Abstract
The tropical estuarine ecosystem is fascinating for studying the dynamics of water quality and phytoplankton diversity due to its frequently changing hydrological conditions. Most importantly, phytoplankton is the main supplier of ω3 polyunsaturated fatty acids (PUFA) in the coastal food web for fish as they could not synthesize PUFA. This study evaluated seasonal variations of water quality parameters in the Meghna River estuary (MRE), explored how phytoplankton diversity changes according to hydro-chemical parameters, and identified the major phytoplankton groups as the main source of PUFA for hilsa fish. Ten water quality indicators including temperature, dissolved oxygen, pH, salinity, dissolved inorganic nitrogen (DIN = nitrate, nitrite, ammonia) and phosphorus, dissolved silica and chlorophyll-a were evaluated. In addition, phytoplankton diversity was assessed in the water and hilsa fish gut. Principal component analysis (PCA) was used to analyze the spatio-temporal changes in the water quality conditions, and the driving factors in the MRE. Four main components were extracted and explained 75.4% variability of water quality parameters. The most relevant driving factors were dissolved oxygen, salinity, temperature, and DIN (nitrate, nitrite and ammonia). These variabilities in physicochemical parameters and dissolved inorganic nutrients caused seasonal variations in two major groups of phytoplankton. Peak abundance of Chlorophyta (green algae) occurred in water in nutrient-rich environments (nitrogen and phosphorus) during the wet (36%) season, while Bacillariophyta (diatoms) were dominant during the dry (32%) season that depleted dissolved silica. Thus, the decrease of green algae and the increase of diatoms in the dry season indicated the potential link to seasonal changes of hydro-chemical parameters. The green algae (53.7%) were the dominant phytoplankton group in the hilsa gut content followed by diatoms (22.6%) and both are contributing as the major source of PUFAs for hilsa fish according to the electivity index as they contain the highest amounts of PUFAs (60 and 28% respectively).
Similar content being viewed by others
Introduction
An estuary is a semi-enclosed body of water with open or intermittent connections to the sea1. Biophysical and chemical components in a healthy estuary persist within the limits of natural change. The growth rate and dominance of the estuarine phytoplankton, which forms an important food item for hilsa (Tenualosa ilisha) are influenced by the changes in the physicochemical parameters2. Although these parameters vary, they are strongly influenced by local weather and climate change and can be interpreted as seasonal characteristics3. Therefore, studying the interaction between water quality and phytoplankton diversity of tropical estuarine ecosystems due to frequently changing hydrological conditions is very important.
Seasonality determines the variation of physicochemical parameters such as salinity, temperature, pH, nitrate, nitrite, ammonia, silicate and inorganic phosphate, which in turn affect the species composition and diversity of the phytoplankton community in the estuarine ecosystem4. Generally, local rainfall, tidal inflow, and several abiotic and biotic processes play a significant role in temporal fluctuations of the nutrient cycle in estuaries5. The important macronutrients for most phytoplankton species are nitrate and phosphate, although diatoms additionally need silicate to construct their frustules. However, each phytoplankton species has its own favourable environmental conditions for multiplication6. For example, Chlorophyta (green algae) proliferate rapidly in a nutrient rich (especially nitrogen and phosphorus) environment with favourable temperature (> 25 °C)7. Gamier, et al.8 reported that low nitrogen conditions usually limit the reproduction of Chlorophyta species. On the other hand, Cyanobacteria are typically dominant in the low salinity estuarine zone9,10,11. In fact, most cyanobacteria are freshwater species. In addition, silica is taken into account as a primary controlling factor of the diatom-green algae succession because its availability is of vital importance for the occurrence of diatoms. Thus, the local processes related to the physicochemical parameters lead to the pattern of phytoplankton diversity.
The large Meghna River estuarine (MRE) system serves as an important spawning ground for hilsa fish (Tenualosa ilisha) in favourable environmental conditions12,13. For example, hilsa prefers freshwater (salinity < 0.1 PSU) for spawning and nursery activities14,15. Although some research has been carried out on the biophysical assessments and phytoplankton diversity of the MRE16,17, the effects of abiotic parameters on phytoplankton communities have not been studied. In addition, hilsa is the best source of ω3 polyunsaturated fatty acids (PUFAs) for human consumption18, and the primary food source is the phytoplankton19. But fish or crustaceans cannot readily biosynthesize the ω3 and ω6 polyunsaturated fatty acids (PUFAs), and have to obtain them from their diet such as phytoplankton19. The highest proportion of PUFA is found in green algae, with approximately 60% of the total fatty acids19. In contrast, the lowest PUFA is found in blue-green algae (Cyanobacteria) and diatoms (26 and 28% respectively)19. However, information on the major phytoplankton groups having significant contribution to the supply of PUFA to hilsa fish is scarce. The purpose of this study was to (i) to evaluate the spatial and seasonal variation of major water quality parameter in the MRE using multivariate statistical techniques, (ii) to explore how phytoplankton diversity changes with changing hydro-chemical parameters and (iii) to identify the major phytoplankton groups as main source of ω3 polyunsaturated fatty acids (PUFA) for hilsa fish. In this study, an effort has been made to establish link between variations in abiotic parameters influencing water quality and the phytoplanktonic diversity of the MRE, and identify major phytoplankton groups for PUFA.
Material and methods
Study area
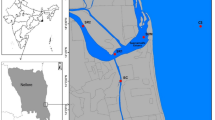
The Meghna River system is the third largest freshwater outlet in the world (Fig. 1). The Meghna River brings huge river discharge of ∼1.5 × 1012 m3 year−1 into the Bay of Bengal. A maximum discharge of approximately 82,000 m3 s−1 occur in the wet season and a minimum of < 10,000 m3 s−1 in the dry season. An annual average of approximately is 32,000 m3 s−1. Huge river discharge and rainfall during the wet season mainly regulate water temperature, salinity, nutrients export and primary productivity of the Meghna River basin. Otero et al.20 revealed that salinity distribution is mainly controlled by river discharge and other atmospheric variable like local rainfall. The presence of marine–brackish–freshwater ecosystems controlled by monsoon river discharge and tide greatly support hilsa fishery in the coastal waters of Bangladesh. The present study was carried out in the large Meghna River estuarine system and its adjacent coastal waters (Fig. 1).
Map of the study area in the Meghna River estuary and its adjacent coastal area. The map was generated using QGIS (version 3.2.1, https://www.qgis.org). Conductivity-temperature-depth (CTD) recorder and water samples collection stations shown as solid circles.
Sampling design
In this study, we established five sections (12 sampling sites) in the upper, middle, lower and sea-side of the Meghna River estuary (Fig. 1). In the sea-side section (OE), three sampling sites were selected at Char Kukrimukri (CK, Longitude: 90.7085, Latitude: 21.9528) and Hatiya Island (HI, Longitude: 91.0824, Latitude: 22.3826). In the upper, middle and lower sections, two sampling sites were selected at Ilisha (UE, Longitude: 90.6922, Latitude: 22.6788), Hakimuddin (ME, Longitude: 90.8191, Latitude: 22.4514) and Charfasson (LE, 90.7962, 22.1851). The along components of the study area are the riverine (UE) and estuarine zone (ME, LE) with six sampling sites and thence the sampling transects extend to the sea-side (CK and HI) of the MRE with six sampling sites. Vertical salinity, water and phytoplankton samples were collected in February, April, July, August, October and December in 2020. In addition, vertical salinity profiles were measured in January, March and June in 2021. Sample collections were conducted under low flow (January, February, March and December: dry season) and high flow (July, August and October: wet season) conditions at twelve sites in the Meghna River estuary.
In situ measurements of water quality parameter
The water quality parameter (such as salinity, temperature, DO etc.) were measured with a conductivity-temperature-depth (CTD) profiler (model: In-situ Aqua TROLL 500, In-situ Inc., USA) in the mouth of the lower Meghna River estuary and its adjacent coastal area (Fig. 1). Speed boats or mechanized boats were used for in situ measurement and to collect water samples. Global positioning system (GPS) was used to collect samples from the accurate sampling stations.
Dissolved inorganic nutrients
Water samples were collected at depths of 0–0.5 m with a water sampler of 1.5 L (Wildco Instruments, USA). Water samples were filtered through a fibreglass filters using a vacuum system and Whatman GF/C filter papers of porosity about 0.45 μm Millipore HA. After filtering, the filtrates were stored in the refrigerator until analyses. The concentration of dissolved inorganic nutrients [Nitrate–N (NO3–N), Nitrite–N (NO2–N), ammonia (NH4+), orthophosphates (PO4-P) and dissolved silicon compounds—Dsi] were analysed with standard spectrophotometric methods21. Spectrophotometer (Model: DR6000 HACH, USA) was used to measure absorbance. The USEPA Ascorbic acid method was used for phosphorus (PO43−), the USEPA Nessler method for ammonium (NH4+)22,23, and the reduced copper cadmium method22 for total oxidised nitrogen (NO3-N and NO2-N). Inorganic nutrients include both dissolved inorganic nitrogen (DIN ≈ NH4+, NO3-N and NO2-N) and phosphorus (DIP ≈ PO4-P).
Primary producers
Phytoplankton biomass
Water samples were collected to determine phytoplankton biomass (measured as chlorophyll-a concentrations) of the MRE. Chlorophyll-a pigment was extracted by filtering 1 L of water through a vacuum machine using Whatman GF/C filter papers of porosity about 0.45 μm Millipore HA. Immediately after completion of filtration, the filters were placed into glass vials containing 10 ml of 95% ethanol (Merck 4111) for 24 h in a refrigerator for extracting chlorophyll-a pigment. Afterwards, pigment extraction was performed by gentle grounding with a homogenizer to speed up the extraction. After homogenizing, the extract was poured into a centrifuge tube and add acetone solution to make the volume up to 10 ml. The solution was centrifuged at 3000 rpm for 10 min. The supernatant solution was measured spectrophotometrically for pigment concentration (DR 6000, USA). The chlorophyll-a concentration was then determined using the SCOR-UNESCO23 equations for each sample.
Phytoplankton community composition
Phytoplankton samples were collected by towing phytoplankton net of mesh size of 20 μm horizontally. The concentrated water samples were then transferred into 15 ml plastic vials and added 10% buffered formalin to preserve in the refrigerator. Thereafter, qualitative analysis of phytoplankton samples was accomplished under a phase-contrast microscope (Primo Star, Carl Zeiss) for the taxonomic rank. For quantitative analysis, Sedgwick Rafter chamber (Wildlife, USA) was used for counting phytoplankton cells. The cells were classified according to different functional groups of algae, i.e. Bacillariophyta (diatoms), Miozoa (dinoflagellates), Cyanobacteria (blue-green algae) and Chlorophyta (green algae). The number of phytoplankton (cells L−1) was computed for each group using the equation defined by Snow et al.24.
Species diversity indices
The species diversity of a habitat is calculated using diversity indices. Phytoplankton diversity indices9 were calculated using the Simpson Diversity Index (D) and Simpson Reciprocal Index (1/D). Simpson Index varies from 0 to 1. Zero denotes a high diversity, while 1 represents a less diverse region6. Simpsons Reciprocal Index is proportionally related to species diversity.
where N is the total number of organisms of all species in an area; n is the total number of organisms of a particular species.
Collection of fish specimens and gut content analysis
Hilsa fish specimens of different sizes were collected randomly from fishermen of the Meghna River estuary. The freshly caught fish specimens were preserved in an insulated box with ice and transported to the laboratory. Ninety fish specimens were taken for gut content analysis. The length varied from 18 to 35 cm and the weight from 109 to 810 g. The alimentary canals from the oesophagus to the anus of the preserved hilsa fish were dissected and preserved in 10% buffered formalin. The gut contents from the stomach to the gizzard of the hilsa fish were then dissolved in water. Thereafter, available food organisms (phytoplankton ) were examined using an electrical microscope (Model: Carl Zeiss, Primo Star, Germany) and took pictures with photogenic devices for qualitative analysis. For the qualitative analysis, phytoplankton were then identified up to the genus level using the keys of Ward and Whipple25,26,27.
Electivity index
Suppose the predator is foraging in an environment (such as water) where the preys consist of two or more environmental prey taxa. Probability \({a}_{i}\) is a randomly selected environmental prey item belongs to taxon i, which we refer to as the “target” taxon for the analysis28. Also assume that the predator ate M prey and let \({g}_{i}\) (gut phytoplankton -) denote the probability that a randomly selected prey from M belongs to taxon i.
The electivity index was calculated from the odds ratio by a logistic transformation,
The index \({X}_{i}\) scales from 0 to 1. The value is 0.5 when the odds ratio is 1, indicating that the fraction of prey (species i) is the same for the environmental (water) prey sample and the gut sample28. The range should be at its maximum when gi = 1 and minimum when gi = 0. Most indices follow this criterion.
Statistical analysis
The R version 4.0.329 was used to perform the multivariate statistical analysis of spatiotemporal variations in the Meghna estuarine habitats. In the present study, 10 physico-chemical factors were used for the multivariate statistical analysis, including water temperature, dissolved oxygen, salinity, pH, chlorophyll-a, nitrate–N, nitrite-N, ammonia, phosphate-p, and dissolved silica. Descriptive statistics were determined for all of the physico-chemical and nutrient variables. As a complement, boxplot analysis was performed by using the ‘ggboxplot’ package. The paired samples Wilcoxon test is a non-parametric substitute to paired t-test used to compare between dry and wet seasons data. In contrast, Kruskal–Wallis test, an alternative of one-way ANOVA, is a non-parametric test used to compare the spatial variations. The Wilcoxon and Kruskal–Wallis tests were made using the ‘ggplot2’ package. The principal component analysis (PCA) was performed to relate the environmental factors (physico-chemical, dissolved nutrients and chlorophyll-a). The correlation matrix and the factorial axes analysed using PCA were showed significantly higher eigenvalues compared to those produced by matrices of the same dimension30. To confirm the presence of spatiotemporal variation among environmental factors along and across the MRE, PCAs were executed by using the ‘FactoMineR’ package using Euclidean distance method30,31. Furthermore, the contributions of the variables to the principal components (PCs) were observed to identify which environmental parameter were greatly differed among the different compartments of the MRE habitats. The four PCs (Dim1 ~ 4) were considered in this study to describe most of the variability. All the PCA were made using the ‘ggplot2’ package32. The correlations among the environmental factors were tested and plotted using the “Performance Analytics” packages33.
Results
Spatial and temporal variation of vertical salinity
Salinity is a useful indicator to understand the hydrodynamic parameters of estuaries, including stratification34,35, flushing36, the distribution patterns of ecological parameters37. Water column stratification was assessed using the stratification parameter (\({n}_{s}=\partial S/{s}_{m}^{\prime}\) where \(\partial S\) = Sbot-Ssur, Sm = 1/2(Sbot-Ssur), with Ssur is the salinity at the surface and and Sbot the salinity at the bottom of the water column. The water column is well mixed when \({n}_{s}\) is < 0.1, partially mixed when 0.1< \({n}_{s}\) < 1.0 and stratified when \({n}_{s}\) > 1.0 38. According to the stratification parameter (< 0.01), the MRE is a well-mixed estuary during the dry and wet seasons. The MRE can be characterized as a macrotidal estuary based on the tidal range criterion39, resulting the distribution of ecological parameters is homogeneous. The tidal range supports the well mixed condition of the MRE. In addition, the spatial distribution of salinity in the MRE showed the expected variations related to the annual rainfall regime and tide (Fig. 2). During the dry season, the maximum salinity value was 13 PSU at the downstream region, approximately 100 km seawards from the Ilisha ghat and the minimum salinity was < 0.15 PSU at the upstream region (Fig. 2). During the wet season, the salinity decreased to < 0.15 along the MRE. The salinity difference between the dry and wet seasons was 13 PSU in the MRE. The saline water persists for several months (December -June) in the MRE during the dry season (Fig. 2 and 3). By contrast, the saline waters of the MRE retreated to the coastal area during the wet season (July–October) due to increasing freshwater discharge from upstream and the MRE became fresh condition. The salinity section recorded in December showed a transition period for the MRE reversal from a freshwater system to a brackish water system (Fig. 2). In contrast, the June salinity section showed a transition period from the brackish water to freshwater system as the river discharge decreased (Fig. 3). The position of a near-bottom isohaline (2 PSU) along the MRE depends primarily on freshwater discharge and secondarily on tide. Hilsa shad prefers freshwater of < 0.1 PSU for spawning, 0–1 PSU estuarine water for nursing of the juveniles and 0–2 PSU estuarine and coastal water for brood fish14. Thus, the temporal variation in vertical salinity profiles indicated that the MRE is a suitable hilsa spawning and nursery habitat during the wet season (Fig. 3). However, hilsa spawn all the year round with a major spawning season during the wet season (September–October) under the full moon phase40. In contrast, during the dry season, the upper MRE (above Ilisha ghat, UE) will act as suitable hilsa spawning and nursery habitat all the year round as per vertical salinity distribution of the MRE.
Seasonal and spatial variation of water quality
Physicochemical parameters
Water quality parameter showed significant seasonal variations and insignificant spatial variations (Table 1; Figs. 4, 5, 6). To understand the spatial variations, the monitoring stations were arranged from the upstream to the downstream (Figs. 5, 6). Water temperature varied significantly between the dry (22.5 °C) and wet (30.8 °C) seasons. The median water temperature of 22.5 °C during the dry season was significantly different (p < 0.01) from that of the wet season (30.5 °C). The spatial concentrations of salinity and pH were generally low at the upstream stations (UE), gradually increasing at the mid (ME) and downstream (LE) stations (Table 1, Fig. 4). The lowest salinity of 0.9 PSU was observed during the wet months (August and October), and the highest salinity of 13.0 PSU was measured in the dry months (Figs. 2, 3, 4, 5 and 6). The mean salinity values differed significantly (p < 0.01) between the wet and dry seasons due to large variations in river discharge (Fig. 4). According to the salinity ranges41,42, an estuary can be classified into five Venice salinity classes viz. euhaline (salinity > 30 PSU), polyhaline (salinity 18–30 PSU), mesohaline (salinity 5–18 PSU), oligohaline (salinity 0.5–5 PSU) and freshwater system (salinity < 0.5 PSU). The MRE acts as freshwater system during the wet season on the basis of Venice salinity classes (Table 1). Significant (p < 0.01) spatiotemporal variation in pH were not found in the water samples (Fig. 4). pH showed an increasing trend from fresh to marine zone during the wet season (Table 1, Fig. 4)). The lowest value of 6.8 was found at the fresh zone (UE) and the highest value (8.6) at marine zone HI. The median values of pH were 7.1 during the dry season and 8.3 during the wet season. The DO concentration was 8.3 mg/l during the dry season and 6.9 mg/l during the wet season. The observed DO level during the dry season was significantly (p < 0.01) higher than the wet season (Fig. 3). It is assumed that higher values of DO were observed during the dry seasons due to higher photosynthetic activity with low turbidity, and lower values during the wet season due to oxidation of organic matter with high turbidity. In addition, more organic waste enters the estuarine waters during the wet season along with huge freshwater runoff. Significant differences were observed in temperature, salinity and DO between the wet and dry seasons (Fig. 4). Among these parameters, only the salinity showed significant spatial variation (Figs. 5, 6). In addition, salinity showed significant positive correlation with DO (p < 0.001, Fig. 7). Relevantly, salinity was negatively correlated with temperature (p < 0.001) and chlorophyll-a (p < 0.01).In addition, there was a distinct negative correlation between pH and NO3−/NH4+. Although DO was inversely correlated with chlorophyll-a, indicating that biological processes are not only the factor affecting DO in the estuary. Negative correlations between temperature and DO were highly significant (p < 0.001), and highly significant positive correlations was found between NH4+ and DIN (p < 0.001, Fig. 7).
Temporal variations of major hydro-chemical parameter, dissolved inorganic nutrients and chlorophyll-a in the lower Meghna River estuary (UE = Upper estuary, ME = Middle estuary, LE = lower estuary, CK = Char Kukrimukri, HI = Hatiya Island). Note: the top, middle and bottom lines of the Box plot denote the upper quartiles, median and lower quartiles, respectively. The vertical line extending upward and downward denotes the range of data distribution. A data point located outside the whiskers of the box plot is called an outlier.
Spatial distributions of major hydro-chemical parameter, dissolved inorganic nutrients and chlorophyll-a in the lower Meghna River estuary during the dry season (UE = Upper estuary, ME = Middle estuary, LE = lower estuary, CK = Char Kukrimukri, HI = Hatiya Island). Note: the same for the Fig. 4.
Spatial distributions of major hydro-chemical parameter, dissolved inorganic nutrients and chlorophyll-a in the lower Meghna River estuary during the wet season (UE = Upper estuary, ME = Middle estuary, LE = lower estuary, CK = Char Kukrimukri, HI = Hatiya Island). Note: the same for the Fig. 4.
Nutrients
Among the major nutrients, dissolved inorganic nitrogen (DIN) values ranged from 0.29 mg/l during the wet season to 0.18 mg/l during the dry season. During the wet season, high concentrations of NO3 -N were found at HI and UE areas (Table 1) due to inflow of nutrient-rich waters from the upstream. The NO3 -N concentrations were higher during the dry season. DIN and NH4+ showed higher concentrations during the wet season as well as higher values in estuarine and marine zones compared to the dry season (Table 1, Figs. 5, 6). The wet season was characterized by high DIN levels (Table 1). The NH4+ was the major inorganic form of DIN during both the wet and dry seasons (Table 1 and Fig. 4). The NH4+ concentrations attained the maximum percentage of 51.6 and 80.7% in the dry and wet seasons, respectively.
Dissolved inorganic phosphorus (DIP) values varied from 0.46 mg/l during the wet season to 0.34 mg/l during the dry season (Table 1). Higher values were observed at the Char Kukrimukri (CK) compared to other areas (Table 1). Significant differences were observed in the PO4-P between the wet and dry seasons (Fig. 4). DIP did not fluctuate significantly throughout the sampling station (Figs. 5, 6). DSi values were higher in the riverine zone (UE) than in the estuarine (LE) and marine zones (HI and CK). Dissolved silica (DSi) loadings in coastal water enhance the production of diatoms. This trend was found during the dry and wet seasons (Table 1, Figs. 5, 6). In the wet season, the DSi concentrations were generally higher (5.99 mg/l). In the lower portion (CK, HI) of the MRE, DSi values decreased to the lowest concentration of 3.1 mg/l where salinity values were higher. The dissolved silica (DSi) concentration did not differ significantly between the dry and wet seasons (Fig. 4). The distributions of silica did not show significant spatial variations (Figs. 5, 6). Nutrients were low in concentration, consistent with tropical conditions.
Chlorophyll-a (Chl-a)
Significant differences were observed in the chlorophyll-a between wet and dry seasons (Fig. 4). Mean chlorophyll-a concentrations were higher at the Char Kukrimukri area (CK), followed by the estuarine (UE, ME, LE) zone. The highest chlorophyll-a values were found at CK during the wet season. The vertical salinity distribution across the mouth of the lower Meghna River estuary also showed the westward outflowing of freshwater to the CK that induced chlorophyll-a production at CK zones of the MRE (Figs. 4, 5, 6). The highest chlorophyll-a values were also found at Mangalore coast in India in the wet season8. The Char Kukrimukri (CK) zone may be characterized as mesotrophic estuarine zone (LE, ME, UE) based on chlorophyll-a concentration8,43. Based on 80th percentiles values of chlorophyll-a44, an estuary can be classified into three states: Oligotrophic (Chlorophyll-a: 0–5 µg/l), mesotrophic (Chlorophyll-a: 5–20 µg/l) and eutrophic (Chlorophyll-a: 20 ~ −60 µg/l). The highest chlorophyll-a value was found during the wet season (Table 1, Fig. 4). The MRE can be classified as mesotrophic based on chlorophyll-a concentration. Chl-a values differed significantly between the wet (6.8 µg/l) and dry (5.6 µg/l) seasons (Table 2). However, Chl-a values showed a significant correlation with PO4-P (p < 0.05) and insignificant correlation with DIN (p > 0.05) (Fig. 7). A significant negative correlation was found between Chl-a and salinity (p < 0.01). The Chl-a variation showed an opposite pattern to salinity (Figs. 5, 6). In addition, chlorophyll-a also showed different spatial patterns between the two seasons (Table 1 and Fig. 4). The dry period was characterised by concentration at the upper portion (UE). During the rainy (wet) season, the largest chlorophyll a peak (5.9 µg/l) occurred in higher-salinity zone of the lower portion (Char Kukrimukri) of the MRE (Fig. 6).
Phytoplankton community composition and diversity in water
In this study, we encountered twenty-seven phytoplankton species belonging to Chlorophyta (green algae), Cyanobacteria (blue-green algae), Miozoa (Dinoflagellates) and Bacillariophyta (diatoms). Among these phyla, Chlorophyta was the most dominant class (Table 3). Two species of blue-green algae, Oscillatoria sp. and Microcystis sp., a diatom, Lialoma sp., and a green alga, Pediastrum sp. were found in all the sampling stations. Spirogyra sp. and Oscillatoria sp. dominated in upper (UE) and middle estuary (ME) during the dry season. Chlorophyta was the dominant group ranging from 36% during the wet season to 26% during the dry season (Table 3). In addition, Chlorophyta was the dominant group at all the sampling stations ranging from 27% (CK) to 35% (UE) during the wet season. In the dry season, phytoplankton density varied from 16.2 × 103 to 94.1 × 103 cells L−1, with the highest count observed in the upper estuarine region (UE). In contrast, in the wet season, phytoplankton density varied from 10.3 × 103 to 215.1 × 103 cells L−1, the highest count was observed in the Char Kukrimukri (CK) and Hatiya Island (HI) regions. Phytoplankton community structure was governed by Spirogyra sp. in upper estuary (UE) and Pediastrum sp. in the Char Kukrimukri (CK) and Hatiya Island (HI) regions during the wet season. In general, the Chlorophyta was the dominant phyla during both the dry and wet seasons (Table 3) when nitrogen and phosphorus concentrations were optimal for their abundance. In contrast, Bacillariophyta was the second dominant phyla in the wet season under oligohaline (salinity < 0.5 PSU) condition. However, Bacillariophyta succeeded Chlorophyta in the dry season and consequently depleted dissolved silica (Fig. 4). Thus, the seasonal succession of phytoplankton between Bacillariophyta and Chlorophyta occurred in the dry season in the MRE. Simpsons Reciprocal Index is directly proportionate to species diversity (Table 4). The highest diversity was found in the MRE during both the dry (D < 0.16, 1/D > 6.0) and wet (D < 0.19, 1/D > 5.2) seasons.
Phytoplankton composition in the hilsa fish gut as the source of polyunsaturated fatty acids
Phytoplankton are producers and major suppliers of polyunsaturated fatty acids in estuary and coastal ecosystems, and are important for the function and quality of the entire coastal food web. A meta-analysis of more than 160 fatty acid profiles from seven marine phytoplankton phyla reveals a highly class-specific PUFA production by marine phytoplankton19. Among them, the highest amount of PUFA is found in Chlorophyta, which accounts for 60% of the total fatty acids19. The lowest PUFA is found in Cyanobacteria and diatoms (26 and 28% respectively). In this study, a mixed population of Chlorophyta (45.8 and 61.5% during the dry and wet seasons, respectively), diatoms (21.6 and 23.5% in the dry and wet seasons, respectively) and Cyanobacteria (14.1 and 12% in the dry and wet seasons, respectively) contributed approximately 89.3% to the composition of gut phytoplankton in the hilsa fish of the MRE. The phytoplankton composition indicates that Chlorophyta was the principal source of PUFA for hilsa fish, followed by diatoms and Cyanobacteria.
Discussion
Seasonal and spatial water quality
Variation of water quality is represented by sampling points (spatial effect) and sampling months (seasonal effect)47. Among water quality parameter, dissolved oxygen is an important indicator48. Decreased DO levels during the rainy (wet) season are related to the amount of oxygen consuming compounds entering from nearby industrial or agricultural areas through estuary river runoff. Low salinity during the rainy (wet) season was due to the outflow of fresh water. In contrast, during the dry season, the upper region (UE) remained oligohaline and the remaining sections (ME, LE, CK and HI) become mesohaline41. It is interesting that hilsa shad, an anadromous fish, can tolerate a wide range of salinity as it travels to different areas to find the best salinity to suit the different stages of its life cycle49,50,51. For example, hilsa prefers freshwater for spawning and nursing of the juveniles, the young (pre-adult called jatka) ones need estuarine and coastal water and the adult requires high saline marine water. However, the ideal salinity for the spawning and nursery activities of hilsa is < 0.1 PSU14,15. Thus, the entire MRE is suitable for hilsa spawning and nursery habitat during the wet season (Figs. 2, 4, 5, 6) when hilsa migrate to the MRE for spawning52. In contrast, only the upper region (above Ilisha ghat, UE) of the MRE acts as suitable hilsa spawning and nursery habitat during the dry season as hilsa fish breeds all the year round40.
The nutrient dynamics and primary productivity (indicated by Chl-a) of the MRE were strongly influenced by the seasonal fluctuations in precipitation. The MRE area is characterized by numerous distributaries. Annual rainfall is characterized by a typical monsoon with a well-defined maximum and minimum period, facilitating nutrient outflow from the Ganges–Brahmaputra-Meghna River system53. Similar seasonal patterns have been identified on the Brazilian coast54 and several other tropical estuarine areas54,55.
The input of large amounts of anthropogenic DIN (mainly NO3− and NH4+), the form of nitrogen that phytoplankton use directly, alters the estuarine and coastal marine ecosystem structure and function (species composition, abundance, distribution, and production, species diversity, and dynamics) of many coastal ecosystems56. The effects of different sources on the nutrient dynamics and chlorophyll-a distribution in the MRE can be explained by the results of a principal component analysis (PCA) performed separately in the dry and wet seasons. The first variable factor (DIM1) explained 27.9% of the variations in water quality fluctuations and contained the most information (Fig. 8). DIM1 clearly showed a clear positive correlation with temperature, DIN and NH4+ (Fig. 8). Inorganic source of dissolved nitrogen in the rainy (wet) season, might be due to human activity, and can be interpreted as an effect from non-point sources, such as freshwater discharges. Inorganic nitrogen fertilizer use, and nitrogen fixation in agricultural systems comprise the largest sources of the N inputs57. Seasonally, the nutrient concentrations in freshwater may be even lower than those found in the coastal ocean, which lead to complex management problems58. Although, the DIN export in rivers to the coastal ocean nearly doubled over the past four decades of the twentieth century globally, DIN species are in low concentrations in tropical coastal water59. DIN variation among Indian coastal ecosystems can be caused by differences in the freshwater input pulse59. Ammonium is the dominant N form in estuarine and coastal areas except in polluted estuaries and eutrophic and coastal upwelling regions59,60. This scenario is true for most of the Indian mangroves; however, some mangroves have nitrate as the major DIN pool. The dominant DIN pool varies depending on source, processing, and other environmental factors. High river discharge and runoff plays a key role in bringing these nutrients (DIN and NH4+) to this system during the wet season. Temporally, the impact of DIN and NH4+ on the phytoplankton biomass production were greater in the wet season than in the dry season (Fig. 8). In addition, DIM1 had a moderate correlation with chlorophyll-a. Therefore, DIM1 should mainly be interpreted as one kind of dissolved inorganic nitrogen source that is mainly affected by a non-point source.
Factorial plan of the first, second, third and fourth axes of the principal component analysis (PCA) for the spatial (A, B), temporal (C, D, E, F) environmental parameter of the lower Meghna River estuary (UE = Upper estuary, ME = Middle estuary, LE = lower estuary, CK = Char Kukrimukri, HI = Hatiya Island).
Salinity and temperature are key environmental traits for the physical structure of estuaries, those influence phytoplankton (chlorophyll a is proxy for that) communities61. DIM2, which accounts for 22.0% of the total variance, had a negative correlation of chlorophyll a with dissolved oxygen and salinity, and a positive correlation of chlorophyll a with temperature (Fig. 8). Dissolved oxygen levels increase with freshwater runoff and decrease with rise in temperature62. DIM2 can be represented as a physicochemical source due to the natural changes in the aquatic environment and the ionic properties of the water body.
Phytoplankton abundance in coastal waters may be due to fluctuations in the essential nutrients (such as nitrate, phosphate and silicate), from either an upwelling or run-off61. In coastal areas, nitrate is the dominant N form and preferred by diatoms63. The uptake of nitrate in coastal areas can be high and may be reduced if ammonium is available64. The ammonium concentration that suppresses nitrate uptake varies greatly and strongly depends on phytoplankton species. For benthic diatoms, the uptake of other N compounds is suppressed when NH4+ is present65. Silicate is an important factor for the growth of diatoms and leading to the shifts of different phytoplankton groups66. DIM3 and DIM4, which accounts for 25.5% of the total variance, were weighted on dissolved silica, NO3−, PO43− and chlorophyll-a (Fig. 8). The correlation matrix showed that DSi, NO3− and PO43− correlates with chlorophyll-a during the wet season (Fig. 8). Therefore, DIM3 and DIM4 represents the natural source of dissolved inorganic nutrients, primarily reflecting the natural changes in the aquatic environment, and the growth of phytoplankton67. The PCA plot shows a seasonal gradient observed at the sampling site, forming two different groups (Fig. 8). This analysis also showed biochemical processes that occur in the MRE waters during the dry and wet seasons.
Phytoplankton community
Bioavailable nitrogen is the principal limiting nutrient in estuaries for phytoplankton68,69. A mixed population of Chlorophyta, diatoms and blue-green algae contributed approximately 77% to the composition of wet season phytoplankton in the MRE due to favourable temperature and nutrients (Table 3) and 74% during the dry season phytoplankton. The Ganges, Brahmaputra and Meghna are the most important contributors to DIN during the wet season and green algae proliferate due to relatively high nitrogen levels. Dissolved inorganic nitrogen (nitrates, nitrites and ammonia) come mainly from nearby catchment areas and enter into estuaries via adjacent rivers and surface runoff70. In addition, denitrification, tidal flushing and hydraulic loading influence nitrogen loads within the estuary71. In contrast, the bioavailability of phosphorous (P) depends on the river load, the degradation of organic matter, and the ingress of coastal waters into the adjacent estuary during the high tide72, which becomes more frequent during the wet season.
The nutrient in freshwater causing most blue-green blooms in particular is phosphorus61. In addition, Cyanobacteria prefer nitrogen in the form of ammonia and nitrate61. Cyanobacterial abundance was driven by an increased presence of nutrients (ammonia, nitrate and phosphorus) in the MRE during the wet season (Fig. 6 and Table 1) because most phytoplankton, except some species belonging to Cyanobacteria cannot fix atmospheric nitrogen61. Salinity is an additional environmental factor that can have some impact on the algal abundance in the freshwater system61. Information is scarce on the salinity tolerances of most freshwater phytoplankton species. Two species of Cyanobacteria such as Anabaena sp. and Microcystis sp., have a salt tolerances of up to 5–6 PSU before they are killed off by salinity73. In contrast, green algae are the most abundant and diverse of all freshwater algae61. The green algae are primarily a freshwater group, with approximately 62% of representatives occurring in both the dry and wet seasons (Fig. 6).
In the MRE, diatoms predominate after a decrease in river flow during the dry season, and a mixed diatom populations contribute approximately 59% to the composition of phytoplankton in the dry season (Fig. 6). Such type of pattern has also been found in the Meuse River, Belgium74 and in the Thames River, UK8. However, the factors that regulate seasonal periodicity of phytoplankton in estuaries are less well documented and not yet fully understood than those in lakes8. The MRE provides the suitable conditions for the proliferation of diatoms and green algae, the main components of the phytoplankton. Silica must be considered as the first regulator of diatom development8. In the MRE, favourable hydrological conditions are met after the decrease of discharge in the dry season, as a consequence a high level of diatom abundance occurred during the dry season that depleted silica (Figs. 4, 5, 6). Silica content clearly affects the maximum diatom biomass that reaches during the dry season.
During the dry season, diatoms were the dominant group which was replaced by blue green algae (Cyanobacteria) during the wet season. Temperature above 25 °C provides suitable environments for blue-green algal growth rates. In addition, under lower light dose and stronger light fluctuations, net growth rate of Cyanobacteria tends to decrease76. Blue-green algae developed quickly in nutrient rich (high in phosphorus) environments with favorable temperature during the wet season61. Salinity is another environmental factor that may have some effect on algal presence in fresh water system73. Some blue-green algal species have been found to have salt tolerances of up to 5–6 PSU before they are killed off by salinity61. The concentration of silicates is essential for the growth of silicified organism’s diatoms77,78. Increasing silica inputs allowed the diatoms to grow at a higher level, leading to decrease in silica level in the water column. Conversely, drop in major nutrient (SiO4) levels could be observed during the dry season, when diatom flourished and that deplete silica concentration during the dry season8. During the wet season, a strong negative correlation was observed between the diatoms and concentration of silica, as diatoms need silica in addition to phosphate and nitrate to build their frustules. Silica is influencing negatively on the growth of diatoms as they consume silica in cell wall synthesis77.
Polyunsaturated fatty acids (PUFA) content in the major phytoplankton groups
Phytoplankton (microalgae) is principal source of ω3 and ω6 PUFA19. Meta-analyses clearly show that some classes of phytoplankton are a better source of essential PUFAs than others19. Studies have shown that crustaceans and fish cannot easily biosynthesize ω3 and ω6 PUFAs19. These fatty acids have to be obtained from their diet available in the food web. The highest levels of PUFAs are found in green algae (approximately 60% of the total fatty acid)19 and the lowest levels of PUFA are found in blue-green algae (26%) and diatoms (28%). Therefore, the focus was given to the composition of the major phytoplankton group in both water and hilsa fish gut of the MRE. In this study, a mixed population of green algae (53.6%), blue-green algae (13%) and diatoms (22.6%) contributed approximately 89.2% to the composition of gut phytoplankton in the hilsa fish of the MRE. Hilsa fish was selected because it is the national fish of Bangladesh and is an important food fish, rich in ω3 and ω6 polyunsatured fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)79. Fish or fish oil contains omega-3 PUFAs, e.g., DHA and EPA, which are beneficial to human health and reduce the risk of coronary heart diseases80. In addition, the ω3/ω6 and DHA/EPA proportions are high in diatoms19. The contribution of EPA and DHA to the mass of PUFAs differ in both proportion and quantity. Hilsa fish cannot synthesize these essential nutrients that must be obtained from the diet, especially via phytoplankton. Hilsa is omnivorous and mainly eats phytoplankton81. Higher levels of lipids and fatty acids in fish muscle, primarily DHA, are due to the high levels of lipids and DHA in the feed.
Electivity index
By comparing the relative quantity of a possible prey item with its relative predominance in a predator's diet, electivity indices summarize the findings of field-based feeding research. The numbers of distinct prey taxa found in water samples and the guts of hilsa fish were used to calculate a new electivity index based on odds ratios28. The electivity index value of 0.5 for the Chlorophyta and Bacillariophyta indicates the same prey sample in the water and in the hilsa fish gut (Fig. 9). Cyanobacteria is the third most preferable prey to hilsa fish following the hexanauplia (Fig. 9).
Conclusion
We explored the ecological understanding of the seasonal periodicity of phytoplankton in the MRE with its changing hydrological conditions. No significant spatial variations were observed in the water quality parameters except salinity. Considering the salinity distribution, the entire MRE is a suitable hilsa spawning and nursery ground during the wet season and only the upper MRE (upstream of Ilisha ghat) can act as spawning ground during the dry season. The results of the multivariate analysis revealed two distinct groups for the dry and rainy seasons for the water quality criteria. The multivariate analysis explained 75.4% variability of seven physicochemical parameters that caused seasonal variations of three major groups of phytoplankton. The most relevant driving factors were dissolved oxygen, salinity, temperature, and DIN (nitrate, nitrite and ammonia). These variabilities in physicochemical parameters and dissolved inorganic nutrients caused seasonal variations in two major groups of phytoplankton. Peak abundance of green algae occurred in nitrogen and phosphorus-rich environment during the wet season. The diatoms were dominant during the dry season that severely depleted dissolved silica. Thus, phytoplankton diversity showed the potential link to seasonal changes of hydro-chemical parameters and phytoplankton development that was invariably initiated by the decrease of river discharge in the dry season. In addition, the green algae and diatoms were the major phytoplanktonic food for hilsa fish in the MRE food web as well as major source for PUFAs as higher percentage of green algae and diatoms were found in the hilsa fish gut.
Data availability
All data used to support the findings of the study are available from the corresponding author upon request.
References
Valle-Levinson, A. Contemporary Issues in Estuarine Physics (Cambridge University Press, 2010).
Singh, S. Analysis of plankton diversity and density with physico-chemical parameters of open pond in town Deeg (Bhratpur) Rajasthan, India. Int. Res. J. Biol. Sci 4, 61–69 (2015).
Roussel, M., Pontier, D., Cohen, J.-M., Lina, B. & Fouchet, D. Quantifying the role of weather on seasonal influenza. BMC Public Health 16, 1–14 (2016).
Davies, O., Abowei, J. & Tawari, C. Phytoplankton community of Elechi creek, Niger Delta, Nigeria-a nutrient-polluted tropical creek. Am. J. Appl. Sci. 6, 1143–1152 (2009).
Choudhury, S. & Panigrahy, R. Seasonal distribution and behavior of nutrients in the Greek and coastal waters of Gopalpur, East coast of India: Mahasagar. Bull. Natl. Inst. Oeanogr 24, 91–88 (1991).
Ratheesh, K., Krishnan, A., Das, R. & Vimexen, V. Seasonal phytoplankton succession in Netravathi-Gurupura estuary, Karnataka, India: Study on a three tier hydrographic platform. Estuar. Coast. Shelf Sci. 242, 106830 (2020).
Deng, Y., Tang, X., Huang, B. & Ding, L. Effect of temperature and irradiance on the growth and reproduction of the green macroalga, Chaetomorpha valida (Cladophoraceae, Chlorophyta). J. Appl. Phycol. 24, 927–933 (2012).
Gamier, J., Billen, G. & Coste, M. Seasonal succession of diatoms and Chlorophyceae in the drainage network of the Seine River: Observation and modeling. Limnol. Oceanogr. 40, 750–765 (1995).
Meng, F. et al. Phytoplankton alpha diversity indices response the trophic state variation in hydrologically connected aquatic habitats in the Harbin Section of the Songhua River. Sci. Rep. 10, 1–13 (2020).
Köhler, J. Growth, production and losses of phytoplankton in the lowland River Spree. I. Population dynamics. J. Plankton Res. 15, 335–349 (1993).
Murrell, M. C. & Caffrey, J. M. High cyanobacterial abundance in three northeastern Gulf of Mexico estuaries. Gulf Caribbean Res. 17, 95–106 (2005).
Haldar, G., Rahman, M. & Haroon, A. Hilsa, Tenualosa ilisha (Ham.) fishery of the Feni River with reference to the impacts of the flood control structure. J. Zool. 7, 51–56 (1992).
Hossain, M. S., Sarker, S., Chowdhury, S. R. & Sharifuzzaman, S. Discovering spawning ground of Hilsa shad (Tenualosa ilisha) in the coastal waters of Bangladesh. Ecol. Model. 282, 59–68 (2014).
Bhaumik, U. & Sharma, A. The fishery of Indian Shad (Tenualosa ilisha) in the Bhagirathi-Hooghly river system. Fishing Chimes 31, 21–27 (2011).
Mitra, G. & Devsundaram, M. P. On the hilsa of Chilka Lake with note on the Hilsa in Orissa. J. Asiatic Soc. Sci. 20, 33–40 (1954).
Abdul, W., Phillips, M. & Beveridge, M. (WorldFish (WF), 2020).
Hasan, K. M. M., Wahab, M. A., Ahmed, Z. F. & Mohammed, E. Y. The biophysical assessments of the hilsa fish (Tenualosa ilisha) habitat in the lower Meghna, Bangladesh (International Institute for Environment and Development, 2015).
Begum, M. et al. Fatty acid composition of Hilsa (Tenualosa ilisha) fish muscle from different locations in Bangladesh. Thai J. Agric. Sci. 52, 172–179 (2019).
Jónasdóttir, S. H. Fatty acid profiles and production in marine phytoplankton. Mar. Drugs 17, 151 (2019).
Otero, P., Ruiz-Villarreal, M., Peliz, Á. & Cabanas, J. M. Climatology and reconstruction of runoff time series in northwest Iberia: Influence in the shelf buoyancy budget off Ría de Vigo. Sci. Mar. 74, 247–266 (2010).
Grasshoff, K., Kremling, K. & Ehrhardt, M. Methods of Seawater Analysis (Wiley, 2009).
Parsons, T., Maita, Y. & Lalli, C. A manual of chemical and biological methods for seawater analysis. Pergamon, Oxford sized algae and natural seston size fractions. Mar. Ecol. Prog. Ser. 199, 43–53 (1984).
Scor-Unesco, W. Determination of photosynthetic pigments. Determination of Photosynthetic Pigments in Sea-water, 9–18 (1966).
Snow, G., Bate, G. & Adams, J. The effects of a single freshwater release into the Kromme Estuary. 2: Microalgal response. Water SA-Pretoria 26, 301–310 (2000).
Ward, H. B. & Whipple, G. C. Freshwater Biology Vol. 2, 12–48 (Willey, London, 1959).
Prescott, G. W. Algae of the western Great Lakes area. (1962).
Bellinger, E. G. A Key to Common Algae: Freshwater, Estuarine and Some Coastal Species (Institution of Water and Environmental Management London, 1992).
Kimmerer, W. J. & Slaughter, A. M. A new electivity index for diet studies that use count data. Limnol. Oceanogr. Methods 19, 552–565 (2021).
Pinheiro, J., Bates, D., DebRoy, S. & Sarkar, D. R Development Core Team. nlme: Linear and nonlinear mixed effects models, 2012. http://CRAN.R-project.org/package=nlme. R package version, 3.1–103 (2020).
Lê, S., Josse, J. & Husson, F. FactoMineR: an R package for multivariate analysis. J. Stat. Softw. 25, 1–18 (2008).
Galili, T., O’Callaghan, A., Sidi, J. & Sievert, C. heatmaply: an R package for creating interactive cluster heatmaps for online publishing. Bioinformatics 34, 1600–1602 (2018).
Wickham, H., Chang, W. & Wickham, M. H. Package ‘ggplot2’. Create Elegant Data Visualisations Using the Grammar of Graphics. Version 2, 1–189 (2016).
Peterson, B. G. et al. Package ‘PerformanceAnalytics’. R Team Cooperation (2018).
Lewis, R. E. & Uncles, R. J. Factors affecting longitudinal dispersion in estuaries of different scale. Ocean Dyn. 53, 197–207 (2003).
Shaha, D., Cho, Y.-K., Seo, G.-H., Kim, C.-S. & Jung, K. Using flushing rate to investigate spring-neap and spatial variations of gravitational circulation and tidal exchanges in an estuary. Hydrol. Earth Syst. Sci. 14, 1465–1476 (2010).
Shaha, D. C., Cho, Y.-K., Kim, T.-W. & Valle-Levinson, A. Spatio-temporal variation of flushing time in the Sumjin River Estuary. Terrestr. Atmos. Ocean. Sci. 23, 119 (2012).
Shivaprasad, A. et al. Seasonal stratification and property distributions in a tropical estuary (Cochin estuary, west coast, India). Hydrol. Earth Syst. Sci. 17, 187–199 (2013).
Haralambidou, K., Sylaios, G. & Tsihrintzis, V. A. Salt-wedge propagation in a Mediterranean micro-tidal river mouth. Estuar. Coast. Shelf Sci. 90, 174–184 (2010).
Dyer, K. R. Estuaries: A physical introduction (1973).
Rahman, M. et al. Impact assessment of twenty-two days fishing ban in the major spawning grounds of Tenualosa ilisha (Hamilton, 1822) on its spawning success in Bangladesh. J. Aquac. Res. Dev. 8, 489 (2017).
Alves, A. S. et al. Spatial distribution of subtidal meiobenthos along estuarine gradients in two southern European estuaries (Portugal). J. Mar. Biol. Assoc. U.K. 89, 1529–1540 (2009).
Teixeira, H., Salas, F., Borja, A., Neto, J. & Marques, J. A benthic perspective in assessing the ecological status of estuaries: The case of the Mondego estuary (Portugal). Ecol. Ind. 8, 404–416 (2008).
Garmendia, M. et al. Eutrophication assessment in Basque estuaries: Comparing a North American and a European method. Estuar. Coasts 35, 991–1006 (2012).
Istvánovics, V. Eutrophication of Lakes and Reservoirs. Lake Ecosystem Ecology 47–55 (Elsevier, 2010).
Dodds, W. K. Eutrophication and trophic state in rivers and streams. Limnol. Oceanogr. 51, 671–680 (2006).
Bricker, S., Ferreira, J. & Simas, T. An integrated methodology for assessment of estuarine trophic status. Ecol. Model. 169, 39–60 (2003).
Vega, M., Pardo, R., Barrado, E. & Debán, L. Assessment of seasonal and polluting effects on the quality of river water by exploratory data analysis. Water Res. 32, 3581–3592 (1998).
Huang, Y., Yang, C., Wen, C. & Wen, G. S-type dissolved oxygen distribution along water depth in a canyon-shaped and algae blooming water source reservoir: Reasons and control. Int. J. Environ. Res. Public Health 16, 987 (2019).
Rahman, M. & Cowx, I. Lunar periodicity in growth increment formation in otoliths of hilsa shad (Tenualosa ilisha, Clupeidae) in Bangladesh waters. Fish. Res. 81, 342–344 (2006).
Rahman, M. J. Population Biology and Management of hilsa shad (Tenualosa ilisha) in Bangladesh (University of Hull, 2001).
Milton, D. A. & Chenery, S. R. Movement patterns of the tropical shad hilsa (Tenualosa ilisha) inferred from transects of 87Sr/86Sr isotope ratios in their otoliths. Can. J. Fish. Aquat. Sci. 60, 1376–1385 (2003).
Rahman, S., Sarker, M. R. H. & Mia, M. Y. Spatial and temporal variation of soil and water salinity in the South-Western and South-Central Coastal Region of Bangladesh. Irrig. Drain. 66, 854–871 (2017).
Kida, S. & Yamazaki, D. The mechanism of the freshwater outflow through the Ganges–Brahmaputra–Meghna delta. Water Resour. Res. 56, e2019WR026412 (2020).
Sarma, V. et al. Intra-annual variability in nutrients in the Godavari estuary, India. Contin. Shelf Res. 30, 2005–2014 (2010).
Burford, M. et al. Controls on phytoplankton productivity in a wet–dry tropical estuary. Estuar. Coast. Shelf Sci. 113, 141–151 (2012).
Vitousek, P. M. et al. Towards an ecological understanding of biological nitrogen fixation. Biogeochemistry 57, 1–45 (2002).
Galloway, J. N. & Cowling, E. B. Reactive nitrogen and the world: 200 years of change. Ambio 31, 64–71 (2002).
Kennish, M. & De Jonge, V. in Human-Induced Problems (Uses and Abuses) 113–148 (Elsevier Inc., 2012).
Alongi, D., Boto, K. & Robertson, A. Nitrogen and phosphorus cycles. Coastal and Estuarine Studies, 251–251 (1993).
Wolanski, E., McLusky, D., Laane, R. & Middleburg, J. (Academic Press, 2011).
Suthers, I., Rissik, D. & Richardson, A. Plankton: A Guide to Their Ecology and Monitoring for Water Quality (CSIRO Publishing, 2019).
Mackay, D. W. & Fleming, G. Correlation of dissolved oxygen levels, fresh-water flows and temperatures in a polluted estuary. Water Res. 3, 121–128 (1969).
Lomas, M. W. & Glibert, P. M. Temperature regulation of nitrate uptake: A novel hypothesis about nitrate uptake and reduction in cool-water diatoms. Limnol. Oceanogr. 44, 556–572 (1999).
Dortch, Q. The interaction between ammonium and nitrate uptake in phytoplankton. Mar. Ecol. Prog. Ser. Oldendorf 61, 183–201 (1990).
Admiraal, W., Riaux-Gobin, C. & Laane, R. W. Interactions of ammonium, nitrate, and D-and L-amino acids in the nitrogen assimilation of two species of estuarine benthic diatoms. Mar. Ecol. Prog. Ser. 40, 267–273 (1987).
Rabalais, N., Turner, R., Dortch, Q., Wiseman, W. Jr. & Sen Gupta, B. Nutrient changes in the Mississippi River and system responses on the adjacent continental shelf. Estuaries 19, 386 (1996).
Gholizadeh, M. H., Melesse, A. M. & Reddi, L. Water quality assessment and apportionment of pollution sources using APCS-MLR and PMF receptor modeling techniques in three major rivers of South Florida. Sci. Total Environ. 566, 1552–1567 (2016).
Elser, J. J. et al. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 10, 1135–1142 (2007).
Teichberg, M. et al. Eutrophication and macroalgal blooms in temperate and tropical coastal waters: Nutrient enrichment experiments with Ulva spp. Glob. Change Biol. 16, 2624–2637 (2010).
Valiela, I. & Bowen, J. Nitrogen sources to watersheds and estuaries: Role of land cover mosaics and losses within watersheds. Environ. Pollut. 118, 239–248 (2002).
Woodland, R. J. et al. Nitrogen loads explain primary productivity in estuaries at the ecosystem scale. Limnol. Oceanogr. 60, 1751–1762 (2015).
Howarth, R. et al. Coupled biogeochemical cycles: Eutrophication and hypoxia in temperate estuaries and coastal marine ecosystems. Front. Ecol. Environ. 9, 18–26 (2011).
Winder, J. A. & Cheng, D. M. Quantification of Factors Controlling the Development of Anabaena Circinalis Blooms (Urban Water Research Association of Australia, 1995).
Descy, J.-P. Phytoplankton composition and dynamics in the River Meuse (Belgium). Arch. Hydrobiol. Supplementband. Monographische Beiträge 78, 225–245 (1987).
Robarts, R. D. & Zohary, T. Temperature effects on photosynthetic capacity, respiration, and growth rates of bloom-forming cyanobacteria. NZ J. Mar. Freshw. Res. 21, 391–399 (1987).
Visser, P. M., Ibelings, B. W., Bormans, M. & Huisman, J. Artificial mixing to control cyanobacterial blooms: A review. Aquat. Ecol. 50, 423–441 (2016).
Krishnan, A., Das, R. & Vimexen, V. Seasonal phytoplankton succession in Netravathi-Gurupura estuary, Karnataka, India: Study on a three tier hydrographic platform. Estuar. Coast. Shelf Sci. 242, 106830 (2020).
Srinivas, L., Seeta, Y. & Reddy, M. Bacillariophyceae as ecological indicators of water quality in Manair Dam, Karimnagar, India. Int. J. Sci. Res. Sci. Tech 4, 468–474 (2018).
Mohanty, B. P. et al. Fatty acid profile of Indian shad Tenualosa ilisha oil and its dietary significance. Natl. Acad. Sci. Lett. 35, 263–269 (2012).
De, D. et al. Nutritional profiling of hilsa (Tenualosa ilisha) of different size groups and sensory evaluation of their adults from different riverine systems. Sci. Rep. 9, 1–11 (2019).
Hasan, K. M. M., Ahmed, Z. F., Wahab, M. A. & Mohammed, E. Y. Food and Feeding Ecology of hilsa (Tenualosa ilisha) in Bangladesh’s Meghna River Basin. (International Institute for Environment and Development, 2016).
Acknowledgements
This research was carried out under a sub-project of the ECOFISH-BD activity and was carried out in accordance with a cooperative agreement between WorldFish, the Department of Fisheries Management, and Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur 1706. The Department of Fisheries (DOF), Bangladesh, and the WorldFish, Bangladesh and South Asia Office collaborated to implement the United States Agency for International Development (USAID) funded Enhanced Coastal Fisheries in Bangladesh (ECOFISH-II) activity. Thanks are due to the post graduate students of the department who assisted in sampling and laboratory analyses. The fishermen fishing in the Meghna Estuary supplied the freshly caught hilsa fish to support this research. The partial financial support was provided by the Research Management Wing, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur, Bangladesh.
Author information
Authors and Affiliations
Contributions
Conceptualization, D.C.S.; data curation, S.R.K. and J.H.; formal analysis, J.H. and D.C.S.; investigation, D.C.S.; J.H.; methodology, J.H.; S.R.K. and D.C.S.; resources, D.C.S.; visualization, D.C.S.; writing—original draft, D.C.S.; S.R.K. and J.H.; writing—review and editing, F.M.Y.; M.A.S.; M.K.; F.H.; M.A.; M.J.R.; M.A.W.; J.H. and D.C.S. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shaha, D.C., Hasan, J., Kundu, S.R. et al. Dominant phytoplankton groups as the major source of polyunsaturated fatty acids for hilsa (Tenualosa ilisha) in the Meghna estuary Bangladesh. Sci Rep 12, 20980 (2022). https://doi.org/10.1038/s41598-022-24500-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-24500-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.