy, wind energy, waves energy, and geothermal energy are interesting renewable energy sources. The biofuels as one of the renewable energy resources, can be applied in similar fossil fuel applications because the biofuel specifications can be similar to the diesel fuel. Depending on the type of biofuel and its application, the biofuels can be used as single or in combination with fossil fuels. For example, the neat biodiesel can be applied in a boiler combustion system but the mixture of biodiesel and petrodiesel can be applied in an internal combustion engine3,4,5,6.
Bioethanol is renewable and ecofriendly biofuel which can be produced from sugar, starch, and cellulosic sources by fermentation and hydrolysis processes6. Various raw materials such as sugar, corn, wheat, potato, stem, hay, agricultural wastes, molasses, macroalgae, microalgae, and seaweed are potential feedstocks for the bioethanol production7,8. The feedstocks like sugar, corn, and potato are edible resources that fall into the first generation feedstocks for the biofuel production, which are not recommended for the bioethanol production due to food versus fuel debate. The application of agricultural wastes, molasses, macroalgae, microalgae, and seaweed as biofuel feedstocks could be a proper solution for this problem9. Bioethanol cannot be used as a single fuel in the diesel engines and it should be blended with the petro-diesel1,10. Using diesel/bioethanol blended fuel have some advantages such as increase in the rate of premixed combustion, improvement of the thermal efficiency and also reducing the smoke exhaust1. However, there are some challenges in the bioethanol addition to the diesel fuel such as limited solubility of bioethanol in the diesel, possible fuel phase separation in cold conditions, and negative effect of bioethanol on some fuel specifications such as Cetane number, heat value, and flash point11,12. Addition of emulsifiers or co-solvents is recommended to solve the phase separation issue in the diesel/bioethanol fuel blend. Among different potential co-solvents, the application of esters is recommended because esters can improve the blend properties and covering the negative effects of bioethanol on the fuel blend10,13.
Biodiesel is another type of biofuel that mainly consists of triglyceride methyl esters, which is produced from the plant oils or animal fats by different processes such as micro-emulsion, thermal cracking, and transesterification. Waste fish oil (WFO) is produced as a byproduct in large quantities in the fish processing industries. This byproduct is more valuable compared to other byproducts such as fish silage or fish fertilizer14. It should be noted that WFO is not a good feed for the pharmaceutical and functional food industries due to its low content of Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) and subsequent low omega-3 concentration. WFO is categorized in the second and third generations feedstocks for the biodiesel production including non-edible resources in which the food versus fuel dilemma is not encountered15. The application of WFO in the biodiesel production can reduce the costs of biodiesel production noticeably14. Biodiesel is usually used in combination with petro-diesel fuel in the diesel engine. The application of biodiesel in the engines leads to an increase in NOx emissions8,16,17. According to the literature, the application of the diesel/bioethanol/biodiesel fuel blend has better properties and performance compared to the diesel/biodiesel or the diesel/bioethanol fuel blends18.
Hulwan et al.12 studied the performance, emissions, and combustion characteristics of Jatropha biodiesel/ethanol/diesel fuel blend in a multi cylinder DI diesel engine. According to their results, the presence of biodiesel in the fuel blends results in an increase in the ethanol solubility in the fuel blend. BSFC of fuel blends increased by an increment in the ethanol content of the fuel blend. It was also found that the BTE increases at high load for all fuel blends compared to the neat diesel. Smoke was also decreased noticeably for all fuel blends compared to the neat diesel. According to their results, the CO emission for the fuel blends was increased significantly at low load and decreased slightly at high load compared to the neat diesel12. Kwanchareon et al.19 investigated the emission characteristics of palm oil biodiesel/ethanol/diesel in the diesel engine. According to this study, the biodiesel acts as an effective additive for the stabilization of ethanol in the fuel blend. According to the results, CO and HC emissions of fuel blends reduced significantly at high engine loads compared to the petro-diesel. It was also found that the NOx emission of the fuel blends increases compared to the neat diesel19. Aydin et al.6 have checked the emission and performance of an engine fueled with safflower biodiesel/ethanol/diesel. According to their results, no significant variation was observed in the engine torque and power using the fuel blends compared to the neat diesel. Regarding to the specific fuel consumption (SFC), an increase in the SFC was observed for the fuel blends compared to the neat diesel. CO2 and HC emissions of the fuel blends are generally more than the neat diesel. SO2 emission of the fuel blends is lower compared to the neat diesel6. Guarieiro et al.10 studied the emissions of a diesel engine fueled by ternary blends of (soybean biodiesel or castor biodiesel or residual biodiesel or soybean oil or castor oil)/ethanol/diesel. Based on their results, the utilization of diesel/ethanol blend fuel leads to the highest reduction in the NOx emission in comparison with the neat diesel. Regarding the CO2 emission, the results show that the fuel blends emission was decreased in the range of 5–24% and 4–6% at 1800 rpm and 2000 rpm compared to the neat diesel, respectively. No sensible difference between the CO emission of the blend fuels and the neat diesel was observed. More carbonyl compounds emission was observed for all examined blend fuel samples compared to the neat diesel10. Subbaiah et al.2 studied the emission and performance of an engine using rice bran oil biodiesel/ethanol/diesel fuel blends. Based to their report, BTE and BSFC of all examined fuel blends are higher compared to the neat diesel. It was also found that the NOx emission of all fuel blends were lower compared to the neat diesel at low loads and higher compared to the neat diesel at high loads. CO2 emission of all fuel blends is higher compared to the neat diesel. CO emission of all fuel blends is lower compared to the neat diesel. The HC emission of all fuel blends are higher compared to the neat diesel at low loads and lower compared to the neat diesel at high loads2. Recently, Sathiyaseelan et al.20 studied the performance, emission, and combustion characteristics of diesel/WFO biodiesel/ethanol fuel blends in DI diesel engine at various compression ratio. According to their report, the application of neat diesel result in the highest peak in the cylinder pressure and highest heat release rate (HRR) considering all examined fuel samples except for D91.25B7.5E1.25 at compression ratio of 18. Diesel fuel has the highest BTE and the lowest BSFC compared to the other examined fuel samples. Moreover, the lowest CO, and NOx emission was observed for D86.25B12.5E1.25 fuel blend considering all examined fuel samples. Table 1 shows a brief summary of the previous studies on the performance and emission of biodiesel/bioethanol/diesel fuel blends.
In summary, many researchers have focused on different diesel/biodiesel fuel blends, however, it was found that the addition of bioethanol to the biodiesel/diesel can improve the fuel blends performance such as reduction in NOx emission. Therefore, more researches should be carried out on the ternary fuel blend (i.e., biodiesel/bioethanol/diesel) as an appropriate alternative for the petro-diesel.
Based on our literature survey, the application WFO biodiesel in diesel/biodiesel/bioethanol fuel blends is only limited Sathiyaseelan et al.20 study which recently examined this ternary fuel blends in limited concentration ranges of WFO biodiesel and ethanol in the blend. Therefore, the available data on the physical property specifications, performance, and emission of WFO biodiesel/bioethanol/diesel are limited. In this regard, the present study is conducted to fill this research gap by considering ternary fuel blends of bioethanol, WFO biodiesel, and petro-diesel in more wide concentration ranges. In this regard, the physical property specifications (i.e., density, kinematic viscosity, pour point, cloud point, closed cup flash point, and heat value), the engine performance (torque, power, BSFC, and BTE), exhaust emission (CO, CO2, UHC, and NOx) were investigated for different WFO biodiesel/bioethanol/diesel fuel samples.
Experimental
Material
Neat petro-diesel was supplied by Tehran refining company (Tehran, Iran). Waste Fish Oil (WFO), for biodiesel production, was supplied from Daneh Talayi Chabahar Company (Chabahar, Iran). Methanol (99.8%), for the esterification and the transesterification reactions, and 2-propanol (99.8%), for determination of the free fatty acids content in the WFO, were purchased from Dr. Mojallalli Industrial Chemical complex company (Iran). Bioethanol (99.8%) was supplied from Kimia Alcohol Zanjan Company (Iran). Sulfuric acid (99.6%), as the catalyst in the esterification reactions, and potassium hydroxide (KOH), as the catalyst in the transesterification reactions, were purchased from Merck Company (Germany).
Preparation of waste fish oil (WFO) biodiesel
WFO which is a byproduct of the fish powder production company. WFO was heated in the production stages in the plant and was passed through a micro filter in order to recover the fish powders from the existing in the oil. Therefore, the purchased WFO is free from the solid impurities and water.
In this study, WFO biodiesel was produced by transesterification method. Free fatty acids (FFA) content of WFO is high, which results in undesirable reaction in the transesterification stage. Therefore, the FFA content of WFO should be decreased to < 1% to maximize the efficiency of biodiesel production via transesterification10. FFA content of WFO is determined by a simple acid/base neutralization experiment. FFA is determined by Eq. (1):
In which, A is the required volume of the solution for the titration of WFO in mL, W is the amount of WFO sample in g, N is the concentration of the titration solution in normality scale, and Wcat is the molecular weight of the catalyst. The titration solution is 0.1 N. Potassium hydroxide (KOH) and 2-propanol alcohol were used as the catalyst and solvent in the titration, respectively.
Since the FFA content of WFO is higher than 1%, the esterification processing step is required in one or more stages to reduce the FFA content of WFO < 1%. The esterification of WFO was carried out in a 70 L batch reactor equipped with a mechanical stirrer with 300 rpm stirring rate and a recycle stream. In the esterification reaction, the methanol to WFO ratio was 9 to 1 in the presence of 1 wt% KOH/sulfuric acid solution at 55 °C for 1 h4,14,26. After termination of the esterification reaction, the unreacted methanol and produced water should be separated from the esterified WFO. Therefore, the reactor content was introduced into a decanter for the phase separation. The treated WFO phase was separated after 24 h. After WFO esterification, the FFA content of treated WFO was reduced to 0.96% which is lower than 1%. Therefore, the treated WFO is ready for transesterification. The transesterification of treated WFO was carried out considering the methanol to WFO ratio of 6:1 in the presence of 1 wt% KOH solution at 60 °C for 1 h4,14,26. The products of transesterification reaction are biodiesel and glycerin. The glycerin phase was separated from the WFO biodiesel after 24 h. The final step in the WFO biodiesel preparation was water washing in order to remove the remaining catalyst, alcohol, soap, and glycerin from the WFO biodiesel. In this regard, the WFO biodiesel was mixed with water (water to biodiesel ratio of 2:1) at 60 °C for 1.5 h. The washing water was separated from the WFO biodiesel by decantation. The treated WFO biodiesel was heated at 85 °C for 8 h for separation of the remaining water from the WFO biodiesel. The final WFO biodiesel was used in the fuel blends.
Engine test and emission measurement system
An Air-cooled single-cylinder diesel engine (model 3LD510 from Lombardini Company) equipped with a dynamometer (model WE400 from Mobtakeran Pars Andish Company) was employed in order to determine the rotational speed, the torque, and power of the diesel engine using different fuel blends. A specific software was employed for other engine performance parameter calculations including brake power (BP), specific fuel consumption (SFC), brake specific fuel consumption (BSFC), and brake thermal efficiency (BTE) using the following equations4. The main specifications of the employed diesel engine and dynamometer can be found in the supporting information file.
MAHA-MGT5 device was used to measure the extent of engine emission using different fuel blends. MAHA-MGT5 is capable to determine the CO2, CO, and HC emission by infrared technology as well as O2 and NOx using electrochemical sensors. The detailed specifications of the applied emission sensors including the detection range and accuracy can be found in the supporting information file.
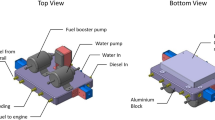
Figure 1 illustrates the single-cylinder diesel engine setup applied for evaluation of the engine performance and emission using different fuel blends.
It should be noted that in each engine test, the engine was held unloaded for 15 min until the oil temperature reaches 70 °C. This condition is so-called warming up the engine. Then, the full load condition was imposed on the engine and the engine speed was set to 1800 rpm. Afterwards, the engine performance and emission were measured at steady state condition. It should be added that the reliability of the experimental measurements was confirmed by repeating the experimental runs for the fuel blends. The relative standard deviation was in the acceptable range of 1–4%. It should be mentioned that the engine tests were carried out in the Renewable Energy laboratory, Bioenergy Research Center, Tarbiat Modares University, Tehran, Iran.
Fuel composition and physico-chemical properties measurement
The fatty acid profile of the WFO biodiesel can affect the engine emission and performance characteristics3,8. In the present study, Clarus 580 gas chromatography (GC) manufactured by Perkin Elmer Company was used to evaluate the fatty acid profile of WFO biodiesel. The GC was equipped with a flame ionization detector (FID) to specify the WFO different biodiesel compounds. A special GC column (model CP 9080 from Varian Company with 30 m length, internal diameter of 0.32 mm, and static phase thickness of 0.25 μm) was employed. Helium was used as the carrier gas. The temperature program of the column was adjusted according to EN 14,103 standard.
Important physicochemical properties of fuels are density, kinematic viscosity, pour point, cloud point, flash point, and heat value3,4,27. The density of different fuel blends was determined using SVM-3000 Stabinger viscometer (Anton paar Company) based on ASTM D4052 with precision of 0.0001 g/cm3. The kinematic viscosity of different fuel blends was determined using SVM-3000 Stabinger viscometer (Anton paar Company) based on ASTM D 455-06 with an accuracy of 0.01 cst. The pour point of fuel blends was determined according to ASTM D97. The measurement of the cloud point of fuel samples was carried out based on ASTM D2500 standard. The flash point of fuel blends was measured based on closed-cup method according to ASTM D93 using SKY1002-I flash point apparatus from Shanghai shenakai with an accuracy of ± 2 °C. The calorific value of the fuel blends was measured using Gallenkamp calorimeter bomb apparatus with an accuracy of ± 0.1%.
Results and discussion
Fuels properties
According to the GC analysis, the main fatty acid esters of WFO biodiesel are palmitic acid, and trans-9-Elaidic acid methyl ester. The presence of Myristic acid methyl ester in the WFO biodiesel is also confirmed. More details regarding the fatty acid profiles and the compositional analysis of the WFO biodiesel can be found in the supporting information file.
In the present study, different fuel blends were prepared by mixing different concentration of WFO biodiesel, bioethanol, and petro-diesel. Table 2 shows the detailed composition of different WFO biodiesel/bioethanol/diesel fuel samples. Afterwards, different specifications such as density, kinematic viscosity, pour point, cloud point, and flash point were measured for different fuel blends according to ASTM D 4052, ASTM D 445-06, ASTM D 97, ASTM D 2500, and ASTM D93 standards, respectively. The results of these measurements are presented in Figs. 2, 3, 4, 5, 6 and 7 for different examined fuel samples. The range of WFO biodiesel in the ternary fuel blends is 5–20%. The range of bioethanol in the ternary fuel samples is 5–15%. These selected concentration ranges are considerably wider compared to Sathiyaseelan et al.20 study.
Figure 2 shows the density of different examined fuel samples. It should be noted that the fuel density directly affect the injection timing, and spray quality of fuels in the combustion chamber28,29. Fuel density also affect the exhaust emissions. Using high density fuel results in increasing the amount of fuel entering the combustion chamber and it gets the oxygen to fuel ratio out of balance, which leads to incomplete combustion and subsequent increment in the emission of unburned hydrocarbons28. According to Fig. 2, the density of fuel blends increases by an increment in the WFO biodiesel content of blends because the density of WFO biodiesel is higher than the neat diesel due to higher molecular weight of WFO biodiesel compared to neat diesel30. The density of biodiesel produced from different sources are in the same range but the WFO biodiesel (density = 892 kg/m3) is more suitable for the fuel blends preparation compared to the soybean biodiesel (density = 913 kg/m3) and the pongamia biodiesel (density = 931 kg/m3)8. On the other hand, the density of ternary fuel blends decreases by an increment in the bioethanol content of blend due to lower density of bioethanol compared to diesel and WFO biodiesel. The addition of bioethanol leads to an improvement in the fuel blend/air mixing due to its low density which results in more complete combustion close to the stoichiometric ratio and subsequent decrease in the exhaust smoke31. According to Fig. 2, it was also found that the bioethanol addition to the ternary fuel blends neutralizes the density increment of WFO biodiesel addition. In this regard, the lowest value of density is observed for B5E15 fuel blends.
Figure 3 shows the kinematic viscosity of different fuel samples. The fuel viscosity is an important parameter which affects the spray quality and consequently the fuel and air mixing14. The viscosity of WFO biodiesel is higher compared to petro-diesel, which may leads to incomplete combustion by reducing the fuel and air mixing quality in the combustion chamber and increment in the exhaust emission3,32. Heat release rate (HRR) and cylinder pressure are the main combustion characteristics which are influenced by the fuel viscosity. Increasing the biodiesel content of the fuel blends leads to poor atomization of the fuel sample due to its high viscosity and results in an increment in the ignition delay and subsequent lower HRR and lower cylinder pressure33,34. On the other hand, the oxygen concentration of fuel blends is another parameter that affects the cylinder pressure. In this regard, the application biofuels leads to earlier combustion and improve the cylinder pressure due to increasing the oxygen content of the fuel blends35. According to Fig. 3, the kinematic viscosity of fuel blends increases by increasing the WFO biodiesel content and decreasing the bioethanol content of fuel blends, respectively. As an advantage, the viscosity of waste fish oil biodiesel is lower compared to calophyllum biodiesel by 87% and pongamia biodiesel by 48%8. It should be also noted that excessive reduction in the viscosity of fuel mixture is not desirable because it can be leads to leakage in the fuel system36. Therefore, the viscosity of fuel blends can be adjusted to the desired value within the standard range by adjusting the bioethanol and WFO biodiesel contents of the ternary fuel blends. It was also found that the B5E15 blends has the lowest value of kinematic viscosity.
Figures 4 and 5 show the pour point and cloud point of different fuel samples, respectively. Pour point and cloud point are important properties for using the fuel samples in cold climate conditions. Lack of attention to these parameters can leads to the crystal formation and subsequent blockage of the fuel lines at low temperatures37. As can be seen, an increment in the WFO biodiesel content of ternary fuel blends results in an increase in the pour point and cloud point of the fuel blends. Pour point and cloud point of WFO biodiesel is higher compared to petro-diesel, which is inappropriate for the engine performance in the cold weather conditions38. Pour point and cloud point of WFO biodiesel is more suitable for preparation of the fuel blends compared to other biodiesel samples. For example, the pour point and the cloud point of palm biodiesel are 15 °C and 16 °C respectively which are obviously higher compared to WFO biodiesel pour point and cloud point8. On the other hand, an increment in the bioethanol content of ternary fuel blends results in a decrease in the pour point and cloud point of fuel blends. Therefore, the cold properties of fuel blends can be tuned within the desired range by adjusting the WFO biodiesel and bioethanol content of the fuel blend. In this regard, the presence of bioethanol in the fuel blends can reduce the incremental impact of WFO biodiesel on the pour point and cloud point of ternary fuel blends to some extent38.
Figure 6 shows the flash point of different fuel samples. The flash point is an important property from safety point of view related to handling, storage, and transportation of fuel blends39. According to Fig. 6, an increment in the WFO biodiesel content of ternary fuel sample results in a slight increment in the flash point of the ternary fuel blends. Residual alcohol content, number of carbon atoms, and double bonds of the biodiesel are the most important factors that influence the biodiesel flash point40. However, increasing the bioethanol content of the fuel blends results in a considerable decrease in the flash point of the fuel blend. Generally, the blend component with the lowest flash point has the dominant effect on the fuel blend flash point. It should be noted that the addition of bioethanol in 0–10% range results in a noticeable reduction in the boiling point range of the fuel blend19,41. As can be observed, the flash point of fuel blends is mainly influenced by the bioethanol content. The average value of flash point for different fuel blends is about 34 °C, which is lower that petro-diesel and WFO biodiesel. Therefore, special cares should be applied in the transportation and storage of the fuel blends containing bioethanol.
Figure 7 shows the heat value of different fuel samples. As can be observed, an increase in the WFO biodiesel and bioethanol contents of the ternary fuel blend results in a decrease in the heat value of fuel blends. This finding can be explained by the lower heat value of WFO biodiesel and bioethanol compared to petro-diesel. The carbon chain length affects the heat value of fuel samples. In this regard, the longer carbon chain length results in the higher heat value of the fuel sample42. Therefore, addition of WFO biodiesel and bioethanol to the ternary fuel blends results in a decrement in the heat value of the fuel blend. In this regard, B5E5 has the highest heat value considering different examined fuel blends. Besides, it was found that the heat value of the ternary fuel blends is decreased by 7% in average compared to the neat petro-diesel.
Figure 8 shows the Cetane index of different examined fuel samples. As can be observed in Fig. 8, the addition of WFO biodiesel and bioethanol to the fuel blend leads to a subsequent decrease in the Cetane index. It should be noted that addition of bioethanol to the fuel blends leads to a considerable decrement in the Cetane index of fuel blends due to its too low Cetane index. Bizzo and Moretti43 also reported Cetane number decrement as a result of increment of the ethanol content of fuel blend. It should be noted that an increment in the Cetane index of fuel blend results in a decrease in the ignition delay and more rapid combustion during the premixed combustion phase, which results in consequent higher cylinder pressure. Therefore, in cylinder pressure of fuel blends is decreased by increasing the bioethanol and WFO biodiesel contents of fuel blends31,44,45.
Engine performance
After evaluation of different physicochemical specifications of the fuel blends, the engine performance parameters including power, torque, brake thermal efficiency, and engine specific fuel consumption were investigated in a single-cylinder diesel engine at the speed of 1800 rpm and full load condition.
Brake power and torque
Figures 9 and 10 show the engine torque and brake power using different ternary fuel blends containing different percentages of diesel, WFO biodiesel, and bioethanol, respectively.
As mentioned in the previous section, the heat value of WFO biodiesel is lower compared to petro-diesel. The heat value of bioethanol is also lower compared to WFO biodiesel. Therefore, an increment in the WFO biodiesel and bioethanol contents of the ternary fuel blends leads to a decrease in the engine torque and brake power. The lower heat value of the fuel blends containing WFO biodiesel and bioethanol leads to lower energy output in the combustion process and subsequent lower brake power and torque4,46. The application of fuel blends containing different concentration of bioethanol at constant concentration of 5%, 12.5%, and 20% of WFO biodiesel results in a reduction in the engine torque by 1.1%, 2.4%, and 3.9%, respectively. For these fuel blends, the engine break power also reduces by 0.8%, 2.3%, and 3.7%, respectively. Using the fuel blends containing different concentration of WFO biodiesel at constant concentration of 5%, 10%, and 15% of bioethanol results in a decrement in the engine torque by 1.9%, 2.4%, and 3% and break power by 1.7%, 2.3%, and 2.9%, respectively.
Brake specific fuel consumption (BSFC)
Figure 11 shows BSFC of different examined ternary fuel blends. Break specific fuel consumption is the ratio of the fuel consumption rate to the generated power. BSFC, as a measure for the evaluation of the fuel efficiency, depends on the heat value, density, and viscosity of fuel4,46,47. As can be seen in Fig. 11, an increment in the WFO biodiesel content of the ternary fuel blends result in a subsequent increase in the BSFC. This can be attributed to the decrement in the efficiency due to lower heat value, higher density, and higher viscosity of WFO biodiesel compared to the petro-diesel. An increment in the bioethanol content of the ternary fuel blends also results in an increase in BSFC. Bioethanol has lower density and viscosity compared to WFO biodiesel and petro-diesel. Therefore, an enhancement in the fuel and air mixing is expected by addition of bioethanol to the fuel blends. However, the considerable lower heat value of bioethanol compared to WFO biodiesel and petro-diesel results in a subsequent increase in the BSFC12,48,49. Fang et al.50, Zhu et al.51, and Al-Hassan et al.52 reported an increase in the BSFC by an increase in the ethanol content of fuel blends. It should be mentioned that WFO biodiesel and bioethanol addition to the ternary fuel blends leads to about 10% on average increment in the BFSC compared to petro-diesel.
Brake thermal efficiency (BTE)
Figure 12 shows BTE for different examined fuel blends. BTE is a parameter that represent the efficiency of the fuel energy conversion to the mechanical energy output12. BTE is influenced by BSFC and heat value of fuel, inversely. As can be observed in Fig. 12, the trend of BTE change for different fuel blends is opposite to the trend of BFSC. It was also found that BTE for B5E5, B5E10, and B12.5E5 fuel blends is higher compared to the neat petro-diesel in spite of their higher BSCF compared to petro-diesel. This can be explained by the lower heat value of these ternary fuel blends in comparison with the petro-diesel, which dominate the effect of BFSC on BTE. The results also show that an increment in the bioethanol content of the ternary fuel blends results in a decrease in BTE.
Engine exhaust emission
CO emission
Figure 13 shows the CO emission in the engine exhaust for different examined ternary fuel samples. Figure 13a shows CO emission in %. Figure 13b shows CO emission in g/kWh, which is calculated by Eq. (6)53:
The emission of CO, as a toxic gas, can be attributed to the incomplete combustion as a result of poor fuel and oxygen mixing. As can be observed in Fig. 13, the CO emission is decreased by an increment in the WFO biodiesel and bioethanol contents of the ternary fuel blends. This can be explained by an increment in the oxygen content of the fuel blends by increasing the WFO biodiesel and bioethanol content of the ternary fuel blends. Enrichment in the oxygen content of the ternary fuel blends results in more complete combustion due to further oxidation of CO in the combustion process. This finding is in agreement with Kwanchareon et al.19, Subbaiah et al.2, Li et al.54, Shi et al.55, and Guarieiro et al.10 studies regarding the CO emission in full load engine performance. According to Fan et al.50 and Zhu et al.51 studies, the CO emission is increased using rape seed oil biodiesel/ethanol/diesel and waste cooking oil biodiesel/ethanol/diesel compared to the neat diesel fuel. However, a noticeable reduction in the CO emission is observed in the present study as an advantage of using WFO biodiesel in the ternary fuel samples. It should be noted that the CO emission of B5E5 (i.e., the fuel blends with the lowest WFO biodiesel and bioethanol content) is 20.22% lower compared to the petro-diesel. Besides, the CO emission of B20E15 (i.e., the fuel blends with the highest WFO biodiesel and bioethanol content) is 70.22% lower compared to the petro-diesel. This is a noticeable reduction in CO emission.
CO 2 emission
Figure 14 shows the CO2 emission in the engine exhaust for different examined ternary fuel samples. Figure 14a shows CO2 emission in volume percent. Figure 14b shows CO2 emission in g/kWh which is calculated by Eq. (7)53.
According to Fig. 14, CO2 emission in the engine exhaust is increased by increment in the WFO biodiesel and bioethanol content of the ternary fuel blend. As mentioned in the previous section, an increment in the oxygen content of the fuel blends as a result of WFO biodiesel and bioethanol addition leads to an enhancement in the complete combustion and subsequent increment in the CO2 emissions. This finding is in agreement with Subbaiah et al.2, Hulwan et al.12, Cheenkachorn et al.56, and Guarieiro et al.10 studies.
Unburned hydrocarbons (UHC) emission
Figure 15 shows the UHC emission in the engine exhaust for different examined ternary fuel samples. Figure 15a shows UHC emission in ppm. Figure 15b shows UHC emission in g/kWh which is calculated by Eq. (8)25.
As can be observed in Fig. 15, the UHC emission is significantly lower for all examined ternary fuel blends in comparison with the petro-diesel. An increase in the WFO biodiesel content of the fuel blends results in more complete combustion and subsequent lower UHC emission. According to the previous studies, the UHC emission is also decreased obviously by an increment in the biodiesel content of the fuel blend samples19,57. It was also found that increasing the bioethanol content of the fuel blends results in an increment in the UHC emission. This can be attributed the decrement in the combustion efficiency as a result of bioethanol increment in the fuel blends. The trend of BSFC with bioethanol content of the fuel blends also confirms this fact. According to Kwanchareon et al.19 and Subbaiah et al.2, this can be attributed to unburned bioethanol emission in the engine exhaust as a result of large ethanol dispersion region in the combustion chamber. According to Fang et al.50, the application of rape seed oil biodiesel in a biodiesel/alcohol/diesel blend results in an increment in UHC emission. However, as a clear advantage, the application of waste fish oil biodiesel in ternary fuel samples has superior performance compared to rape seed oil biodiesel.
Nitrogen oxide (NO x ) emission
Figure 16 shows the NOx emission in the engine exhaust for different examined ternary fuel samples. Figure 16a shows NOx emission in ppm. Figure 16b shows NOx emission in g/kWh which is calculated by Eq. (9)25.
As can be observed in Fig. 16, the NOx emission is increased by an increment in the WFO biodiesel content of the ternary fuel blends. It is also found that an increment in the bioethanol content of the ternary fuel blends results in a decrement in the NOx emission. The dominant mechanism of NOx formation is thermal mechanism. Thermal mechanism includes the endothermic reactions, which stimulated by high temperature. Equation (10) gives NO formation rate based on Zeldovich mechanism which describes the NO formation by thermal mechanism58:
According to Eq. (10), the temperature and oxygen concentration are effective parameters in NO formation. In this regard, an increase in the temperature leads to an increment in NO formation. Increasing the oxygen concentration also leads to increasing NO formation because the oxygen as a reactant for NOx formation reaction, is an essential agent for the endothermic reaction progress3,58.
Increasing biodiesel content in the fuel blends leads to the increment in the fuel blend oxygen content. The normal boiling point of biodiesel is about 350 °C and it ignites before evaporation, which leads to increasing the combustion chamber temperature. Therefore, the increment in the fuel blend oxygen content and combustion chamber temperature due to WFO biodiesel addition to the ternary fuel blends results in an increment in the NOx emission59. Increasing bioethanol in the fuel blends also leads to the fuel blend oxygen content increment. However, the normal boiling point of bioethanol is about 78 °C and its latent heat of evaporation is 846 kJ/kg. It seems that a part of bioethanol content of the fuel blends evaporates during ignition in the combustion chamber, which leads to a noticeable reduction in the combustion chamber temperature. Therefore, the increment in the fuel blend oxygen content and the decrement in the combustion chamber temperature due to bioethanol addition to the fuel blends affect the NO formation rate oppositely. The decreasing trend of NOx formation by increasing the bioethanol content of the fuel blends confirmed that the combustion chamber temperature decrement effect on the NO formation is dominant and therefore, the increment in the bioethanol content of the ternary fuel blends results in a decrement in the NOx emission7,12.
This is an interesting pull–push effect, which can be applied to adjust the engine NOx emission to the desired limit by adjusting the WFO biodiesel and bioethanol contents of the ternary fuel blends. In this regard, as can be observed, the NOx emission of B5E15 fuel blend is in the range of the neat petro-diesel, approximately.
Recommended ternary fuel formulation
According to Euro 5 diesel standard, B5E5, B5E10, and B5E15 fuel blends are the fuel samples which pass the standard criteria. These fuel sample can be considered as the potential candidates for the best fuel blends. B5E5 fuel blend has better performance compared to the other fuel blends considering the engine performance tests. B5E15 fuel blend has better performance compared to the other fuel blends considering the engine exhaust emissions.
According to Kara et al.60, the petro-diesel, and WFO biodiesel costs are 0.91 USD/L, 0.69 USD/L, respectively. According to IRENA reports61, the bioethanol cost is 1.04 USD/L. Therefore, a preliminary cost evaluation can be carried out for different ternary fuel blends. Figure 17 shows the outcome of this preliminary cost evaluation.
According to Fig. 17, cost of all fuel blends is comparable to the neat petro-diesel. It should be noted that the cost of WFO biodiesel, petrodiesel, and bioethanol is varied throughout the world as a results of balance between supply and demand of these fuel, the availability of raw materials and the employed production process. Therefore, more detailed cost analysis in different situations is highly recommended.
Conclusion
In the presents study, the physicochemical specifications, engine performance, and engine exhaust were investigated for different ternary fuel blends containing WFO biodiesel, bioethanol, and petro-diesel. The main findings are as follows:
-
It was found that the viscosity, density, cloud point, and pour point of all examined fuel blends are in standard range and close to the petro-diesel. However, the flash point of the fuel blends containing high bioethanol content is considerably lower compared to the neat petro-diesel due to low flash point of bioethanol. This should be considered in the storage and handling of the fuel blends. The calorific value of the fuel blends containing WFO biodiesel and bioethanol is lower compared to the neat petro-diesel.
-
Regarding the engine performance parameters, it was found that the engine torque and brake power is lower for the ternary fuel blends containing WFO biodiesel and bioethanol compared to the neat petro-diesel. The BSFC was higher for the fuel blends compared to the neat petro-diesel is used. This can be attributed to the lower heat value of the ternary fuel blends compared to the neat petro-diesel.
-
Regarding the engine exhaust emission, a significant decrement in the CO emission and UHC emission was observed for the ternary fuel blends compared to the neat petro-diesel. This is a clear advantage of using WFO biodiesel/bioethanol/diesel fuel blends compared to the neat petro-diesel. It was also found that despite the increment in NOx emission using the fuel blends containing WFO biodiesel and bioethanol, the engine NOx emission could be adjusted to the desired limit by tuning the WFO biodiesel and bioethanol content of the ternary fuel blends.
-
B5E5 fuel blend has the best performance compared to the other fuel blends considering the engine performance parameters. Moreover, B5E15 is the best fuel blend considering the engine exhaust emissions. Investigation of the influence of the engine rpm and load on the performance and emission of different fuel blends is an interesting subject for future studies.
Data availability
It should be justified that “All data generated or analysed during this study are included in this published article [and its supplementary information files]”.
References
Shahir, S. A., Masjuki, H. H., Kalam, M. A., Imran, A. & Ashraful, A. M. Performance and emission assessment of diesel-biodiesel-ethanol/bioethanol blend as a fuel in diesel engines: A review. Renew. Sustain. Energy Rev. 48, 62–78. https://doi.org/10.1016/j.rser.2015.03.049 (2015).
Subbaiah, G. V. V. et al. Rice bran oil biodiesel as an additive in diesel–ethanol blends for diesel engines. Int. J. Recent Res. Appl. Stud. 3, 334–342 (2010).
Bahadorizadeh, O., Sobati, M. A. & Shahnazari, S. Emission characteristics of a semi-industrial boiler fueled by waste cooking oil biodiesel containing different metal oxide nanoparticles. Process. Saf. Environ. Prot. 158, 199–209. https://doi.org/10.1016/j.psep.2021.11.050 (2022).
Khanjani, A. & Sobati, M. A. Performance and emission of a diesel engine using different water/waste fish oil (WFO) biodiesel/diesel emulsion fuels: Optimization of fuel formulation via response surface methodology (RSM). Fuel 288, 119662. https://doi.org/10.1016/j.fuel.2020.119662 (2021).
Bazooyar, B. & Shariati, A. A comparison of the emission and thermal capacity of methyl ester of corn oil with diesel in an experimental boiler. Energy Sources Part A Recover Util. Environ. Eff. 35, 1618–1628. https://doi.org/10.1080/15567036.2010.527902 (2013).
Aydın, F. & Öğüt, H. Effects of using ethanol–biodiesel–diesel fuel in single cylinder diesel engine to engine performance and emissions. Renew. Energy 103, 688–694. https://doi.org/10.1016/j.renene.2016.10.083 (2017).
Balat, M., Balat, H. & Öz, C. Progress in bioethanol processing. Prog. Energy Combust. Sci. 34, 551–573. https://doi.org/10.1016/j.pecs.2007.11.001 (2008).
Dharma, S., Ong, H. C., Masjuki, H. H., Sebayang, A. H. & Silitonga, A. S. An overview of engine durability and compatibility using biodiesel–bioethanol–diesel blends in compression-ignition engines. Energy Convers. Manag. 128, 66–81. https://doi.org/10.1016/j.enconman.2016.08.072 (2016).
Ghobadian, B., Yusaf, T., Najafi, G. & Khatamifar, M. Diesterol: An environment-friendly IC engine fuel. Renew. Energy 34, 335–342 (2009).
Guarieiro, L. L. N., de Souza, A. F., Torres, E. A. & de Andrade, J. B. Emission profile of 18 carbonyl compounds, CO, CO2, and NOx emitted by a diesel engine fuelled with diesel and ternary blends containing diesel, ethanol and biodiesel or vegetable oils. Atmos. Environ. 43, 2754–2761. https://doi.org/10.1016/j.atmosenv.2009.02.036 (2009).
Özgür, T., Özcanli, M. & Aydin, K. Investigation of nanoparticle additives to biodiesel for improvement of the performance and exhaust emissions in a compression ignition engine. Int. J. Green Energy 12, 51–6. https://doi.org/10.1080/15435075.2014.889011 (2015).
Hulwan, D. B. & Joshi, S. V. Performance, emission and combustion characteristic of a multicylinder DI diesel engine running on diesel-ethanol–biodiesel blends of high ethanol content. Appl. Energy 88, 5042–5055. https://doi.org/10.1016/j.apenergy.2011.07.008 (2011).
Fernando, S. & Hanna, M. Development of a novel biofuel blend using ethanol–biodiesel–diesel microemulsions: EB-diesel. Energy Fuels 18, 1695–1703 (2004).
García-Moreno, P. J., Khanum, M., Guadix, A. & Guadix, E. M. Optimization of biodiesel production from waste fish oil. Renew. Energy 68, 618–624. https://doi.org/10.1016/j.renene.2014.03.014 (2014).
Abomohra, A. E. F., Elsayed, M., Esakkimuthu, S., El-Sheekh, M. & Hanelt, D. Potential of fat, oil and grease (FOG) for biodiesel production: A critical review on the recent progress and future perspectives. Prog. Energy Combust. Sci. 81, 100868. https://doi.org/10.1016/j.pecs.2020.100868 (2020).
Shrivastava, N. Experimental investigation of performance, emission, and noise parameters of water-emulsified Karanja biodiesel: A prospective Indian fuel. J. Braz. Soc. Mech. Sci. Eng. 39, 1009–1017 (2017).
Zhang, Z. et al. Effects of low-level water addition on spray, combustion and emission characteristics of a medium speed diesel engine fueled with biodiesel fuel. Fuel 239, 245–262 (2019).
Ramírez-Verduzco, L. F., Rodríguez-Rodríguez, J. E. & del Rayo, J.-J. Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel 91, 102–111 (2012).
Kwanchareon, P., Luengnaruemitchai, A. & Jai-In, S. Solubility of a diesel-biodiesel-ethanol blend, its fuel properties, and its emission characteristics from diesel engine. Fuel 86, 1053–1061. https://doi.org/10.1016/j.fuel.2006.09.034 (2007).
Sathiyaseelan, V., Lakshmana Gowder, S. & Sathyamurthy, R. Comparative assessment of low-concentration ethanol and waste fish oil biodiesel blends on emission reduction and performance improvement in variable compression ratio engine. J. Therm. Sci. 32, 1306–1319. https://doi.org/10.1007/s11630-023-1757-3 (2023).
Paul, A., Panua, R. & Debroy, D. An experimental study of combustion, performance, exergy and emission characteristics of a CI engine fueled by diesel–ethanol–biodiesel blends. Energy 141, 839–852 (2017).
Yilmaz, N., Vigil, F. M., Donaldson, A. B. & Darabseh, T. Investigation of CI engine emissions in biodiesel–ethanol–diesel blends as a function of ethanol concentration. Fuel 115, 790–793 (2014).
Paul, A., Panua, R., Bose, P. K. & Banerjee, R. An experimental study of performance and emission parameters of a compression ignition engine fueled by different blends of diesel–ethanol–biodiesel. In 2013 International Conference on Energy Efficient Technologies for Sustainability 786–791 (IEEE, 2013).
Krishna, S. M., Salam, P. A., Tongroon, M. & Chollacoop, N. Performance and emission assessment of optimally blended biodiesel–diesel–ethanol in diesel engine generator. Appl. Therm. Eng. 155, 525–533 (2019).
Ağbulut, Ü., Sarıdemir, S. & Albayrak, S. Experimental investigation of combustion, performance and emission characteristics of a diesel engine fuelled with diesel–biodiesel–alcohol blends. J. Braz. Soc. Mech. Sci. Eng. https://doi.org/10.1007/s40430-019-1891-8 (2019).
El-Mashad, H. M., Zhang, R. & Avena-Bustillos, R. J. A two-step process for biodiesel production from salmon oil. Biosyst. Eng. 99, 220–227. https://doi.org/10.1016/j.biosystemseng.2007.09.029 (2008).
Ashraful, A. M. et al. Production and comparison of fuel properties, engine performance, and emission characteristics of biodiesel from various non-edible vegetable oils: A review. Energy Convers. Manag. 80, 202–228 (2014).
Hoang, A. T. & Le, A. T. Trilateral correlation of spray characteristics, combustion parameters, and deposit formation in the injector hole of a diesel engine running on preheated Jatropha oil and fossil diesel fuel. Biofuel Res. J. 6, 909–919. https://doi.org/10.18331/BRJ2019.6.1.2 (2019).
Moneib, H. A., Mahfouz, A., El-Fatih, A. & Emara, A. Near-field spray characterization of a spill return atomizer using a PIV laser sheet. Fuel 289, 119792. https://doi.org/10.1016/j.fuel.2020.119792 (2021).
Alptekin, E. & Canakci, M. Determination of the density and the viscosities of biodiesel–diesel fuel blends. Renew. Energy 33, 2623–2630. https://doi.org/10.1016/J.RENENE.2008.02.020 (2008).
Labeckas, G., Slavinskas, S. & Mažeika, M. The effect of ethanol-diesel-biodiesel blends on combustion, performance and emissions of a direct injection diesel engine. Energy Convers. Manag. 79, 698–720. https://doi.org/10.1016/j.enconman.2013.12.064 (2014).
Alptekin, E. & Canakci, M. Characterization of the key fuel properties of methyl ester–diesel fuel blends. Fuel 88, 75–80. https://doi.org/10.1016/J.FUEL.2008.05.023 (2009).
Adhithan, B. & Sachdeva, G. Analysing the emissions and fuel efficiency of fish-waste biodiesel in a compression ignition engine. Int. J. Amb. Energy https://doi.org/10.1080/01430750.2023.2190329 (2023).
Sharma, D. K. & Verma, T. N. Characteristics of fish oil biodiesel with the impact of diesel fuel addition on a ci engine. J. Comput. Appl. Res. Mech. Eng. 10, 245–256. https://doi.org/10.22061/jcarme.2019.4737.1571 (2020).
Mrad, N., Varuvel, E. G., Tazerout, M. & Aloui, F. Effects of biofuel from fish oil industrial residue–diesel blends in diesel engine. Energy 44, 955–963. https://doi.org/10.1016/j.energy.2012.04.056 (2012).
Kwangdinata, R., Raya, I. & Zakir, M. Production of biodiesel from lipid of phytoplankton Chaetoceros calcitrans through ultrasonic method. Sci. World J. https://doi.org/10.1155/2014/231361 (2014).
Wan Ghazali, W. N. M., Mamat, R., Masjuki, H. H. & Najafi, G. Effects of biodiesel from different feedstocks on engine performance and emissions: A review. Renew. Sustain. Energy Rev. 51, 585–602. https://doi.org/10.1016/J.RSER.2015.06.031 (2015).
Hussan, M. J., Hassan, M. H., Kalam, M. A. & Memon, L. A. Tailoring key fuel properties of dieselebiodieseleethanol blends for diesel engine. J. Clean. Prod. 51, 118–125. https://doi.org/10.1016/j.jclepro.2013.01.023 (2013).
Boog, J. H. F., Silveira, E. L. C., De Caland, L. B. & Tubino, M. Determining the residual alcohol in biodiesel through its flash point. Fuel 90, 905–907. https://doi.org/10.1016/J.FUEL.2010.10.020 (2011).
Carareto, N. D. D., Kimura, C. Y. C. S., Oliveira, E. C., Costa, M. C. & Meirelles, A. J. A. Flash points of mixtures containing ethyl esters or ethylic biodiesel and ethanol. Fuel 96, 319–326. https://doi.org/10.1016/J.FUEL.2012.01.025 (2012).
Černoch, M., Hájek, M. & Skopal, F. Relationships among flash point, carbon residue, viscosity and some impurities in biodiesel after ethanolysis of rapeseed oil. Bioresour. Technol. 101, 7397–7401. https://doi.org/10.1016/j.biortech.2010.05.003 (2010).
Oliveira, L. E. & Da Silva, M. L. Comparative study of calorific value of rapeseed, soybean, jatropha curcas and crambe biodiesel. Renew. Energy Power Qual. J. https://doi.org/10.24084/repqj11.411 (2013).
Bizzo, W. & Moretti, R. R. The effects of blending diesel, ethanol, and biodiesel. Energy Sources Part A Recover Util. Environ. Eff. 38, 2111–2118. https://doi.org/10.1080/15567036.2013.832440 (2016).
Shahir, S. A. et al. Feasibility of diesel–biodiesel–ethanol/bioethanol blend as existing CI engine fuel: An assessment of properties, material compatibility, safety and combustion. Renew. Sustain. Energy Rev. 32, 379–395. https://doi.org/10.1016/j.rser.2014.01.029 (2014).
Gharehghani, A., Mirsalim, M. & Hosseini, R. Effects of waste fish oil biodiesel on diesel engine combustion characteristics and emission. Renew. Energy 101, 930–936. https://doi.org/10.1016/j.renene.2016.09.045 (2017).
Koc, A. B. & Abdullah, M. Performance and NOx emissions of a diesel engine fueled with biodiesel-diesel-water nanoemulsions. Fuel Process. Technol. 109, 70–77 (2013).
Jannatkhah, J., Najafi, B. & Ghaebi, H. Energy-exergy analysis of compression ignition engine running with biodiesel fuel extracted from four different oil-basis materials. Int. J. Green Energy 16, 749–762 (2019).
Abdel-Rahman, A. A. On the emissions from internal-combustion engines: A review. Int. J. Energy Res. 22, 483–513 (1998).
Ajav, E. A., Singh, B. & Bhattacharya, T. K. Experimental study of some performance parameters of a constant speed stationary diesel engine using ethanol–diesel blends as fuel. Biomass Bioenergy 17, 357–365 (1999).
Fang, Q., Fang, J., Zhuang, J. & Huang, Z. Effects of ethanol–diesel–biodiesel blends on combustion and emissions in premixed low temperature combustion. Appl. Therm. Eng. 54, 541–548. https://doi.org/10.1016/J.APPLTHERMALENG.2013.01.042 (2013).
Zhu, L., Cheung, C. S., Zhang, W. G. & Huang, Z. Combustion, performance and emission characteristics of a DI diesel engine fueled with ethanol–biodiesel blends. Fuel 90, 1743–1750. https://doi.org/10.1016/J.FUEL.2011.01.024 (2011).
Al-Hassan, M., Mujafet, H. & Al-Shannag, M. An experimental study on the solubility of a diesel–ethanol blend and on the performance of a diesel engine fueled with diesel–biodiesel–ethanol blends. Jordan J. Mech. Ind. Eng. 6, 147–153 (2012).
Pilusa, T. J., Mollagee, M. M. & Muzenda, E. Reduction of vehicle exhaust emissions from diesel engines using the whale concept filter. Aerosol Air Qual. Res. 12, 994–1006. https://doi.org/10.4209/aaqr.2012.04.0100 (2012).
Li, D. G., Zhen, H., Xingcai, L., Wu-Gao, Z. & Jian-Guang, Y. Physico-chemical properties of ethanol-diesel blend fuel and its effect on performance and emissions of diesel engines. Renew. Energy 30, 967–976. https://doi.org/10.1016/j.renene.2004.07.010 (2005).
Shi, X. et al. Emission characteristics using methyl soyate–ethanol–diesel fuel blends on a diesel engine. Fuel 84, 1543–1549. https://doi.org/10.1016/J.FUEL.2005.03.001 (2005).
Cheenkachorn, K. & Fungtammasan, B. Biodiesel as an additive for diesohol. Int. J. Green Energy 6, 57–72 (2009).
Park, S. H., Cha, J. & Lee, C. S. Impact of biodiesel in bioethanol blended diesel on the engine performance and emissions characteristics in compression ignition engine. Appl. Energy 99, 334–343 (2012).
Bazooyar, B., Ebrahimzadeh, E., Jomekian, A. & Shariati, A. NOx formation of biodiesel in utility power plant boilers. Part A: Influence of fuel characteristics. Energy Fuels 28, 3778–3792. https://doi.org/10.1021/ef500001g (2014).
Liu, H. P., Strank, S., Werst, M., Hebner, R. & Osara, J. Combustion emissions modeling and testing of neat biodiesel fuels. In ASME 2010 4th International Conference on Energy Sustainability ES, Vol. 1, 131–140 (2010). https://doi.org/10.1115/ES2010-90038.
Kara, K. et al. Biodiesel production from waste fish oil with high free fatty acid content from Moroccan fish-processing industries. Egypt J. Pet. 27, 249–255. https://doi.org/10.1016/j.ejpe.2017.07.010 (2018).
International Renewable Energy Agency. Road transport: The cost of renewable solutions. Int. Renew. Energy Agency 80 (2013).
Author information
Authors and Affiliations
Contributions
D.T.: Investigation; drawing figures; methodology; formal analysis; software; writing—original draft; visualization. M.A.S.: Conceptualization; supervision; writing—review and editing; project administration. S.S.: Formal analysis; software; writing—original draft; visualization. B.G.: Supervision; writing—review and editing; validation. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tarangan, D., Sobati, M.A., Shahnazari, S. et al. Physical properties, engine performance, and exhaust emissions of waste fish oil biodiesel/bioethanol/diesel fuel blends. Sci Rep 13, 14024 (2023). https://doi.org/10.1038/s41598-023-41280-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-41280-5
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.