Abstract
Spatio-temporal variation in phytoplankton community dynamics in a temporarily open/closed Swarnamukhi river estuary (SRE), located on the South East coast of India was investigated and correlated to that of the adjacent coastal waters. Understanding the seasonal variability of the phytoplankton community and influencing factors are essential to predicting their impact on fisheries as the river and coastal region serve as the main source of income for the local fishing communities. Downstream before the river meets the sea, an arm of the Buckingham Canal (BC), carrying anthropogenic inputs empties into the Swarnamukhi River (SR1). The impact of anthropogenic effects on the phytoplankton community at BC was compared to other estuarine stations SR2 (upstream), SR1 (downstream), SRM (river mouth) and coastal station (CS). In BC station, harmful algal blooms (HABs) of Chaetoceros decipiens (2940 × 103 cells L−1) and Oscillatoria sp. (1619 × 103 cells L−1) were found during the southwest monsoon and winter monsoon, respectively. These HABs can be linked to the anthropogenic input of increased nutrients and trace metals. The HABs of Oscillatoria sp. were shown to be induced by elevated concentrations of nitrate (10.18 µM) and Ni (3.0 ppm) compared to ambient, while the HABs of C. decipiens were caused by elevated concentrations of silicate (50.35 µM), nitrite (2.1 µM), and phosphate (4.37 µM). Elevated nutrients and metal concentration from the aquaculture farms, and other anthropogenic inputs could be one of the prime reasons for the recorded bloom events at BC station. During this period, observed bloom species density was found low at other estuarine stations and absent at CS. The formation of bloom events during the closure of the river mouth could be a major threat to the coastal ecosystem when it opens. During the Osillatoria sp. bloom, both the Cu and Ni levels were higher at BC. The elevated concentration of nutrients and metals could potentially affect the coastal ecosystem and in turn fisheries sector in the tropical coastal ecosystem.
Similar content being viewed by others
Introduction
Phytoplankton play a crucial role in the marine food web. Composition and the spatio-temporal variability of phytoplankton community reflect both short- and long-term environmental changes in the aquatic ecosystem1. Several environmental factors, including temperature, nutrient availability, and river runoff2,3 determine the growth, distribution, and composition of phytoplankton in the coastal and marine environment. Estuarine systems, which are influenced by both natural fluctuation (tidal and seasonal) and anthropogenic activities (land and river-runoff) are complex and dynamic environmental niches in terms of their hydrological conditions & community composition. The land runoffs usually increase the load of pathogens, nutrients, trace metals, detergents, and pesticides in the receiving water body4. In closed estuarine systems increased supply of nutrients results in eutrophication that could trigger harmful algal blooms (HABs) which release toxic secondary metabolites and alter the ecosystem. The occurrence of HABs is increasing in frequency and intensity around the world5,6,7. The occurrence and persistence of algal blooms depends upon a combination of interaction with physical, chemical, and biological factors8,9.
On the other hand, pollutants brought by rivers and land runoff are considered major threats to marine ecosystems and might alter food-web dynamics in coastal environments4,10. Trace metals enter the estuarine system through natural processes and various anthropogenic activities such as mineral weathering, mining, fossil fuel combustion, industrial discharges, urban development, sewage and agricultural discharge11,12. Some trace metals are essential for phytoplankton growth and survival, however, their concentration beyond the permissible level will harm marine ecosystem and seafood safety through persistent bio-accumulation1,11. Therefore, both natural and anthropogenic inputs play an important role in controlling the physicochemical and biological factors of an estuarine ecosystem.
Open-closed estuaries are the most complex and important aquatic ecosystems, which differ from other estuaries, in that mixing does not occur during the closure of the river mouth13. Swarnamukhi River Estuary is a temporarily open/closed estuarine system, situated along the Pamanji coastal village of Nellore District, Andhra Pradesh, on the southeast coast of India. River mouth closure and the connection between the river and sea is mostly interrupted for 3–5 months during the late summer monsoon to early north-east monsoon (June–October) due to the massive amount of sand deposition. The duration of mouth closure depends on the precipitation during that particular period14,15, mostly the river mouth opens during the NEM due to high rainfall. It is of interest to study the phytoplankton community dynamics and the factors influencing them in such temporarily open/closed estuaries. Bloom incidents occurring in open/closed estuaries have not been well-documented16,17. Several studies have addressed the phytoplankton community structure in relation to physico-chemical parameters18,19,20, however, there are few studies which have investigated the levels of heavy metals and their role in phytoplankton community structure21. In view of these lacunae the present study has been carried out in the Swarnamukhi River Estuarine system which offers a unique habitat to investigate phytoplankton community structure. Objectives of the present study are (1) to investigate the spatio-temporal changes in hydrographical parameters and heavy metals concentration, (2) to investigate the spatio-temporal variations of phytoplankton community and their response to changing environmental variables, and (3) to investigate the influence of heavy metals and other environmental variables on harmful algal blooms.
Materials and methods
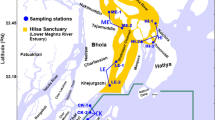
Sampling was carried out in the temporarily open-closed Swarnamukhi river estuary (SRE) and adjacent coastal waters of Pamanji, Nellore, Southeast coast of India. Duplicate samples were collected for 2 years (2018 and 2019) during four different seasons, i.e., winter monsoon (WM; Jan–Mar), summer monsoon (SM; Apr–May), southwest monsoon (SWM; Jun–Sep), and northeast monsoon (NEM; Oct–Dec). Five sampling stations covering, Coastal Station (CS), Swarnamuki River Estuary (SRM: river mouth, SR1: river downstream, and SR2: river upstream), and Buckingham Canal (BC) were selected (Fig. 1).
Study locations along the Swarnamukhi River Estuary, southeast coast of India. (Study area map created by ArcGIS ver. 10.1 software, https://www.esri.com/en-us/home).
Monthly mean rainfall data for the sampling period was collected from Indian Meteorological Department (IMD). Water temperature (WT), salinity, and pH were measured using an in situ calibrated portable multi-parameter water quality instrument (Hana HI 9829). Water samples were collected from the surface (~ 0.5 m) using a 5 L Niskin sampler (Hydrobios-Kiel) for measuring dissolved oxygen (DO), biochemical oxygen demand (BOD), total suspended solids (TSS), chlorophyll-a (Chl-a), nutrients, and trace metals. The DO and BOD were measured by modified Winkler’s method22. To determine TSS, a measured volume of sample was filtered through pre-dried and pre-weighed (0.45 mm, Millipore GF/C) filter paper and washed with Milli-Q water to remove salt contents23. Water samples collected for dissolved nutrient analysis were filtered by GF/F filter paper for removing the particulate matter and the filtrate was frozen at − 20 °C until further analysis. All nutrients (Ammonium (NH4+), nitrite (NO2−), nitrate (NO3−), phosphate (PO43–), silicate (SiO42–), total nitrogen (TN), and total phosphorus (TP)) were analyzed using standard spectrophotometric procedures24. The detection limits of NH4+, NO2−, NO3−, PO43–, SiO42–, TN and TP were ± 0.02, ± 0.01, ± 0.02, ± 0.01, ± 0.02, ± 0.02 and ± 0.01 μM, respectively. For trace metal analysis, unfiltered water samples were collected in acid-cleaned polypropylene bottles and immediately acidified to pH 2–3 using supra-pure HNO3 and kept at 4 °C until analysis. For digestion, 5 mL of water sample was added with 5 mL of concentrated HNO3 and digested in microwave. The digested samples were transferred to centrifuge tubes, and made the volume up to 50 mL by adding Milli-Q water25. Trace metals concentrations were determined by using Inductively Coupled Plasma Mass Spectrometry (ICP-MS; Agilent-7500). The digestion and analytical procedures were carried out according to the method given by the US Environmental Protection Agency26. The samples, certified reference materials (CRM), and duplicate analytical blanks were examined for any potential contamination as per the protocol followed by Jha et al. 27,28. The reference value (seawater), analyzed mean and standard deviation of the elemental values obtained for the NIST CRM QC3163 (seawater), is adopted from Jha et al. 27. By comparing the analyzed value to the CRM value, the precision and correctness of the analysis were confirmed. The metal concentration in water sample was represented as ppb.
For Chl-a analysis, a known volume of water sample was filtered through Whatman GF/F filter and preserved at − 20 °C until analysis. Chl-a in the filter was extracted with 90% acetone at 4 °C in the dark for 24 h and analysed spectrofluorometrically29. For phytoplankton analysis, water sample was collected 5 L for coastal station, and 1 L for estuarine stations, and preserved with 4% Lugol’s iodine solution. Utermohl’s sedimentation method was used to count the phytoplankton under microscope (NIKON Eclipse E100), and identification was carried out with the standard identification keys30,31,32.
Shannon–Wiener’s diversity index (H′), Margalef’s species richness (d) and Pielou’s evenness (J′) were determined by using PRIMER-7. The statistical significance of diversity indices and environmental parameters were assessed using one-way analysis of variance (ANOVA) on XLSTAT software. Pearson correlation analysis was performed for environmental variables with phytoplankton biomass and abundance by XLSTAT statistical software. Principal component analysis (PCA) was used to analyse the spatio-temporal distributions of the environmental parameters. Redundancy analysis (RDA) was used to examine the relationship between the dominant phytoplankton species and environmental variables. PCA and RDA were employed by using CANACO statistical software.
Results and discussion
Spatio-temporal variation of hydrographic parameters and heavy metals
In the present study, maximum rainfall was recorded during the NEM followed by SWM (Fig. 2). High rainfall (337.7 mm) was recorded during the NEM (Oct 2019). The average rainfall in the Nellore coastal region ranges from 762 to 1270 mm33, which was about 50% lesser during 2018 in the present study area. Most of the environmental parameters showed a significant spatial variability between the seasons (Table 1). The salinity was comparatively high during the SM (33.76 PSU) than the WM (27.71 PSU). However, salinity did not show a significant seasonal variation in the SRE, which could be due to the low rainfall during the study period. Further, limited freshwater discharge and presence of reservoirs/dams at upstream result in low runoff which could be attributed to high salinity observed, unlike other estuarine systems34. In the present investigation, comparatively higher salinity was recorded in the CS, whereas it was low in BC and SR2 stations. The WT ranged from 26.6 to 32.9 °C with the higher WT recorded during the SM in SR2 station and lower during the WM at CS. However, during the NEM, SRM station recorded high WT, which might be due to the closure of river mouth and lack of water exchange15. TSS ranged from 5.3 to 20.8 mg L−1, with the mean concentration being higher during the SWM and lower during the WM. TSS levels in BC and SRM were consistently high throughout the year (Table S1), attributed to continuous discharge from shrimp farms, and sediment disturbance due to runoff & mixing at the convergence zone of sea and river water, respectively14,27. The low DO at BC during all the seasons, could be due to the organic enrichment from aquaculture effluents discharge35. Dissolved inorganic nutrient levels were fluctuating among the seasons (Table 1). The NO3− concentration was higher during the SWM (11.23 µM) and lower during the SM (0.31 µM). A similar trend was observed for NO2− and NH4+. Spatially, increased NO2− and \({\text{NO}}_{{3}}^{ - }\) concentration was observed in the BC and SR2 might be due to the anthropogenic input in the region19,36. Higher NH4+ concentration was recorded in SRM during the SWM could be attributed to non-mixing of oceanic water due to closure of the SRE mouth14. During all the seasons, most of the nutrient concentration were close to optimal level in the CS, and increased gradually from downstream to upstream and BC, which could be attributed to high land runoff into the river. Estuarine-coastal ecosystems are usually characterized with increased nutrient inputs from untreated domestic sewage, industrial waste, and agricultural runoff4,7. Spatially, the SiO42–and PO43–concentrations (Table S1) were always 2–3 times higher at BC than the other stations. The mean SiO42–and PO43–concentrations were high during the SWM. Increased PO43– concentration during the SWM might be due to the usage of phosphorus compounds for the aquaculture and agriculture operations37.
Result of heavy metal levels measured in the present study is given in Table 2. The mean concentrations of heavy metals in seawater decreased in the following order in the present study area: Fe (104.6 ± 26.4 ppb) > Al (61.0 ± 12.7) > Mn (35.6 ± 14.3 ppb) > Zn (8.5 ± 1.4 ppb) > Cu (1.5 ± 0.2 ppb) > Cr (1.5 ± 0.4 ppb) > Ni (1.4 ± 0.2 ppb) > Pb (0.7 ± 0.1 ppb) > Co (0.3 ± 0.04 ppb) > Hg (BDL). Most of the trace metal concentrations were higher during the SM followed by SWM in the river upstream and BC stations. In the CS, Fe and Cr showed elevated levels during the SM, and Mn during the WM. The transportation of iron ores from Krishnapatnam port may be a potential source for the elevated levels of Fe (483.73 ppb) observed in the coastal water38. The high concentration of Cu at BC station during the WM corroborated with an earlier report and was attributed to the use of biocides in ships and boats, as well as organic inputs from agriculture and industries27,38. Ni found to be above water quality index39,40 (Table 2) at BC station during the SWM followed by the WM and NEM. Prime source of Ni might be due to wastewater effluent41.
The spatio-temporal distributions of environmental parameters were examined using principal component analysis (PCA) (Fig. 3). PCA result indicates a clear spatial variability in the study area. The first and second axis depicts 68.5% and 21.5% of the environmental variation in the SRE. The SR2 and BC stations were characterised by high WT (r2 = 0.63, p < 0.01), TSS (r2 = 0.67, p < 0.01) and nutrients (r2 = 0.58, p < 0.01). Among these environmental factors, SiO42–, TN and TP were found to be the most influenced variables with higher values in the SR2 and BC stations. Hence, SR2 and BC stations were clearly distinguished by high nutrients, TSS attributed to anthropogenic runoff and mixing of sea & river water14,35.
Principal compound analysis (PCA) for water quality parameters. Stations W-(BC, SR2, SR1, SRM, and CS) indicates the WM, A- (BC, SR2, SR1, SRM, and CS) indicates the SM, S-(BC, SR2, SR1, SRM, and CS) indicates the SWM, and N-(BC, SR2, SR1, SRM, and CS) indicates the NEM. Dotted circle indicates the upper estuarine stations.
Spatio-temporal variations of phytoplankton community
During the present study area, phytoplankton biomass (Chl-a) varied from 0.17 to 17.18 mg m−3 between the seasons. SR2 and BC recorded higher biomass during the SWM and NEM, respectively (Table 1). Throughout the year about 5 to 6 times higher biomass was recorded at BC and SR2 compare to other stations (Table S1). Sewage-borne nutrients at BC and SR2 might have enhanced the phytoplankton biomass42. Low biomass was observed in the CS during the SM and SWM. A total of 81 phytoplankton species were identified in the present study, including 53 diatoms (32 centrales and 21 pennales), 24 dinoflagellates, 1 silicoflagellate, and 3 Cyanophycae (Table S2). The phytoplankton density varied from 5697 to 30,01,835 cells L−1, the mean density was higher during the SWM followed by WM and lower during the NEM and SM. Spatio-temporal variation of abundant phytoplankton species was presented in Fig. S1. Phytoplankton density was positively correlated with PO43– (r = 0.87, p < 0.01) and negatively correlated with pH (r = − 0.51, p < 0.05). During all the seasons, phytoplankton density was recorded maximum at BC station, which alone contributed 79–90% of total phytoplankton abundance, whereas in CS recorded only 1–5%. Spatially, the total phytoplankton density was recorded high in estuarine stations like BC (87%) followed by SR2 (7%) and low in SR1 (3%), CS (2%) and SRM (1%). In BC, 80–98% of the total abundance was contributed by a single species in each season, which shows the mono phytoplankton bloom or domination. In BC, the phytoplankton community was dominated by Oscillatoria sp. (96%), Scrippsiella trochoidea (87%), Chaetoceros decipiens (98%), and Skeletonema costatum (79%) during the WM, SM, SWM, and NEM, respectively. In all the 4 seasons, CS exhibited higher number of phytoplankton taxa and lower phytoplankton density, whereas in other estuarine stations high phytoplankton density and low taxa was observed. Higher nutrient concentrations by runoff and anthropogenic activities caused some phytoplankton species to dominate and resulting in low taxa and high density19,43. Diatoms found to be the most abundant group during the SWM and NEM, contributing 99% and 94%, respectively. Cyanophyceae (84%) and Dinophyceae (73%), dominated during the WM and SM, respectively. Phytoplankton density in BC station was high throughout the year (295.4 to 3001.8 × 103 cells L−1). Diatoms showed positive correlation with PO43– (r = 0.92, p < 0.01), NO2− (r = 0.62, p < 0.01), and negative correlation with pH (r = − 0.55, p < 0.05). Whereas the dinoflagellate resulted in negative correlation with nutrients, which reflected in lower abundance during all the seasons except the SM. Phytoplankton diversity ranged from 0.14 to 2.81, the higher diversity was found during the SWM at CS and lower diversity recorded during the WM at BC station. The species evenness ranged from 0.05 to 0.80 with higher and lower evenness observed during the SWM and NEM, respectively. During the SWM, the higher (3.47) and lower (0.74) species richness was observed at CS and BC, respectively. Throughout the season, the phytoplankton taxa was recorded high in CS (43) and low in estuarine region particularly at SR2 (10) and BC (12). In the CS, optimal hydrographic conditions and lower nutrient concentration lead to higher phytoplankton taxa and lower abundance, respectively. Whereas in estuarine stations, higher nutrient concentrations resulted in dominance of some phytoplankton species and higher TSS limiting the phytoplankton taxa20,44,45.
Spatio-temporal distribution of dominant phytoplankton and HABs in relation to environmental variables
During the study period, 14 phytoplankton species (listed in Fig. 5) were found to be dominant, among these 6 species (Chaetoceros decipiens, Oscillatoria sp., Scripsiella trochoidea, Skeletonema costatum, Cylindrotheca closterium, and Thalassiosira decipiens) contributed 90–95% to the total phytoplankton abundance (Fig. 4). All these dominant phytoplankton species were observed at BC station except C. closterium, which was found at SR2. Throughout the seasons, the most abundant phytoplankton species were S. trochoidea, followed by C. closterium and T. decipiens. In BC, HABs of C. decipiens (2940 × 103 cells L−1) and Oscillatoria sp. (1619 × 103 cells L−1) occurred during the SWM (Jul 2019) and WM (Feb 2019), respectively. During the NEM, dominance of Skeletonema costatum (233.7 × 103 cells L−1) was observed at BC might be attributed to higher silicate concentration (58.74 µM). Earlier study at Gurupura estuary also noted the bloom of Skeletonema costatum during the monsoon due to hefty silicate loading (> 100 µM)46. In comparison to other stations, BC was found to be very turbid and polluted, with high levels of TSS, heavy metals (Fe, Cu, Ni, Pb, Al, and Cd), and all the nutrients owing to agricultural runoff and frequent effluent discharge from shrimp farms. Earlier study also identified BC as a high-risk zone due to the various anthropogenic wastes such as fertilizers, algaecides, fungicides, and molluscicides in the vicinity27. Lack of riverine and oceanic influx due to low rainfall and the closure of the bar mouth, respectively resulted in the azoic condition (no species) at BC station35. Moderately high NH4+ concentration was found during the bloom period, which could be due to un-grazed and decomposition of the Oscillatoria sp. bloom47,48. Usually during the bloom of Oscillatoria sp., discolouration of water occurs and some instances causes mortality of fish, due to oxygen deficiency49. However, there were no fish kills sighted during the present study.
The redundancy analysis (RDA) was used to investigate the relationship between dominant phytoplankton species and environmental factors (Fig. 5). The physico-chemical variables in the first two axis explained 93.8% of the total variance of species distribution. The species-environmental factors in the first two axis explained 98.8% of the total variances with the eigenvalues of 0.720 and 0.217. In axis 1, Chaetoceros decipiens bloom was positively correlated with elevated nutrients (PO43–, NO2−, and SiO42–) and negatively correlated with salinity. During the C. decipiens bloom, higher WT (30.87 °C), PO43– (4.37 µM), and SiO42–(50.35 µM) were recorded at BC. Earlier studies also revealed that Chaetoceros spp. bloom when the temperature, PO43–, and SiO42– increases50,51. C. decipiens correlated positively with PO43– (r = 0.93, p < 0.01). Increased PO43– and SiO42– due to aquaculture and agriculture runoff might have triggered the bloom of C. decipiens. Chl-a was negatively correlated to salinity and positively correlated with PO43–, Si, and NH4+. The majority of dinoflagellates and Cyanophyceae were positively correlated with nutrients and trace metals while being negatively correlated with salinity, WT, and TSS. In axis 2, bloom of Oscillatoria sp. was positively correlated to NO3−, Cu, Ni, and negatively correlated with salinity, where the high concentration of metals (Ni, Cu, and Cd) and low salinity were recorded at BC. Elevated Cd concentration observed in the bloom station could be attributed to the decomposition of detrital material produced by the Oscillatoria sp. bloom52. Rodriguez and Ho53 also reported that trace metal availability plays an important role in relieving the stress of Oscillatoria sp. induced by low red-light condition and promotes enhanced growth conditions. During the bloom period, higher Cu (4.24 ppb) and Ni (3.0 ppb) concentrations were observed in BC. The RDA results also revealed that Ni and Fe were positively correlated with Oscillatoria sp., which could have triggered bloom formation along with elevated nutrients at BC. Fluvial input is the main source for Ni in the estuarine region and it plays a vital role in algal growth54,55,56. Several studies examined the role of Ni and Fe in Oscillatoria growth53,57,58,59. Rodriguez and Ho59 found increased Oscillatoria growth in the presence of sufficient Ni concentration and high light, resulting in increased N2 fixation rates. Furthermore, the Cu concentration was high in BC, which is more sensitive to Oscillatoria60,61. Therefore, it can be concluded that high nutrients and presence of some heavy metals is playing a crucial role in phytoplankton productivity and bloom formation in the study region. Heavy metals are essential for phytoplankton growth at trace levels but, toxic at high levels61. Even though high Ni plays an important role in Oscillatoria sp. bloom increase in free Cu2+ concentration may be detrimental to Oscillatoria sp. growth60,61). However, more research is needed to determine the combination of certain metals in higher concentrations may have detrimental effect on the phytoplankton community and subsequent tropical level.
Redundancy analysis (RDA) for dominant phytoplankton species with associated environmental variables. The Chl-a, and phytoplankton species (Cd, Chaetoceros decipiens; Os, Oscillatoria sp.; Gd, Guinardia delicatula; Sc, Skeletonema costatum; Td, Thalassiosira decipiens; Ag, Asterionellopsis glacialis; Cc, Cylindrotheca closterium; Nd, Navicula delicatula; Ps, Pseudo-nitzschia sp.; Cf, Ceratium furca; St, Scrippsiella trochoidea; Ns, Noctiluca sp.; Pq, Peridinium quinquecorne and Pp, Protoperidinium pallidum), stations {1–5 (BC, SR2, SR1, SRM, and CS of WM), 6–10 (BC, SR2, SR1, SRM, and CS of SM), 11–15 (BC, SR2, SR1, SRM, and CS of SWM), and 16–20 (BC, SR2, SR1, SRM, and CS of NEM)}, and environmental parameters {WT, water temperature; TSS, total suspended solids; Sal, salinity; NO2, nitrite; NO3, nitrate; NH4, ammonia; PO4, phosphate; Sil, silicate; Cu, copper; Ni, nickel; Pb, lead; Fe, iron and Cr, chromium}.
Conclusion
In the present study, optimal hydrographic conditions and lower nutrient concentrations at coastal station was characterised by higher phytoplankton taxa and lower abundance, whereas in the estuarine region higher nutrient and trace metal concentrations from anthropogenic sources favoured the dominance of few phytoplankton species and lead to low phytoplankton diversity. Increased concentrations of NO3− and Ni potentially triggered the Oscillatoria sp. bloom, whereas PO43– and SiO42– induced the bloom of Chaetoceros decipiens. Only the BC station documented the observed bloom episodes with the main cause as increased nutrient and metal content from aquaculture and other anthropogenic inputs. The present study will aid in anticipating their future response to climatic change, and it emphasizes the impact of trace metals in HABs, and future bloom events could harm zooplankton followed by fish and higher trophic level. Hence, treatment system and continuous monitoring are needed for the present study region.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
05 February 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41598-024-53429-x
References
Uddin, S., Fowler, S. W., Behbehani, M. & Metian, M. 210Po bioaccumulation and trophic transfer in marine food chains in the northerns Arabian Gulf. J. Environ. Radioact. 174, 23–29 (2017).
Cloern, J., Foster, Q. & Kleckner, E. Phytoplankton primary production in the world’s estuarine-coastal ecosystems. Biogeosciences 11, 2477–2501 (2014).
Eyre, B. & Balls, P. A comparative study of nutrient behavior along the salinity gradient of tropical and temperate estuaries. Estuaries 22, 313–326 (1999).
Halpern, B. S. et al. A global map of human impact on marine ecosystems. Science 319(5865), 948–952 (2008).
Acharyya, T. et al. Reduced river discharge intensifies phytoplankton bloom in Godavari estuary. India. Mar. Chem. 132, 15–22 (2012).
Al-Shehhi, M. R., Gherboudj, I. & Ghedira, H. An overview of historical harmful algae blooms outbreaks in the Arabian Seas. Mar. Pollut. Bull 86, 314–324 (2014).
Xu, H., Paerl, H. W., Qin, B., Zhu, G. & Gaoa, G. Nitrogen and phosphorus inputs control phytoplankton growth in eutrophic Lake Taihu, China. Limnol. Oceanogr. 55, 420–432 (2010).
Anderson, D. M. Toxic algal blooms: A global perspective. In Red tides: Biology, Environmental Science and Toxicology (eds Okaichi, T. et al.) 11–16 (Elsevier, 1989).
Jyothibabu, R. et al. Trichodesmium blooms and warmcore ocean surface features in the Arabian Sea and the Bay of Bengal. Mar. Pollut. Bull. 121, 201–215 (2017).
Rombouts, I. et al. Food web indicators under the Marine Strategy Framework Directive: From complexity to simplicity?. Ecol. Indic. 29, 246–254 (2013).
Annabi-Trabelsi, N. et al. Concentrations of trace metals in phytoplankton and zooplankton in the Gulf of Gabès, Tunisia. Mar. Pollut. Bull. 168, 112392 (2021).
Demirak, A., Yilmaz, F., Tuna, A. L. & Ozdemir, N. Heavy metals in water, sediment and tissues of Leuciscuscephalus from a stream in southwestern Turkey. Chemosphere 63, 1451–1458 (2006).
Navas-Parejo, J. C. C., Corzo, A. & Papaspyrou, S. Seasonal cycles of phytoplankton biomass and primary production in a tropical temporarily open-closed estuarine lagoon—the effect of an extreme climatic event. Sci. Total Environ. 723, 138014 (2020).
Ratnam, K., Jha, D. K., Prashanthi, D. M. & Dharani, G. Evaluation of physicochemical characteristics of coastal waters of nellore, southeast coast ofIndia, by a multivariate statistical approach. Front. Mar. Sci. 9, 141. https://doi.org/10.3389/fmars.2022.857957 (2022).
Sreenivasulu, G. et al. Coastal morphodynamics of Tupilipalem Coast, Andhra Pradesh, southeast coast of India. Curr. Sci. 112(4), 823–829 (2017).
Human, L. R. D. et al. Natural nutrient enrichment and algal responses in near pristine micro-estuaries and micro-outlets. Sci. Total Environ. 624, 945–954 (2018).
Lawrie, R. A., Stretch, D. D. & Perissinotto, R. The effects of wastewater discharges on the functioning of a small temporarily open/closed estuary. Estuar. Coast. Shelf Sci. 87(2), 237–245 (2010).
Caron, D. A. & Hutchins, D. A. The effects of changing climate on microzooplankton grazing and community structure: Drivers, predictions and knowledge gaps. J. Plankton Res. 35(2), 235–252 (2013).
Kumar, P. S. et al. Influence of nutrient fluxes on phytoplankton community and harmful algal blooms along the coastal waters of southeastern Arabian Sea. Cont. Shelf Res. 161, 20–28 (2018).
Kumar, P. S. et al. Multivariate approach to evaluate the factors controlling the phytoplankton abundance and diversity along the coastal waters of Diu, northeastern Arabian Sea. Oceanologia 64(2), 267–275 (2022).
Rochelle-Newall, E. J. et al. Phytoplankton distribution and productivity in a highly turbid, tropical coastal system (Bach Dang Estuary, Vietnam). Mar. Pollut. Bull. 62(11), 2317–2329 (2011).
Carrit, D. E. & Carpenter, J. H. Recommendation procedure for Winkler analyses of sea water for dissolved oxygen. J. Plankton Res 24, 313–318 (1966).
APHA. Standard methods for the examination of water and wastewater, 22nd edition 2540 D. In Total Suspended Solids Dried at 103–105C. Washington, DC: APHA 2–56 (2012).
Grasshoff, K., Kremling, K. & Ehrhardt, M. Methods of seawater analysis -chapter 10- nutrients. Methods Seawater Anal. 1999, 159–228. https://doi.org/10.1002/9783527613984 (1999).
Cortada, U. & Collin, M. The nature and contamination of sediments in the Plentziaestuary (Biscay province, Spain). Geogaceta 54, 147–150 (2013).
USEPA. Methods for collection, storage and manipulation of sediments for chemical and toxicological analyses: Technical manual. In EPA-823-B-01-002. Office of Water, Washington, DC (2001).
Jha, D. K. et al. Evaluation of trace metals in seawater, sediments, and bivalves of Nellore, southeastcoast of India, by using multivariate and ecological tool. Mar. Pollut. Bull. 146, 1–10 (2019).
Jha, D. K. et al. Evaluation of factors influencing the trace metals in Puducherry and Diu coasts of India through multivariate techniques. Mar. Pollut. Bull. 167(6), 2021 (2021).
Parson, T. R., Maita, Y. & Lalli, C. M. A Manual of chemical and biological methods for seawater. Analysis https://doi.org/10.1016/B978-0-08-030287-4.50002-5 (1984).
Subrahmanyan, R. A systematic account of the marine plankton diatoms of the Madras coast. Proc. Acad. Sci. 24, 85–197 (1946).
Taylor, F. J. R. Dinoflagellates from the international Indian Ocean expedition. A report on material collected by R.V. Anton Bruun 1963–1964. In Plates 1–46 (1976).
Tomas, C. R. Identifying Marine Phytoplankton (Academic Press, 1997).
Sreenivasulu, G., Jayaraju, N., Sundara-Raja-Reddy, B. C., Lakshmanna, B. & LakshmiPrasad, T. Influence of coastal morphology on the distribution of heavy metals in the coastal waters of Tupilipalem coast, Southeast coast of India. Rem. Sens. Appl.: Soc. Env. 10, 190–197 (2018).
Janardanan, V. et al. Salinity response to seasonal runoff in a complex estuarine system (Cochin estuary, west coast of India). J. Coast. Res. 31(4), 869–878 (2015).
Pandey, V. et al. Assessment of ecological health of Swarnamukhi river estuary, southeast coast of India, through AMBI indices and multivariate tools. Mar. Pollut. Bull. 164, 112031 (2021).
Martin, G. D. et al. Fresh water influence on nutrient stoichiometry in a tropical estuary, southwest coast of India. Appl. Ecol. Environ. Res. 6, 57–64 (2008).
Lallu, K. R. et al. Transport of dissolved nutrients and chlorophyll a in a tropical estuary, southwest coast of India. Environ. Monit. Assess. 186, 4829–4839 (2014).
Anbuselvan, N., Senthil-Nathan, D. & Sridharan, M. Heavy metal assessment in surface sediments off Coromandel Coast of India: Implication on marine pollution. Mar. Pollut. Bull. 131, 712–726 (2018).
CONAMA. Proyecto def initivo de normas de calidad primaria para la protección de las aguas marinas. In Comisión Nacional de Medioambiente, Santiago, Chile 20 (2003).
Shanmugam, P. et al. Assessment of the levels of coastal marine pollution of Chennai city, Southern India. Water Resour. Manag. https://doi.org/10.1007/s11269-006-9075-6 (2006).
Bedsworth, W. W. & Sedlak, D. L. Sources and environmental fate of strongly complexed nickel in estuarine waters: The role of ethylene diamine tetraacetate. Environ. Sci. Technol. 33(6), 926–931 (1999).
Sarma, V. V. S. S., Krishna, M. S. & Srinivas, T. N. R. Sources of organic matter and tracing of nutrient pollution in the coastal Bay of Bengal. Mar. Pollut. Bull. 159, 111477 (2020).
Ratnam, K. et al. Seasonal variations influencing the abundance and diversity of plankton in the Swarnamukhi River Estuary, Nellore, India. J. Threatened Taxa 14(2), 20615–20624 (2022).
Sarma, V. V. S. S. et al. Intra-annual variability in nutrients in the Godavari estuary, India. Continent. Shelf Res. 30(19), 2005–2014 (2010).
Wu, N., Schmalz, B. & Fohrer, N. Distribution of phytoplankton in a German lowland river in relation to environmental factors. J. Plankton Res. 33(5), 807–820 (2011).
Krishnan, A., Das, R. & Vimexen, V. Seasonal phytoplankton succession in Netravathi-Gurupura estuary, Karnataka, India: Study on a three tier hydrographic platform. Estuar. Coast. Shelf Sci. 242, 106830 (2020).
Devassy, V. P., Bhattathiri, P. M. A. & Qasim, S. Z. Trichodesmium phenomenon. Indian J. Mar. Sci. 7, 168–186 (1979).
Steven, D. M. & Glombitza, R. Oscillatory variation of a phytoplankton population in a tropical ocean. Nature 237, 105–107 (1972).
D’Silva, M. S., Anil, A. C., Naik, R. K. & D’Costa, P. M. Algal blooms: A perspective from the coasts of India. Nat. Hazards 63(2), 1225–1253 (2012).
Kesaulya, I., Rumohoira, D. R. & Saravanakumar, A. The Abundance of Gonyaulax polygramma and Chaetoceros sp. causing blooming in Ambon Bay, Maluku, Indonesian. J. Mar. Sci. Ilmu Kelautan 27(1), 25 (2022).
Somsap, N., Gajaseni, N. & Piumsomboon, A. Physico-chemical factors influencing blooms of Chaetoceros spp. and Ceratium furca in the Inner Gulf of Thailand. Agric. Nat. Resourc. 49(2), 200–210 (2015).
Krishnan, A., Anoop, P. K., Krishnakumar, M. & Rajagopalan, F. Trichodesmium erythraeum (Ehrenberg) bloom along the southwest coast of India (Arabian Sea) and its impact on trace metal concentrations in seawater. Estuar. Coast. Shelf Sci. 71, 641–646 (2007).
Rodriguez, I. B. & Ho, T. Y. Interactive effects of spectral quality and trace metal availability on the growth of Trichodesmium and Symbiodinium. Plos One 12(11), e0188777 (2017).
Price, N. M. & Morel, F. M. M. Co-limitation of phytoplankton growth by nickel andni- trogen. Limnol. Oceanogr. 36, 1071–1077 (1991).
Cameron, V. & Vance, D. Heavy nickel isotope compositions in rivers and the oceans. Geochim. Cosmochim. Acta 128, 195–211. https://doi.org/10.1016/j.gca.2013.12.007 (2014).
Middag, R., De Baar, H. J., Bruland, K. W. & Van Heuve, S. M. The distribution of nickel in the west-Atlantic Ocean, its relationship with phosphate and a comparison to cadmium and zinc. Front. Mar. Sci. 7, 105 (2020).
Ho, T. Y. Nickel limitation of nitrogen fixation in Trichodesmium. Limnol. Oceanogr. 58(1), 112–120 (2013).
Ho, T. Y., Chu, T. H. & Hu, C. L. Interrelated influence of light and Ni on Trichodesmium growth. Front. Microbiol. 4, 139 (2013).
Rodriguez, I. B. & Ho, T. Y. Diel nitrogen fixation pattern of Trichodesmium: The interactive control of light and Ni. Sci. Rep. 4(1), 1–5 (2014).
Held, N. A. Mechanisms and heterogeneity of in situ mineral processing by the marine nitrogen fixer Trichodesmium revealed by single-colony meta proteomics. ISME Commun. 1(1), 1–9 (2021).
Mann, E. L., Ahlgren, N., Moffett, J. W. & Chisholm, S. W. Copper toxicity and cyanobacteria ecology in the Sargasso Sea. Limnol. Oceanogr. 47(4), 976–988 (2002).
Acknowledgements
The authors are grateful to the Ministry of Earth Sciences, Govt. of India, for providing financial support to carry out the present study. We are thankful to Director, NIOT, Chennai, for his constant encouragement and support. We also thank scientific and supporting staffs of NIOT and NCCR, for their support in the field and laboratory during the course of this study.
Author information
Authors and Affiliations
Contributions
P.S.K.: conceptualization, sample collection, laboratory analysis, data processing, and writing-original manuscript. G.D.: reviewing the manuscript, suggestion, and project administration, D.K.J.: data validation, and manuscript review. K.R.: data validation, and manuscript review. S.J.: data validation, and manuscript review. V.P.: field investigation. V.N.S.: field investigation. A.J.: data validation, suggestion, and manuscript review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error in the name of the author Ponnusamy Sathish Kumar which was incorrectly given as Sathish Kumar Ponnusamy.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kumar, P.S., Gopal, D., Jha, D.K. et al. Impact of anthropogenic accumulation on phytoplankton community and harmful algal bloom in temporarily open/closed estuary. Sci Rep 13, 23034 (2023). https://doi.org/10.1038/s41598-023-47779-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-47779-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.