Abstract
TBX1, which encodes a T-box transcription factor, is considered a candidate gene for DiGeorge syndrome, velocardiofacial syndrome, and conotruncal anomaly face syndrome. Transduction of TBX1 decreases cell proliferation in epithelial cancer cells and Tbx1 ablation induces epithelial proliferation during palatal development. Here, we report that TBX1 regulates stem cell properties and epithelial differentiation through the transcriptional activation of microRNAs. Stable expression of TBX1 induces microRNA-200 (miR-200), whose members repress the epithelial-to-mesenchymal transition and induce epithelial differentiation. TBX1 rescues ZEB2-dependent transcriptional inhibition of the miR-200b/200a/429 cluster, whose promoter region contains conserved overlapping cis-regulatory motifs of the ZEB-binding E-box and TBX-binding element. Consequently, TBX1 activates the expression of both miR-200 and stemness-inhibitor miR-203 to inhibit their common targets, BMI1 and ZEB2. Moreover, Tbx1 ablation affects the differentiation of the palatal epithelium and perturbs the expression of miR-200, miR-203, and their target genes. We propose that TBX1 links stem cell properties and epithelial differentiation by inducing miR-200 and miR-203. Thus, targeting of the ZEB2–miR-200 axis by TBX1 may have potential therapeutic implications in miR-200-associated tumors and cleft palate.
Similar content being viewed by others
Introduction
TBX1, which encodes a T-box transcription factor, is considered a candidate gene for DiGeorge syndrome (OMIM #188,400), velocardiofacial syndrome (OMIM #192,430), and conotruncal anomaly face syndrome (OMIM #217,095)1,2,3. Tbx1-null (Tbx1Δ/Δ) mice exhibit craniofacial and cardiovascular phenotypes, including cleft palate phenotypes, in patients with DiGeorge syndrome4,5,6,7. Tbx1 is transiently expressed in the undifferentiated oral epithelium, and its expression decreases upon subsequent palatal fusion4. Tbx1 ablation increases the number of basal cells in the palatal epithelium and induces abnormal oral epithelial differentiation and intraoral epithelial adhesion4. In addition, TBX1 reduces cell growth in epithelial cancer cell lines4,8. These findings suggest that TBX1 is linked to both tissue morphogenesis and tumor growth.
The epithelial-to-mesenchymal transition (EMT) is critically important for the development and progression of carcinomas or disease conditions and is linked to the gain of stem cell properties9,10. EMT is associated with marked changes in cell–cell adhesion, polarity, motility, and migration, suggesting that EMT drives epithelial tumor invasion, embryonic development, and tissue remodeling9,10. EMT is typically characterized by the downregulation of the epithelial marker E-cadherin/CDH1 and upregulation of the mesenchymal marker N-cadherin/CDH29,10,11. The EMT program is regulated by multiple networks of microRNAs (miRNAs) and EMT transcription factors, including the ZEB (ZEB1 and ZEB2/SIP1/ZFHX1B), Twist, and Snail families, whose expression and functional importance depend on the tissue type10.
The miR-200 family (miR-200) induces epithelial differentiation, thereby suppressing EMT in epithelial cancers, and is associated with reduced invasion, metastasis, and embryonic development10. miR-200 has five members derived from two microRNA clusters: miR-200b/200a/429 are encoded on human chromosome 1p36.33 and miR-141/200c on chromosome 12p13.31. miR-200 inhibits mRNA translation of the EMT-activators ZEB1 and ZEB2, which harbor conserved miR-200 sites in their 3′ untranslated region (3′UTR)12,13. Conversely, miR-200 expression can be directly suppressed by ZEB2 and ZEB1, indicating that miR-200 and ZEB proteins reciprocally regulate each other via a feedback loop12,14,15,16. Zeb1 ablation induces cleft palate in 50% of mice17, and embryos having the compound genotype of Zeb1- and Zeb2-deficient mice exhibit midfacial cleft, suggesting that ZEB1 and ZEB2 interact synergistically in midfacial development18. miR-200b, which is expressed in the palatal epithelium, represses the expression of Zeb1 and Zeb2 during palatogenesis19. Moreover, miR-200b targets angiogenesis-related genes, vascular endothelial growth factor-A (VEGFA) and its receptors FLT1/VEGFR1 and KDR/VEGFR2/FLK20,21. VEGF signaling plays a crucial role in pathological and physiological angiogenesis, contributing to tumor progression from dormant in situ lesion to metastasis22. Along with miR-200, ZEB1 promotes tumorigenicity by repressing the stemness-inhibiting, skin-specific miR-203, which induces differentiation of skin stem cells into suprabasal cells23,24. Tumor suppressor miR-203 expression was enriched in keratin-high cells from cervical squamous cancers25. Despite the importance of EMT- and stemness-inhibiting microRNAs, a limited number of transcription factors have been reported to induce their expression, and the regulators that contribute to mesenchymal-to-epithelial transition (MET) remain to be elucidated.
Here, we investigated the role of the relationship among TBX1, EMT-inhibiting miRNAs, and the EMT-activator ZEB2 in the control of stemness. We found that TBX1 inhibited stem cell properties in cervical carcinoma cells by regulating the expression of miR-200, miR-203, and their target genes. Moreover, Tbx1 ablation in mice inhibited epithelial differentiation, accompanied by the reduction of miR-200 and miR-203 and the induction of their target genes.
Results
TBX1 is a regulator of epithelial differentiation and stemness
Tbx1 ablation induces epithelial proliferation during palatal development, and transduction of TBX1 decreases cell proliferation in epithelial cancer cells4,8. Based on these findings, we speculated that TBX1 not only inhibits epithelial proliferation, but also affects tumorigenesis. To examine the role of TBX1 in epithelial carcinoma, we first investigated TBX1 expression in carcinoma using human tumor bulk RNA-sequencing data from different origins. Stratification of patients by TBX1 expression revealed increased overall survival for cervical cancer patients with higher TBX1 expression (Fig. 1A). In contrast, no significant correlation was observed in head, neck, and lung squamous cell carcinomas (Supplementary data, Fig. S1A). Interestingly, TBX1 expression was significantly lower in cervical cancer specimens than in matched normal tissues, which was different from the case in other squamous cell carcinomas (Fig. 1B and Supplementary data, Fig. S1B). Therefore, we speculated that TBX1 might be associated with EMT phenotypes in cervical cancer. Stable expression of TBX1 in HeLa cells, a cervical cancer cell line, induced strong upregulation of TBX1 levels (Fig. 1C). Using control (HeLa-vector) and HeLa-TBX1 stably transfected cells, we investigated whether TBX1 contributes to the inhibition of invasive capacity and anchorage-independent cell growth. TBX1 inhibited cell migration (Fig. 1D), invasive capacity (Fig. 1E), and colony-forming capacity (Fig. 1F) compared to controls.
TBX1 inhibits cell migration, invasion, and soft agar colony formation. (A) Kaplan–Meier curve representing overall survival in patients with primary cervical cancer (n = 288) with high, medium, or low TBX1 expression. Two-sided P-values were computed using a log-rank (Mantel-Cox) test. (B) Box plots were obtained from the GEPIA using the GTEx database to compare the expression of TBX1 in cervical cancer (n = 306) and matched normal specimens (n = 13). TPM, transcripts per million. ***P ≤ 0.001; one-way ANOVA. (C) Expression of TBX1 was examined by qPCR in HeLa cells stably transfected with control pEBMulti plasmid (vector) or pEBMulti-TBX1 plasmid (TBX1). n = 6. (D) Comparison of the relative migration of HeLa cells stably transfected with empty vector (HeLa-vector) or TBX1 (HeLa-TBX1) towards a 10% serum gradient for 24 h. n = 3. (E) In vitro invasion assays for HeLa cells stably transfected with empty vector or TBX1. Representative images of invaded cells are shown below the bar plot. n = 9. (F) Soft agar colony formation assays with HeLa cells stably transfected with the empty vector or TBX1. Colony-forming efficiency was determined after culturing for 3 weeks in the presence of hygromycin in three independent assays (n = 10). Representative images of colonies are shown below the bar plot. (G) Western blot analysis of extracts derived from HeLa cells stably transfected with empty vector or TBX1 for EMT-related markers. The full-length blots are shown in Supplementary data, Fig. S13. (H) Expression of ZEB1 and ZEB2 was examined with qPCR in HeLa-vector or HeLa-TBX1 cells (n = 6, *P ≤ 0.05; NS, not significant). For (C-F) and (H), the results are presented as mean ± s.e.m.; unpaired two-tailed Student’s t-test.
To analyze whether TBX1 overexpression alters the expression of EMT-related markers, we analyzed expression of the epithelial markers E-cadherin (CDH1), β-catenin, and Desmoglein1 (DSG1) as well as the mesenchymal markers vimentin and N-cadherin (CDH2). Stable expression of TBX1 increased the expression of E-cadherin, a master regulator of epithelial polarity and EMT in epithelial cancers26, whereas the levels of N-cadherin, β-catenin, Desmoglein1, and vimentin were unaffected (Fig. 1G). Analysis of cervical cancer and normal tissue data in The Cancer Genome Atlas (TCGA) database revealed a statistically significant correlation between TBX1 and E-cadherin gene CDH1, whereas there was no correlation between TBX1 and CDH2 expression (Supplementary data, Fig. S2). These findings suggest that TBX1 overexpression partially reverts the mesenchymal phenotype to the epithelial phenotype in cervical cancer cells. ZEB proteins induce EMT by binding to E-boxes within the CDH1 promoter and repressing its transcription26. Since TBX1 markedly reduced invasive capacity, accompanied by the reduced expression of E-cadherin/CDH1 (Fig. 1E–G), we speculated that the expression of TBX1 might be inversely correlated with that of EMT-related ZEB transcription factors. To address this possibility, we examined the expression of ZEB1 and ZEB2 in HeLa cells stably transfected with TBX1. ZEB2 expression was significantly reduced in TBX1-transfected HeLa cells relative to that in the control cells (Fig. 1H), which suggested that TBX1 may be involved in maintaining the epithelial phenotype by inhibiting ZEB2 expression.
To elucidate whether TBX1 can also influence EMT-associated stem cell properties, we compared CD44+CD24− stem cell populations in HeLa cells stably transfected with a control vector or TBX1. CD44 is upregulated during EMT and in stem cells, and promotes cancer progression and metastasis27. As measured by flow cytometry, stable expression of TBX1 significantly diminished the percentage of CD44+CD24− stem cells (Fig. 2A). Proliferation was also reduced in TBX1-overexpressing cells (Fig. 2B; Supplementary data, Fig. S3A-B). Furthermore, TBX1-overexpressing clones showed reduced sphere-forming capacity (Fig. 2C), associated with reduced expression of stem cell factors, BMI1 and SOX2 (Fig. 2D). In addition, TBX1 induced the expression of differentiation marker KRT17 (Supplementary data, Fig. S3C). These findings suggest that TBX1 suppresses stem cell properties and induces epithelial differentiation.
TBX1 suppresses stem cell properties. (A) Flow cytometric analysis of CD24 and CD44 expression in empty vector (HeLa-vector) and TBX1 stably transfected HeLa cells (HeLa-TBX1). The percentage of CD44+CD24− stem cell population (lower right box) is indicated (n = 6). (B) Flow cytometric analysis of HeLa‑vector and HeLa‑TBX1 cells labeled with CFSE fluorescent dye. A representative day 2 histogram (left) and the mean ± s.e.m. of quantified results from three independent experiments with replicate wells (right) are shown (see also Supplementary data, Fig. S3A). MFI, mean fluorescence intensity. (C) Sphere-forming capacity of HeLa-vector and HeLa-TBX1 cells (n = 3). (D) Expression of stem cell markers was examined by qPCR in control HeLa‑vector cells and HeLa‑TBX1 cells (n = 6). For (A-D), the results are presented as mean ± s.e.m.; *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001; NS, not significant; unpaired two-tailed Student’s t-test.
TBX1 is a regulator of miR-200
The stem cell factors BMI1 and SOX2 are target genes of miR-20024. Given the association of TBX1 with epithelial differentiation and stem cell properties following reduced expression of miR-200 target genes (ZEB2, BMI1, and SOX2) we surmised that TBX1 may induce the expression of miR-200, which otherwise triggers E-cadherin/CDH1 by downregulating ZEB226. To address this possibility, we focused on the EMT-suppressor miR-200b-200a-429 as a potential novel target gene of TBX1. miR-200b, miR-200a, and miR-429 are clustered on human chromosome 1p36.33 (Fig. 3A). The miR-200b-200a-429 promoter contains cis-regulatory motifs of the ZEB-binding E-box15. Using JASPAR, we identified a putative TBX1-binding element (TBE) within − 110/ + 120 of the miR-200b-200a-429 transcription start site (Fig. 3A,B). Overlapping cis-regulatory E-box and TBE motifs were conserved among primates (Fig. 3C). Expression of pri-miR-200b-200a-429 was significantly elevated in TBX1-overexpressing cells relative to control cells (Fig. 3D). Among the miR-200 family members, miR-429 was the most elevated by TBX1 overexpression, although the increase was not statistical significance (Fig. 3E). Because the miR-200b-200a-429 promoter contains a TBE motif and TBX1 can induce the expression of pri-miR-200b-200a-429 (Fig. 3A–D), we speculated that the observed effects were at least partly due to the activation of the miR-200b-200a-429 promoter. As expected, TBX1 mildly activated miR-200 promoter constructs (Fig. 3F). We also searched for TBX1 functional domains for miR-200b-200a-429 promoter activation, using TBX1 deletion mutants. Mutants with deletions of the activation domain (ΔAD) and T-box DNA-binding domain (ΔT-box) failed to activate the miR-200b-200a-429 transactivation function (Fig. 3G), suggesting that the activation and DNA-binding domains are essential for miR-200b-200a-429 promoter activation. Interestingly, TBX1[∆(295–488) + NLS] (hereafter TBX1∆) activated the miR-200b-200a-429 promoter effectively (Fig. 3G). Chromatin immunoprecipitation (ChIP) showed that TBX1 directly bound to the − 110/ + 19 region of the miR200b-200a-429 promoter (Fig. 3H). Mutations in TBE inhibited the stimulation of the miR-200b-200a-429 promoter by TBX1Δ (Fig. 3I). In situ proximity ligation assay (PLA) showed a very close proximity between TBX1 and ZEB2 at the nucleus in HeLa cells and A549 human lung epithelial cells (Fig. 3J and Supplementary data, Fig. S4). Consistent with the previous reports15, overexpression of ZEB2 suppressed the activity of the miR-200b-200a-429 promoter in HeLa and A549 cells (Fig. 3K). Inhibition of the miR-200b-200a-429 promoter by ZEB2 was rescued by TBX1 in a dose-dependent manner in these cells (Fig. 3K), but not in MCF7 breast cancer cells (Supplementary data, Fig. S5). These findings demonstrate that TBX1 specifically rescues ZEB2-dependent inhibition of the miR-200b-200a-429 promoter.
TBX1 induces pri-miR-200b-200a-429. (A) Schematic of the upstream promoter region of the human miR-200b/200a/429 cluster on chromosome 1p36.33. The luciferase construct of the miR-200b-200a-429 promoter with the location of the predicted TBE (blue box), as well as ZEB-binding E-boxes (red boxes)15 and SP1-binding sites (black boxes)49, are shown. (B) Sequence of TBE/E-box from the miR-200b-200a-429 promoter and the JASPAR sequence logos of TBE and E-box motifs. The overlapping core sequence, CACCT, is boxed. (C) Nucleotide conservation of the cis-overlapping motifs of TBE and E-box. Asterisks indicate evolutionarily conserved nucleotides. (D) qPCR analysis of pri-miR-200b-200a-429 expression in control HeLa‑vector cells and HeLa‑TBX1 cells (n = 15, *P ≤ 0.05; unpaired two-tailed Student’s t-test). (E) qPCR analysis of miRNAs in HeLa‑vector cells and HeLa‑TBX1 cells. Expression was normalized to U6 and compared with that of the control (n = 6; Student’s t-test). (F) Relative luciferase activity of miR-200b-200a-429 promoter reporters in HeLa cells (n = 9; Student’s t-test). (G) Top, schematic representation of TBX1 functional domains. Bottom, relative luciferase activity of the miR-200b-200a-429 promoter constructs (− 321/ + 120) transiently transfected with various TBX1 deletion mutants (n = 6, *P ≤ 0.05, ***P ≤ 0.001; one-way ANOVA). AD, activation domain; NLS, nuclear localization signal. (H) Binding of TBX1 to the miR-200b-200a-429 promoter (− 110/ + 19) detected by a ChIP assay using HeLa cells. (I) Relative luciferase activity of miR-200b-200a-429 promoter constructs (− 64/ + 120) for wild-type (WT) or mutant (Mut) TBE transiently transfected with TBX1[∆(295–488) + NLS] in HeLa cells. Fold activity was measured in the absence of TBX1 and normalized to 1.0 (n = 8, two-way ANOVA). (J) In situ PLA detection of the TBX1 and ZEB2 colocalization. Constructs encoding TBX1 and ZEB2 were transfected into HeLa or A549 cells. PLA showed a very close proximity between TBX1 and ZEB2 at the nucleus, indicated by fluorescent spots (red). Nuclei were counterstained with DAPI (see also Supplementary data, Fig. S4). (K) Relative luciferase activity of HeLa or A549 cells transiently co-transfected with the miR-200b-200a-429 promoter construct (− 321/ + 120), ZEB2 (150 ng), and the indicated amount of TBX1 expression vector (n = 6, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001; NS, not significant; one-way ANOVA).
TBX1 is a regulator of miR-203
miR-203 is a stemness-inhibiting miRNA that is highly expressed in the epithelium23 and is downregulated in the stem cell population28,29. The polycomb repressor BMI1, whose expression is repressed in TBX1-overexpressing cells (Fig. 2D), is a common target of miR-200 and miR-20324. Therefore, we surmised that miR-203 may be an additional downstream target of TBX1 along with miR-200b-200a-429. The miR-203 promoter contains E-boxes24 and a putative TBE motif, which is conserved among primates (Fig. 4A–C). As expected, miR-203a-3p expression was mildly elevated in HeLa cells transfected with TBX1 (Fig. 4D). ChIP analysis showed that TBX1 directly bound within the − 298/ − 66 region of the human miR-203 promoter (Fig. 4E). TBX1∆ activated the miR-203 promoter constructs, which include a TBE motif (Fig. 4F). Mutations in TBE inhibited the stimulation of miR-203 promoter (− 228/ + 77) by TBX1∆ (Fig. 4G). Consistent with previous reports24, overexpression of ZEB2 mildly suppressed miR-203 promoter activity (Fig. 4H). TBX1 rescued ZEB2-dependent inhibition of the miR-203 promoter in a dose-dependent manner (Fig. 4H). We also examined the effects of ZEB2 and TBX1 on the activities of artificial promoter, p4×RE-Luc, which contained four tandem copies of overlapping cis-regulatory E-box and TBE motifs (Supplementary data, Fig. S6). Overexpression of ZEB2 suppressed the p4×RE-Luc activity (Supplementary data, Fig. S6C). TBX1 hardly affected the ZEB2-dependent inhibition of p4×RE-Luc (Supplementary data, Fig. S6C). These findings suggest that TBX1 reverses the inhibitory activity of ZEB2 through TBE, but the TBE sequence alone is not sufficient to rescue ZEB2 activity.
TBX1 induces miR-203. (A) Schematic of the upstream promoter region of the hsa-mir-203 stem-loop on chromosome 14q32.33 and the luciferase construct of the miR-203 promoter with the location of the predicted TBE (blue box), as well as ZEB1-binding E-boxes (red boxes)24 and an IRF1-binding site (black box)50. (B) Nucleotide conservation of the miR-203 promoter, including TBE. Asterisks indicate evolutionarily conserved nucleotides in all five species. (C) Sequence of TBE from the human miR-203 promoter and the JASPAR sequence logos of TBE. (D) Expression of miR-203a-3p examined using qPCR in control HeLa‑vector cells and HeLa‑TBX1 cells. The graph shows the relative miRNA quantities (n = 3, *P ≤ 0.05; unpaired two-tailed Student’s t-test). (E) Binding of TBX1 to the miR-203 promoter (− 298/ − 66) as detected by ChIP assay using HeLa cells. Chromatin was immunoprecipitated with normal rabbit IgG or anti-TBX1 antibodies. Input control is also shown. (F) Relative luciferase activity of miR-203 promoter reporters in HeLa cells (n = 9, *P ≤ 0.05, ***P ≤ 0.001, ****P ≤ 0.0001; one-way ANOVA). (G) Relative luciferase activity of miR-203 promoter constructs (− 228/ + 77) for wild-type (WT) or mutant (Mut) TBE transiently transfected with TBX1Δ (n = 8, two-way ANOVA). (H) Relative luciferase activity of HeLa or A549 cells transiently co-transfected with the miR-203 promoter construct (− 667/ + 77), ZEB2 (150 ng), and the indicated amount of TBX1 expression vector (*P ≤ 0.05, **P ≤ 0.01, ****P ≤ 0.0001; NS, not significant; one-way ANOVA).
TBX1 links the expression of epithelial miRNAs and their target genes in vivo
To provide physiological evidence for the regulation of epithelial miRNAs and proliferation/differentiation balance by TBX1, we validated our findings in the palatal development of Tbx1Δ/Δ mice, which exhibit cleft palate as the consequence of abnormal fusion between the epithelia covering the palatal shelves and the mandible4. As previously reported4, Tbx1Δ/Δ embryos exhibit intraoral epithelial adhesions (Fig. 5A). The palatal epithelium of Tbx1Δ/Δ neonates showed basal cell hyperplasia and multiple layers of the palatal epithelium (Fig. 5A). Tbx1 ablation significantly reduced the expression of miR-200a-3p, miR-200b-3p, miR-200b-5p, miR-429-3p, and miR-203-3p in palatal shelves compared to controls (Fig. 5B). Palatal shelves from Tbx1Δ/Δ mice also showed significantly increased expression of Zeb2 and Zeb1 (Fig. 5C). These findings demonstrate that Tbx1 is required for the proper expression of miR-200, miR-203, and their targets, Zeb1 and Zeb2, during palatogenesis.
TBX1-mediated miRNA regulation engages epithelial differentiation in vivo. (A) Hematoxylin and eosin staining of the palate from wild-type and Tbx1Δ/Δ embryos at the indicated age. Irregularities in the shape and multiple layers of the epithelium are evident in the Tbx1Δ/Δ embryos (arrows). The broken yellow line traces the basal lamina. m, mandible; p, palatal shelf/palate; e, epithelium; s, stroma. Scale bars, 25 µm. (B) qPCR analysis of miRNAs in wild-type and Tbx1Δ/Δ palatal shelves (n = 8 per genotype) at E13.5. Expression was normalized to that of U6 and compared with that in wild-type littermates (n = 6). (C) qPCR analysis of Zeb2 and Zeb1 expression in wild-type and Tbx1Δ/Δ palatal shelves (n = 8 per genotype) at E13.5. Expression was normalized to that of Actb and compared with that in wild-type littermates (n = 6). (D) qPCR analysis of Vegfa, Kdr, and Flt1 expression in wild-type and Tbx1Δ/Δ palatal shelves (n = 8 per genotype) at E13.5 (n = 6). (E) qPCR analysis of the expression of epithelial markers in wild type and Tbx1Δ/Δ palatal shelves (n = 8 per genotype) at E13.5 (n = 3). (F) Heat map representing the pairwise correlation coefficients of the expression of TBX1 and epithelial differentiation markers51 in the skin: sun-exposed (+ ; lower leg, n = 701) and unexposed (− ; suprapubic, n = 604). Pearson correlation coefficients (r) were calculated according to the color scale. Dark red denotes high positive correlation (r = 0.5), dark blue denotes high negative correlation (r = − 0.5), and white denotes a lack of correlation (r = 0). The statistical source data are provided in Supplementary data, Figs S9–S11. For (B-E), the results are presented as mean ± s.e.m.; *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001; NS, not significant; unpaired two-tailed Student’s t-test.
The predicted target genes of miR-200 and miR-203 include human and mouse genes associated with syndromic cleft lip and/or palate (CL/P) (Supplementary data, Fig. S7). VEGFA and its receptor genes, KDR and FLT1, are known RNA targets of miR-200b-3p and miR-429-3p20,21. Vegfa is highly expressed in the midline of the developing palate, and Vegfa ablation causes cleft palate and craniofacial defects in mice30. These defects are reminiscent of those found in patients with DiGeorge syndrome30, suggesting that VEGF signaling may act in the genetic pathway of TBX1. Based on these findings, we speculated that TBX1 may indirectly affect the expression of Vegfa and its receptor genes, Kdr and Flt1, via miR-200 and miR-203 in palatal shelves. The predicted miR-200 and miR-203 binding sites in the 3′UTR of these genes were conserved throughout evolution (Supplementary data, Figs. S8 and S9). In the Tbx1Δ/Δ palatal shelves, the expression of Vegfa, Kdr, and Flt1 was significantly upregulated compared to that in the controls (Fig. 5D), suggesting that TBX1 regulates the genes that are involved in VEGF signaling. Moreover, loss of Tbx1 correlates with an increase in the expression of epithelial basal cell markers (Krt15 and Krt5) with poor epithelial differentiation (based on Krt18, Krt19, Krt17 expression), indicating that Tbx1 is essential for oral epithelial differentiation (Fig. 5E). Consistent with the Tbx1Δ/Δ palatal model, we noted that TBX1 expression was positively correlated with the expression levels of basal cell markers and negatively correlated with the expression levels of differentiation markers in skin cells (Fig. 5F, Supplementary data, Figs S10-12). The spatio-temporal regulation of differentiation of palatal epithelium is essential in preventing abnormal adhesion of oral epithelial surfaces during palatogenesis31. Tbx1Δ/Δ palatal shelves exhibit a hyperproliferative epidermis that fails to undergo differentiation leading to cleft palate4. Together, our findings indicate that TBX1 is associated with miRNAs involved in regulating EMT and stemness, both pathologically in epithelial cancer cells and physiologically in epithelial differentiation (Fig. 6).
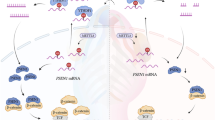
Proposed model of TBX1-mediated regulation of stemness and differentiation in epithelial cells through the miR-200–ZEB2 axis. ZEB2 and miR-200 reciprocally repress each other’s expression and are linked in a double-negative feedback loop12,13,15. TBX1 rescues ZEB2-dependent inhibition of the miR-200b-200a-429 promoter and induces the expression of miR-200, which controls the expression of EMT- and VEGFA-related molecules. In addition, TBX1 induces miR-203 expression, which, together with miR-200, inhibits the expression of stem cell factors. In conclusion, TBX1 links the maintenance of stemness and epithelial differentiation, pathologically in epithelial cervical cancer, and physiologically in palatal development.‡, molecules associated with cleft palate in humans and/or mice.
Discussion
The miR-200–ZEB feedback loop has been extensively studied in various tumors to understand the mechanisms regulating miR-200 expression, which controls EMT and stem cell properties in epithelial cancer cells9,32,33. In this study, we discovered that TBX1 acts as a stemness inhibitor by rescuing ZEB2-dependent transcriptional inhibition of the promoters of miR-200 and miR-203. Our finding that TBX1 induces miR-200b-200a-429 expression further substantiates the notion that miR-200 target genes, ZEB2, BMI1 and SOX2, are downstream of TBX1. TBX-binding TBE and ZEB-binding E-box motifs have an overlapping core sequence of CACCT; therefore, the T-box family of transcription factors may have a counter-regulatory effect on ZEB-target genes, similar to miR-200b-200a-429. Regulatory mechanisms of TBX1 and the expression of ZEB1 and ZEB2 may differ among tumor types, organs, or species; however, the complete molecular mechanism underlying this effect is unknown. TBX1∆ activates the promoters of miR-200b-200a-429 and miR-203 effectively compared to wild-type TBX1; however, the molecular mechanisms and functions of TBX1∆ remain unknown. Additional studies are needed to identify novel common target genes and regulatory mechanisms of TBX1 and ZEB proteins.
CL/P is one of the most frequent craniofacial congenital disorders in humans34. Understanding the mechanisms regulating the balance between epithelial proliferation and differentiation during palatogenesis may have implications for preventing CL/P. The hyperproliferative oral epithelium in Tbx1Δ/Δ mice failed to undergo epithelial differentiation, which may result in pathogenic intraoral adhesions4, similar to Irf6-mutant mice35. We provide evidence that Tbx1 has molecular interactions with miR-200, miR-203, and their target genes, Zeb2 and Zeb1, in mouse palatogenesis. Interestingly, both Tbx1Δ/Δ and Zeb2+/Δ;Zeb1Δ/Δ mouse embryos exhibit unique CL/P phenotypes of varying severity, ranging from incomplete to complete cleft palate4,18. In humans, missense mutations in ZEB2 induce Mowat–Wilson syndrome associated with CL/P (OMIM #235,730)36,37. In addition to ZEB2 and Zeb1, predicted targets of miR-200 include disease genes related to CL/P in humans and mice. These results suggest that primary defects in regulators and target genes of miR-200 are significant contributors to CL/P. Genes that play essential roles in tumorigenesis are often involved in cell proliferation and differentiation during embryogenesis38. For individuals born with CL/P, an increased risk of breast cancer was observed39. Thus, the TBX1-mediated link between miR-200 and its target genes may not be a unique phenomenon in selected tumors; rather, it may be a regulatory system already active in embryonic development. Tbx1 ablation decreased miR-200 and miR-203 in palatal shelves and was associated with increased expression of their target genes, Vegfa, Kdr, and Flt1. Vegfa ablation causes cardiovascular, craniofacial, thymic, and parathyroid defects in mice, reminiscent of the birth anomalies found in Tbx1Δ/Δ mice30. Tbx1 may downregulate VEGF signaling, at least in part, through miR-200 and miR-203. Since most anti-angiogenic strategies focus on inhibiting VEGF and its receptors22, controlling the miR-200–ZEB2–VEGF signaling axis by TBX1 may be an essential pathway for influencing tumor progression. The repressive influence of transiently-expressed Tbx1 may be released temporally and spatially in order for the expression of miR-200 and miR-203 to regulate their target genes and execute epithelial differentiation during palatogenesis. Because our data were derived from ectoderm-derived epithelium in the developing palate, further validation of miR-200 in Tbx1-expressing endoderm-derived epithelia is needed to confirm our findings.
In conclusion, TBX1 directly or indirectly links the expression of miRNAs and EMT/stemness-related factors pathologically in epithelial cervical cancer and physiologically in palatal development. We propose that TBX1 induces epithelial differentiation and inhibits stemness by activating the expression of EMT/stemness-inhibiting miRNAs and consequently repressing the expression of their target genes. The finding that TBX1 regulates stem cell properties through association with miR-200–ZEB2 suggests possible strategies for developing personalized medicine for treating patients with cervical cancer by maintaining a differentiated epithelial cell phenotype. In addition, the miR-200–ZEB2 axis is involved in TBX1-mediated control of epithelial differentiation during palatogenesis, contributing to our understanding of CL/P etiology.
Materials and methods
Bioinformatic analysis
Survival analysis was performed by stratifying patients into high, medium, or low TBX1 expression groups, and Kaplan–Meier plots were generated and analyzed using the UCSC Xena program (https://xenabrowser.net)40 The mRNA expression data of tumors, matched normal specimens, sun-exposed skin, and unexposed skin were obtained from GEPIA (http://gepia.cancer-pku.cn/detail.php)41using the GTEx (https://gtexportal.org/home) or TCGA (http://cancergenome.nih.gov) database. The JASPAR database (http://jaspar.genereg.net) of transcription factor binding site motifs42 was used to predict putative TBEs and ZEB consensus E-box motifs. The predicted target genes of miRNAs were identified using TargetScan 7.2 (http://www.targetscan.org). Correlations between mRNA–mRNA pairs of the gene set were analyzed by calculating the Pearson correlation coefficient.
Plasmids
The pEBMulti-Hyg-TBX1 vector4 was generated from the EBNA1-based episomal pEBMulti-Hyg vector (Fujifilm Wako), which included a hygromycin-resistant gene. Expression vectors of Myc-tagged TBX1, its deletion mutants, and p4×RE-Luc were previously described43,44. To construct the ZEB2 plasmid, the cDNA sequence was amplified and cloned into pFlag-CMV3.1 (ThermoFisher Scientific). The miR-200b-200a-429 promoter (− 1574/ + 120) and miR-203 promoter (− 667/ + 77) were amplified from human genomic DNA; full-length DNA, and deletion mutants were then directionally cloned into the pGL2-Basic vector (Promega). Primer sequences used to construct the vectors are shown in Supplementary data, Table S1.
Cell culture and stable transfection of cell lines
The cell lines HeLa (Tohoku University cell bank), A549 (RIKEN cell bank), and MCF7 (a kind gift from Dr. K. Ito) were cultured in DMEM (Nacalai Tesque) supplemented with 1% penicillin–streptomycin and 10% fetal bovine serum (FBS). Cells were transfected with pEBMulti-Hyg or pEBMulti-Hyg-Myc-TBX1 vectors using the Avalanche-Everyday transfection reagent (Integral) or FuGENE HD (Promega). The transfected cells were then selected using 300 μg/mL (HeLa) or 400 μg/mL (MCF7) hygromycin (Nacalai Tesque).
Mouse strains
All animal care and experiments were conducted in accordance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines. Heterozygous mice (Tbx1neo/+) were mated with More-Cre mice (a kind gift from Dr. M. Tallquist), resulting in the heterozygous Tbx1-null allele (Tbx1+/Δ) and Tbx1 homozygous-null Tbx1Δ/Δ mice, as described previously4,6. Wild-type littermates were used as controls. All experimental animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Tokyo Medical and Dental University (Permit Number 0126215C, February 24, 2016). All experiments and methods were performed in accordance with relevant guidelines and regulations.
Migration and invasion assays
Assays were conducted in 24-well Transwell chambers (6.5 mm, 8.0 μm pore size; Corning) pre-coated without (for migration assays) or with (for invasion assays) 50 µL of Matrigel (BD Biosciences). For migration assays, 1.0 × 105 cells in 100 μL of serum-free medium were placed in the upper compartment of the Transwells. The lower compartment was filled with 600 μL of the complete culture medium. After incubation at 37 °C for 24 h, the migrated cells that passed through the pores on each insert were counted. For the invasion assay, the cells that penetrated the Matrigel-coated membrane into the lower side were fixed with 3.7% formaldehyde in phosphate-buffered saline (PBS) and stained with 0.1% crystal violet. Invaded cells were imaged in three individual fields on each insert using a BX41 microscope (Olympus) and counted using Fiji (https://imagej.net/software/fiji).
Soft agar colony formation assay
Anchorage-independent growth of HeLa-vector and HeLa-TBX1 stably transfected cells was estimated using a soft agar colony formation assay, as described previously45. Cells were suspended in 1.5 mL of DMEM containing 10% FBS, 300 μg/mL hygromycin, and 0.3% agarose and plated at 7500 cells/well in triplicate over a 1.5 mL 0.6% agarose base in 6-well plates. Fresh medium (300 μL) was added to the plate for 3 weeks. The colonies in the plate were imaged using a BX41 microscope and analyzed using Fiji.
Quantitative real-time PCR (qPCR) analysis
Palatal shelves were dissected from wild-type and Tbx1Δ/Δ mice (n = 8 per genotype) on embryonic day (E)13.5. Cultured cells and secondary palatal shelves were processed for total RNA extraction using TRIzol (ThermoFisher) and the RNeasy Mini Kit (Qiagen). mRNA analysis was carried out using the 1st strand cDNA synthesis kit for RT-PCR (AMV) (Sigma-Aldrich) and PowerUP SYBR Green PCR Master Mix (ThermoFisher). mRNA expression was normalized to that of TBP or Actb. miRNA analysis was performed as previously described46. Briefly, cDNA was synthesized from total RNA, and miRNAs were amplified using an All-in-One miRNA qRT-PCR Detection Kit (GeneCopoeia) with stem-loop primers specifically designed to analyze mature miRNAs. The expression of miRNAs was normalized to the endogenous control U6 (GeneCopoeia). Amplification and detection of mRNAs and miRNAs were performed using the StepOnePlus Real-Time PCR System (ThermoFisher), and the relative quantity was calculated using the 2^ − ΔΔCt method47. Experiments were performed in at least three replicates for each sample and each gene. The primer sequences used for qPCR are shown in Supplementary data, Table S2.
Western blotting
HeLa cells stably transfected with the indicated plasmids were lysed using RIPA lysis buffer (50 mM Tris–HCl [pH 7.5], 150 mM NaCl, 1 mM EDTA, 1% Triton X-100 supplemented with 1 × cOmplete protease inhibitor cocktail (Sigma-Aldrich). The cell lysates were electrophoresed via SDS–PAGE, transferred onto PVDF membranes (Amersham), blocked with 5% non-fat dried milk (BD Biosciences), and incubated with appropriate primary and HRP-conjugated secondary antibodies (Bio-Rad Laboratories). Immunoblots were visualized using an ECL detection system (Santa Cruz Biotechnology). The Western blots were imaged using ChemiDoc MP (Bio-Rad). The following primary antibodies were used: rabbit anti-Myc (A14; Santa Cruz, 1:1,000), rabbit anti-E-cadherin (24E10; Cell Signaling Technology, 1:1,000), anti-vimentin (D21H3; Cell Signaling, 1:1,000), anti-N-cadherin (#32; BD Biosciences, 1:1,000), anti-β-catenin (6B3; Cell Signaling, 1:1,000), anti-β-tubulin (9F3; Cell Signaling, 1:1,000), and anti-DSG1 (H-290; Santa Cruz, 1:1,000). The full-length blots are shown in Supplementary data, Fig. S13.
Flow cytometric analysis
Cells were collected with 0.05% trypsin–EDTA solution, washed, and resuspended at 5.0 × 106/mL in PBS with 2% FBS (staining medium) containing PE-conjugated anti-CD24 (ML5, BD Biosciences) and FITC-conjugated anti-CD44 (G44-26, BD Biosciences) antibodies. Cells were incubated at 4 °C for 60 min, washed with PBS, resuspended in staining medium, and analyzed using FACSLyric (BD Biosciences). Side scatter and forward scatter profiles were used to eliminate debris and cell doublets. Standard compensation was performed using single-stained cells incubated with a labeled isotype antibody. A total of 10,000 viable cells were counted.
Cell proliferation assays
For the vital carboxyfluorescein N-hydroxysuccinimidyl ester (CFSE) dye dilution assay, the CellTrace CFSE cell proliferation kit (ThermoFisher) was used according to the manufacturer’s instructions. Briefly, stable cell lines were collected with 0.05% trypsin–EDTA solution, washed with PBS, and resuspended in PBS at a final density of 1.0 × 106/mL. Next, the cells were mixed with the CFSE fluorescent dye in PBS (final concentration 5 uM) and incubated for 20 min at 37 °C. Cells were then washed with serum-free medium, seeded in 10-cm dishes at 20% confluency, and cultured in DMEM containing 10% FBS. CFSE dilution resulting from cell division was evaluated using FACSLyric. Side scatter and forward scatter profiles were used to eliminate debris and cell doublets. For cell cycle analysis, cells were analyzed using the Muse Cell Cycle Kit (Merck). A total of 10,000 viable cells were counted. Each experiment was repeated three times using independent samples.
Sphere-formation assay
To test sphere-forming capacity, cells were resuspended in DMEM containing 0.5% methylcellulose #1500 (Nacalai), 10% FBS, 300 μg/mL hygromycin, and 1% penicillin–streptomycin. 10,000 single cells were seeded into individual wells of 6-well clear flat-bottom ultra-low attachment plates (Coster, #3471). Colonies with a diameter greater than 80 µm were counted after 12 days.
Reporter assays
HeLa, A549, and MCF7 cells were plated in 24-well plates and co-transfected with 150 ng of firefly luciferase reporter and expression plasmids using TransFectin lipid reagent (Bio-Rad Laboratories) or TransIT-2020 Transfection Reagent (Mirus Bio) along with 50 ng of a pRSV-β-galactosidase expression plasmid to monitor transfection efficiency. After 48 h of incubation, cells were lysed in passive lysis buffer (Promega), and luciferase activity was measured using the Luciferase Reporter Assay System (Promega). All data are expressed as mean ± standard error of the mean (s.e.m.) from at least six separate experiments.
Chromatin immunoprecipitation (ChIP) assay
The ChIP assay was performed as previously described48, with minor modifications. After cells were formaldehyde cross-linked and lysed, sheared chromatin was immunoprecipitated with anti-TBX1 antibody (34–9800, ThermoFisher), and DNA was isolated and analyzed by PCR. The gels were imaged using BioDoc-It System (analytik jena US). The primer sequences are listed in Supplementary data, Table S3. The full-length gel is shown in Supplementary data, Fig. S14.
Proximity ligation assay (PLA)
PLA was performed with the Duolink In Situ Detection Reagents-Red kit (Sigma-Aldrich) following the manufacturer’s instructions. Antibodies used for PLA were rabbit anti-Myc (A14; Santa Cruz) and mouse anti-Flag (M2; Sigma-Aldrich). Nuclei were counterstained with DAPI. Images were acquired by microscopy (BX-61; Olympus) and processed with Fiji.
Statistical analysis
The log-rank test was used to compare the Kaplan–Meier survival curves. Correlation of mRNA-mRNA pairs of the gene set in cell lines or human tumors was analyzed by calculating the Pearson correlation coefficient. The experiments were repeated at least three times independently. Data were analyzed using PRISM 9.0 software (GraphPad) and were presented as mean ± s.e.m. for n experiments. Unpaired two-tailed Student’s t-tests were applied to compare two groups of independent samples. A one-way ANOVA with Dunnett’s post hoc test was used to analyze differences among three or more groups. Statistical significance is presented as follows: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 and ****P ≤ 0.0001.
Data availability
The Affymetrix expression data from GTEx analysed during the current study are available in the GEO repository, GSE45878. Other datasets generated during and/or analysed during the current study are included in this published article (and its Supplementary Information files) or available from the corresponding author on reasonable request.
References
Yagi, H. et al. Role of TBX1 in human del22q11.2 syndrome. Lancet (London, England) 362, 1366–73 (2003).
Ogata, T. et al. TBX1 mutation identified by exome sequencing in a Japanese family with 22q11.2 deletion syndrome-like craniofacial features and hypocalcemia. PLoS One 9, e91598 (2014).
Zweier, C., Sticht, H., Aydin-Yaylagül, I., Campbell, C. E. & Rauch, A. Human TBX1 missense mutations cause gain of function resulting in the same phenotype as 22q11.2 deletions. Am. J. Hum. Genet. 80, 510–517 (2007).
Funato, N., Nakamura, M., Richardson, J. A., Srivastava, D. & Yanagisawa, H. Tbx1 regulates oral epithelial adhesion and palatal development. Hum. Mol. Genet. 21, 2524–2537 (2012).
Funato, N., Nakamura, M., Richardson, J. A., Srivastava, D. & Yanagisawa, H. Loss of Tbx1 induces bone phenotypes similar to cleidocranial dysplasia. Hum. Mol. Genet. 24, 424–435 (2015).
Hu, T. et al. Tbx1 regulates fibroblast growth factors in the anterior heart field through a reinforcing autoregulatory loop involving forkhead transcription factors. Development 131, 5491–5502 (2004).
Lindsay, E. A. Chromosomal microdeletions: dissecting del22q11 syndrome. Nat. Rev. Genet. 2, 858–868 (2001).
Trempus, C. S. et al. A novel role for the T-box transcription factor Tbx1 as a negative regulator of tumor cell growth in mice. Mol. Carcinog 50, 981–991 (2011).
Nieto, M. A., Huang, R.Y.-J., Jackson, R. A. & Thiery, J. P. EMT: 2016. Cell 166, 21–45 (2016).
Thiery, J. P., Acloque, H., Huang, R. Y. J. & Nieto, M. A. Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–890 (2009).
Aban, C. E. et al. Downregulation of E-cadherin in pluripotent stem cells triggers partial EMT. Sci. Rep. 11, 2048 (2021).
Burk, U. et al. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 9, 582–589 (2008).
Park, S. M., Gaur, A. B., Lengyel, E. & Peter, M. E. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 22, 894–907 (2008).
Gregory, P. A. et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 10, 593–601 (2008).
Bracken, C. P. et al. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res. 68, 7846–7854 (2008).
Christoffersen, N. R., Silahtaroglu, A., Orom, U. A., Kauppinen, S. & Lund, A. H. miR-200b mediates post-transcriptional repression of ZFHX1B. RNA 13, 1172–1178 (2007).
Takagi, T., Moribe, H., Kondoh, H. & Higashi, Y. DeltaEF1, a zinc finger and homeodomain transcription factor, is required for skeleton patterning in multiple lineages. Development 125, 21–31 (1998).
Miyoshi, T. et al. Complementary expression pattern of Zfhx1 genes Sip1 and deltaEF1 in the mouse embryo and their genetic interaction revealed by compound mutants. Dev. Dyn. 235, 1941–1952 (2006).
Shin, J. O. et al. MiR-200b regulates cell migration via Zeb family during mouse palate development. Histochem. Cell Biol. 137, 459–470 (2012).
McArthur, K., Feng, B., Wu, Y., Chen, S. & Chakrabarti, S. MicroRNA-200b regulates vascular endothelial growth factor-mediated alterations in diabetic retinopathy. Diabetes 60, 1314–1323 (2011).
Choi, Y. C., Yoon, S., Jeong, Y., Yoon, J. & Baek, K. Regulation of vascular endothelial growth factor signaling by miR-200b. Mol. Cells 32, 77–82 (2011).
Ellis, L. M. & Hicklin, D. J. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat. Rev. Cancer 8, 579–591 (2008).
Yi, R., Poy, M. N., Stoffel, M. & Fuchs, E. A skin microRNA promotes differentiation by repressing ‘stemness’. Nature 452, 225–229 (2008).
Wellner, U. et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat. Cell Biol. 11, 1487–1495 (2009).
Burk, R. D. et al. Integrated genomic and molecular characterization of cervical cancer. Nature 543, 378–384 (2017).
Comijn, J. et al. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol. Cell 7, 1267–1278 (2001).
Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J. & Clarke, M. F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. U. S. A. 100, 3983–3988 (2003).
Chang, C. J. et al. P53 regulates epithelial-mesenchymal transition and stem cell properties through modulating miRNAs. Nat. Cell Biol. 13, 317–323 (2011).
Shimono, Y. et al. Downregulation of miRNA-200c Links breast cancer stem cells with normal stem cells. Cell 138, 592–603 (2009).
Stalmans, I. et al. VEGF: a modifier of the del22q11 (DiGeorge) syndrome?. Nat. Med. 9, 173–182 (2003).
Richardson, R. J., Dixon, J., Jiang, R. & Dixon, M. J. Integration of IRF6 and Jagged2 signalling is essential for controlling palatal adhesion and fusion competence. Hum. Mol. Genet. 18, 2632–2642 (2009).
Brabletz, S. & Brabletz, T. The ZEB/miR-200 feedback loop–a motor of cellular plasticity in development and cancer?. EMBO Rep. 11, 670–677 (2010).
Mani, S. A. et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715 (2008).
Tolarova, M. M. & Cervenka, J. Classification and birth prevalence of orofacial clefts. Am. J. Med. Genet. 75, 126–137 (1998).
Richardson, R. J. et al. Irf6 is a key determinant of the keratinocyte proliferation-differentiation switch. Nat. Genet. 38, 1329–1334 (2006).
Cacheux, V. et al. Loss-of-function mutations in SIP1 Smad interacting protein 1 result in a syndromic Hirschsprung disease. Hum. Mol. Genet. 10, 1503–1510 (2001).
Wilson, M. et al. Further delineation of the phenotype associated with heterozygous mutations in ZFHX1B. Am. J. Med. Genet. A 119A, 257–265 (2003).
Monk, M. & Holding, C. Human embryonic genes re-expressed in cancer cells. Oncogene 20, 8085–8091 (2001).
Bille, C. et al. Cancer risk in persons with oral cleft - A population-based study of 8,093 cases. Am. J. Epidemiol. 161, 1047–1055 (2005).
Goldman, M. J. et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 38, 675–678 (2020).
Tang, Z. et al. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 45, W98–W102 (2017).
Fornes, O. et al. JASPAR 2020: update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 48, D87–D92 (2020).
Funato, N., Srivastava, D., Shibata, S. & Yanagisawa, H. TBX1 regulates chondrocyte maturation in the spheno-occipital synchondrosis. J. Dent. Res. 99, 1182–1191 (2020).
Weintraub, H., Davis, R., Lockshon, D. & Lassar, A. MyoD binds cooperatively to two sites in a target enhancer sequence: Occupancy of two sites is required for activation. Proc. Natl. Acad. Sci. U. S. A. 87, 5623–5627 (1990).
Cifone, M. A. & Fidler, I. J. Correlation of patterns of anchorage-independent growth with in vivo behavior of cells from a murine fibrosarcoma. Proc. Natl. Acad. Sci. U. S. A. 77, 1039–1043 (1980).
Funato, N. et al. The transcription factor HAND1 is involved in cortical bone mass through the regulation of collagen expression. Int. J. Mol. Sci. 21, 8638 (2020).
Schmittgen, T. D. et al. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal. Biochem. 285, 194–204 (2000).
Shang, Y., Hu, X., DiRenzo, J., Lazar, M. A. & Brown, M. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103, 843–852 (2000).
Kolesnikoff, N. et al. Specificity protein 1 (Sp1) maintains basal epithelial expression of the mir-200 family: Implications for epithelial-mesenchymal transition. J. Biol. Chem. 289, 11194–11205 (2014).
Mao, L., Zhang, Y., Mo, W., Yu, Y. & Lu, H. BANF1 is downregulated by IRF1-regulated MicroRNA-203 in cervical cancer. PLoS ONE 10, 1–14 (2015).
Bragulla, H. H. & Homberger, D. G. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J. Anat. 214, 516–559 (2009).
Acknowledgements
We would like to thank Eriko Matsumoto and Yuki Yokomachi for technical assistance, Deepak Srivastava and Michelle Tallquist for mice, Masataka Nakamura for reagents, Kinji Ito for cells, the DD Unit project team of the Tokyo Medical and Dental University for consultations, and Editage (www.editage.com) for English language editing. A549 cell line (RCB0098) was provided by the RIKEN BRC through the National BioResource Project of the MEXT, Japan. This work was supported by grants from the Astellas Foundation for Research on Metabolic Disorders (N.F.), the Japan Society for the Promotion of Science (JSPS) [grant number 20K09901] (N.F.). This study was additionally supported by the collaborative research fund program for women from the TMDU, funded by the “Initiative for realizing diversity in the research environment” from the Japan Science and Technology Agency (JST) in 2017.
Author information
Authors and Affiliations
Contributions
N.F. designed the study and analyzed the data; N.F. and H.Y. performed the experiments for mice and wrote the paper. All authors discussed the results and implications and commented on the manuscript at all stages.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Funato, N., Yanagisawa, H. TBX1 targets the miR-200–ZEB2 axis to induce epithelial differentiation and inhibit stem cell properties. Sci Rep 12, 20188 (2022). https://doi.org/10.1038/s41598-022-24604-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-24604-9
This article is cited by
-
Unveiling the mechanisms and challenges of cancer drug resistance
Cell Communication and Signaling (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.