Abstract
The present study evaluated the predictive role of baseline radiographic change and disease activity on drug retention and clinical response in patients with rheumatoid arthritis (RA) treated with tumor necrosis factor inhibitor (TNFi). Korean Observational Study Network for Arthritis (KORONA) registry was evaluated to identify RA patients treated with a TNFi. Disease activity score-28 (DAS28) was evaluated at baseline and 1 year after TNFi initiation or at termination of TNFi due to inefficacy (within 1 year). The retention rate of TNFi was compared in patients with and without bony erosions. The hazard ratio (HR) for drug retention was evaluated by Cox regression analysis, as was the odds ratio (OR) for achieving remission (DAS28 < 2.6). This study included 109 RA patients, including 97 (89%) women and 30 (27.5%) with erosions, who were treated with a TNFi. Higher baseline DAS28 was negatively associated with achievement of remission (OR = 0.56, 95% CI 0.35–0.88). The TNFi retention rate was significantly lower in RA patients with than in those without erosions (p = 0.04). Factors significantly associated with drug discontinuation included the presence of erosions (HR = 2.45, 95% CI 1.08–5.51) and higher time-averaged DAS28 (HR = 2.17, 95% CI 1.47–3.20), whereas concomitant methotrexate was associated with lack of drug discontinuation (HR = 0.40, 95% CI 0.17–0.95). The presence of erosions and high time-averaged disease activity could predict poor retention of TNFi by RA patients. Higher baseline DAS28 was associated with a reduced clinical response in patients with RA.
Trial registration Clinical Research Information Service of South Korea https://cris.nih.go.kr: KCT0000086, registered May 26, 2009.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is an autoimmune-mediated systemic arthritis, with proper management needed to improve symptoms and prevent structural damage to joints1. Initial therapy consists of treatment with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), singly or in combination, but about 40–50% of RA patients fail to respond to these csDMARDs2,3. Guidelines of the European League Against Rheumatism (EULAR), the American College of Rheumatology (ACR), and the Korean College of Rheumatology (KCR) recommend the use of biologic (bDMARDs) or targeted synthetic (tsDMARDs) drugs in patients who fail to respond to csDMARDs4,5,6. Tumor necrosis factor inhibitors (TNFis) are the most widely used class of bDMARDs, with five TNFis, etanercept, adalimumab, infliximab, golimumab, and certolizumab pegol, currently approved for the treatment of RA. TNFis have shown better clinical efficacy than csDMARDs and improved the prognosis of extra-articular manifestations of RA, such as cardiovascular disease7,8.
RA is characterized by erosion, joint space narrowing (JSN), subluxation, and juxta-articular osteopenia1. Reduction of disease activity is the primary endpoint of clinical trials in RA9. The EULAR has defined a good response as a > 1.2 point reduction of disease activity score-28 joints (DAS28), and remission as the achievement of a DAS28 < 2.610. In addition to reducing disease activity, another important treatment target in RA is the prevention of joint destruction, as joint destruction is irreversible and significantly reduces patient quality of life11. Long-term TNFi treatment in patients with early RA was found to slow radiographic structural damage12, and the achievement of remission by TNFi treatment showed beneficial effects on the radiographic progression of RA13. To date, however, the effect of baseline radiographic destruction in RA on TNFi retention rate has not been determined.
Real-world data have both advantages and disadvantages when compared with randomized clinical trials (RCTs). The most important advantage of real-world data is the inclusion of a wider variety of patients, including those with intractable disease. The heterogeneity of these populations can be an obstacle when evaluating the net effect of a specific medication on disease progression, but can also increase overall knowledge when considered along with RCTs14. In addition, most RCTs have limited follow-up duration, whereas real-world studies are observational, with generally longer total follow-up duration14. The Korean Observational Study Network for Arthritis (KORONA) registry enrolled only RA patients in Korea who were treated at university-based tertiary hospitals15,16. Data collected annually by the KORONA registry have included vast amounts of RA-related information, such as DAS28, HAQ, laboratory data, and radiographic changes of the hands and feet.
The present study aims to find predictors of TNFi discontinuation, and achievement of remission (DAS28 < 2.6).
Patients and methods
Data source
The KORONA registry, established by the Clinical Research Center for Rheumatoid Arthritis (Hanyang University Hospital), enrolled RA patients from 23 university-based tertiary hospitals from July 2009 to December 2011 (Clinical Research Information Service of South Korea approval number: KCT0000086), and annual data collection was done until February 2017. The study was designed as prospective observational cohort study. Patients were included if they fulfilled the 1987 ACR classification criteria17, were aged > 18 years, and initiated or restarted TNFi treatment within 3 months of enrollment in the KORONA registry. Patients without disease activity parameters or follow-up data were excluded, as were patients treated with bDMARDs or tsDMARDs other than TNFis, and patients who initiated or restarted TNFi treatment more than 3 months prior to KORONA registry enrollment (Fig. 1). The reporting of this study conforms to the STROBE statement18.
Flow chart for inclusion and exclusion of enrolled patients. RA rheumatoid arthritis, KORONA Korean Observational Study Network for Arthritis, bDMARD biologic disease-modifying antirheumatic disease, tsDMARD targeted synthetic disease-modifying antirheumatic drug, TNFi tumor necrosis factor inhibitor, DAS28 disease activity score-28 joints.
Data collection and outcomes
Demographic and clinical features, laboratory and clinical data, and health-related outcomes were collected at baseline and annually15,16. DAS28 was calculated based on the erythrocyte sedimentation rate (ESR), tender joint count, swollen joint count, and visual analog scale (VAS) for patient’s global assessment (PtGA)19. Smoking status at baseline was recorded as nonsmoker, ex-smoker, or current smoker. Regular exercise was recorded as a dichotomous variable (yes or no) by asking whether the patients exercise at least 3 times a week. The presence of erosions or JSN on the hands or feet was determined radiographically at baseline. Swollen and tender joints were assessed by physical examination. The VAS for physician’s global assessment (PhGA) and PtGA each ranged from 0 to 100. ESR (mm/hr), C-reactive protein (CRP) concentration (mg/dL), and positivity for rheumatoid factor (RF) and anticitrullinated peptide Ab (ACPA) were recorded at enrollment. The time-averaged DAS28 and health assessment questionnaire (HAQ) were calculated from their respective areas under the curve during total follow-up via dividing area under ROC curve of DAS28 and HAQ with total follow up days16,20,21. The area under ROC curve was calculated by using parameters (DAS28, HAQ) of each time points and duration between dates when each parameter was checked. Baseline medications were recorded, including nonsteroidal anti-inflammatory drugs (NSAIDs), daily doses of glucocorticoids, and csDMARDs (methotrexate, hydroxychloroquine, leflunomide, and sulfasalazine). Patients were followed-up until they discontinued TNFi or through the end of the KORONA registry study (until February 2017). The reason for TNFi discontinuation was also recorded.
Statistical analyses
Continuous data were assessed using the Kolmogorov–Smirnov test to determine the normality of distribution, with normally distributed data reported as the mean ± SD and non-normally distributed data as the median with interquartile range. Continuous variables were compared with Student T test or Mann Whitney U test. Categorical variables were expressed as numbers and percentages and compared by chi-square test. The Kaplan–Meier method was utilized to determine the cumulative incidence of TNFi discontinuation due to inefficacy, with incidence in RA patients with and without baseline erosions compared using log-rank tests. Logistic regression analysis was performed to find predictors of achieving clinical remission. All variables with p value under 0.05 in univariate logistic regression analysis were included in multivariate logistic regression analysis, except for variable with variance inflation factor > 10. TNFi discontinuation due to other causes than inefficacy was censored. Factors associated with the inefficacy of TNFi were evaluated by univariate Cox proportional regression analyses and presented as hazard ratios (HRs) and 95% confidence intervals (CI). Variables with p-values < 0.05 on univariate analyses, except for multicollinear variables, were included in multivariate Cox regression analysis. All statistical analyses were performed using R software (R for Windows 3.3.2; The R Foundation for Statistical Computing, Vienna, Austria), with p-values < 0.05 considered statistically significant.
Ethics approval and consent to participate
The present study conformed to the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by the institutional review boards of each participating hospital. Written informed consent was obtained from each patient prior to enrollment. The KCR provided data from the KORONA registry, and approval for analysis was obtained from the Institutional Review Board of Konkuk University Medical Center (2020-05-053).
Results
Baseline characteristics of RA patients prior to TNFi initiation
Patients who restarted a TNFi after previous treatment with a TNFi, however, were included. Median patient age was 52.0 years; 97 (89%) patients were women, and 12 (11%) were men. At baseline, bony erosions was present in 30 (27.5%) patients and JSN in 39 (35.8%). Ninety-two (84.4%) patients concomitantly used methotrexate, and 13 (11.9%) had been previously treated with a TNFi. Ninety (82.6%) patients were positive for RF, and 79 (72.5%) were positive for ACPA. Other baseline characteristics are presented in Table 1.
Achieving remission in TNFi users
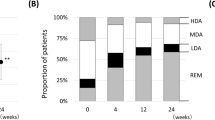
Evaluation of DAS28 at baseline and after 1 year, or at the time of TNFi discontinuation in patients who stopped treatment, showed that 31 (28.4%) patients, 26 women and five men, achieved remission (DAS28 < 2.6). Univariate logistic regression analysis showed that baseline PtGA (odds ratio [OR] = 0.98, 95% confidence interval [CI] 0.96–0.99), DAS28 (OR = 0.49, 95% CI 0.31–0.71), and HAQ (OR = 0.31, 95% CI 0.13–0.66) were significantly associated with the achievement of remission. Multivariate analysis, however, showed that baseline DAS28 (OR = 0.56, 95% CI 0.35–0.88) was the only factor independently associated with the achievement of remission (Table 2).
Reasons and predictors of TNFi discontinuation
Forty-four TNFi discontinuations occurred during 370.1 person-years (PYs) of the study. Reasons for TNFi discontinuation included inefficacy (n = 26), side effects (n = 4), improvements in RA symptoms (n = 2), patient desire (n = 1), and other reasons (n = 11). Only TNFi discontinuation events due to inefficacy were included in drug retention analysis. Kaplan–Meier analysis showed that drug retention rate was significantly lower in RA patients with than without baseline erosions (Fig. 2, p = 0.04).
Cox regression analysis was performed to assess the associations of each variable with TNFi discontinuation due to inefficacy. Univariate analysis showed that baseline erosions (HR = 2.19, 95% CI 1.00–4.77), JSN (HR = 2.17, 95% CI 1.00–4.68), PtGA (HR = 1.03, 95% CI 1.01–1.04), PhGA (HR = 1.04, 95% CI 1.02–1.06), time-averaged DAS28 (HR = 2.93, 95% CI 2.09–4.11), and time-averaged HAQ (HR = 2.79, 95% CI 1.66–4.69) were significantly associated with increased risk of TNFi discontinuation, whereas treatment with MTX was significantly associated with a reduced risk of TNFi discontinuation (HR = 0.31, 95% CI 0.14–0.69). HR of sex was not calculated because none of the male RA patients discontinued TNFi due to inefficacy. The HR for TNFi discontinuation between monoclonal antibody form of TNFi and TNF receptor-Fc portion of IgG (etanercept) was not significant (data not shown). Multivariate Cox regression analysis included all variables with significance, except for those with multicollinearity (baseline erosions and JSN). Multivariate regression analysis was performed using two models, with model 1 including the presence of erosions and model 2 including the presence of JSN. In model 1, baseline erosions (HR = 2.45, 95% CI 1.08–5.51) and higher time-averaged DAS28 (HR = 2.17, 95% CI 1.47–3.20) were significantly associated with increased risk, and concomitant MTX (HR = 0.40, 95% CI 0.17–0.95) was significantly associated with a reduced risk, of TNFi discontinuation. In model 2, only time-averaged DAS28 was significantly associated with TNFi discontinuation, whereas JSN was not significant (Table 3).
Discussion
The present study showed that RA patients with bony erosions and higher time-averaged DAS28 have higher risk for TNFi discontinuation. These findings support the importance of controlling disease activity in aspect of drug retention, and possible poor retention rate of TNFi in RA patients with baseline joint destruction.
In present study, higher time-averaged DAS28 and baseline bony erosions were found as predictors for TNFi discontinuation for the first time. Several factors have been shown to affect the rate of bDMARD continuation. For example, bDMARD subtype22,23, sex22,23,24, baseline HAQ22, disease duration25,26, and concomitant csDMARDs22 have been associated with TNFi retention rate in RA patients. Although several studies found that etanercept had a higher retention rate than adalimumab or infliximab22,23,26,27, however, the present study did not show significant difference between monoclonal antibody form of TNFi and etanercept. Concomitant use of MTX was found to reduce the risk of TNFi discontinuation24,26,27. Female sex was found to significantly increase HR for TNFi discontinuation22, with one meta-analysis showing that the pooled HR for TNFi discontinuation was increased in women23. In contrast, other studies found that sex was not significant in predicting drug retention of TNFi in RA patients24,25,26. Longer disease duration prior to TNFi initiation was found to result in a significantly lower HR for TNFi discontinuation25,26, whereas other studies showed that disease duration was not associated with TNFi retention23,24. Higher baseline HAQ score, an indicator of poor health-related function, was reported to be associated with an increased risk of TNFi discontinuation22, whereas baseline DAS28 was not24,26. One study showed that DAS28 score at 6 months after TNFi initiation could predict long term drug response28. However, none of the studies included time-averaged DAS28 and HAQ in their analysis of TNFi retention rate in patients with RA. In the present study, disease duration and sex were not significantly associated with TNFi discontinuation, and time-averaged HAQ was significant only in univariate Cox regression analysis. A study from Spanish demonstrated that RA patients with multiple bDMARDs non-response were positively associated with baseline bony erosions29. Although previous studies analyzed factors associated with TNFi discontinuation in RA, those studies did not analyze the impact of radiographic joint destruction or time-averaged parameters on TNFi retention. In the present study, erosions in the hands or feet and higher time-averaged DAS28 score were associated with increased risk of TNFi discontinuation due to inefficacy. Moreover, consistent with earlier findings24,26,27, the present study found that the concomitant use of MTX lowered the risk of TNFi discontinuation in RA patients.
The present study found that higher baseline DAS28 score was associated with a significantly reduced likelihood of achieving remission, other factors, such as sex, age, BMI, disease duration, ESR/CRP, and HAQ, were not significantly associated with clinical response. An evaluation of clinical response of RA patients to etanercept treatment found that age < 49 years, body mass index (BMI) > 28.5 kg/m2, longer disease duration, and lower ESR/PhGA/Td joint count/HAQ were associated with achievement of remission at week 2430. Moreover, remission at week 128 was significantly associated with younger age, higher mental health scale score on the SF-36, higher swollen joint counts, and lower pain VAS30. Male gender has been associated with the likelihood of achieving good EULAR response31. Moreover, the percentage of patients achieving good EULAR response 12 months after TNFi initiation was lower in patients with high (baseline DAS28 > 5.1) than moderate (baseline DAS28 3.2–5.1) disease activity31. Nonresponse to TNFi has been observed more frequently in obese than in nonobese patients32, and the probability of remission was found to be lower in patients with higher than lower ESR33. Additional studies including a larger sample size and longer duration are needed to determine the baseline characteristics associated with clinical response to TNFis in RA patients.
The present study had several limitations. First, the results were drawn from observational real-world data (KORONA registry), which have intrinsic limitations when compared with RCTs. Second, about 90% of enrolled patients were female. Sex is an important factor in predicting drug retention and achieving clinical response in RA, but sex did not show significant difference on drug retention nor achieving clinical remission in the present study, primarily because the number of males in this study was small, as were the events being analyzed (i.e., TNFi discontinuation due to inefficacy or remission). Moreover, most of the enrolled RA patients were nonsmokers, limiting the ability to determine the effects of smoking status on drug retention and clinical response. Third, the KORONA registry was started in 2009, but most of the TNFis were later approved for use in Korea, making the number of TNFi initiators relatively small. Fourth, other bDMARDs or tsDMARDs were not included in analysis because, in 2009, these agents were approved for use only in RA patients who failed one or more TNFis, thus limiting the numbers of patients in the KORONA registry who received other bDMRADs or tsDMARDs. Fifth, radiographic evidence of damage to the hands or feet was not assessed semiquantitatively, as the KORONA registry did not record modified total sharp scores34, the most widely used method of semiquantitatively evaluating joint destruction in patients with RA. Finally, although the present study only included RA patients who initiated or restarted TNFi within 3 months prior to enrollment in the KORONA registry, 41 (37.6%) of the 109 patients had started taking a TNFi prior to enrollment in the KORONA registry. Because these patients may have achieved good EULAR responses before baseline data were collected in the KORONA registry, good EULAR response was not analyzed in the present study. However, the present study also has some strengths: the first study (1) which used time-averaged parameters as a predictor on TNFi retention, and (2) revealed association between baseline bony erosions and TNFi retention.
Conclusions
In conclusion, higher baseline DAS28 reduced the likelihood of achieving remission in RA patients treated with TNFis. Erosions on the hands or feet at baseline was associated with a lower TNFi retention rate, increasing the likelihood of drug discontinuation due to inefficacy. Higher time-averaged DAS28 was also associated with a significantly increased risk of TNFi discontinuation due to inefficacy, whereas concomitant use of MTX reduced the risk of TNFi discontinuation. Radiographic evidence of joint destruction and higher DAS28 may have negative effects on drug retention and clinical response to TNFis, whereas treatment with MTX may reduce the risk of TNFi discontinuation due to inefficacy in RA patients.
Data availability
All data generated during this study are available from the corresponding author on reasonable request.
Abbreviations
- RA:
-
Rheumatoid arthritis
- DMARD:
-
Disease-modifying antirheumatic drug
- EULAR:
-
The European League Against Rheumatism
- ACR:
-
American College of Rheumatology
- KCR:
-
Korean College of Rheumatology
- TNFi:
-
Tumor necrosis factor inhibitor
- JSN:
-
Joint space narrowing
- DAS28:
-
Disease activity score-28 joints
- RCT:
-
Randomized clinical trial
- KORONA:
-
Korean Observational Study Network for Arthritis
- ESR:
-
Erythrocyte sedimentation rate
- VAS:
-
Visual analog scale
- PtGA:
-
Patient’s global assessment
- PhGA:
-
Physician’s global assessment
- CRP:
-
C-reactive protein
- RF:
-
Rheumatoid factor
- ACPA:
-
Anticitrullinated peptide Ab
- HAQ:
-
Health assessment questionnaire
- HRs:
-
Hazard ratios
- CI:
-
Confidence interval
- OR:
-
Odds ratio
References
Smolen, J. S. et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers. 4, 18001. https://doi.org/10.1038/nrdp.2018.1 (2018).
Aramaki, T. et al. Clinical predictors of inadequate response to conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) including methotrexate (MTX) in untreated rheumatoid arthritis patients: A single-center observational study. Mod. Rheumatol. 30, 50–57. https://doi.org/10.1080/14397595.2018.1553265 (2020).
Sergeant, J. C. et al. Prediction of primary non-response to methotrexate therapy using demographic, clinical and psychosocial variables: Results from the UK Rheumatoid Arthritis Medication Study (RAMS). Arthritis Res. Ther. 20, 147. https://doi.org/10.1186/s13075-018-1645-5 (2018).
Eun-Jung, P. et al. The use of biological disease-modifying antirheumatic drugs for inflammatory arthritis in Korea: Results of a Korean Expert Consensus. J. Rheum. Dis. 27, 4–21. https://doi.org/10.4078/jrd.2020.27.1.4 (2020).
Fraenkel, L. et al. 2021 American College of Rheumatology Guideline for the treatment of rheumatoid arthritis. Arthritis Rheumat. (Hoboken, N.J.). 73, 1108–1123. https://doi.org/10.1002/art.41752 (2021).
Smolen, J. S. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 79, 685–699. https://doi.org/10.1136/annrheumdis-2019-216655 (2020).
Ozen, G., Pedro, S. & Michaud, K. The risk of cardiovascular events associated with disease-modifying antirheumatic drugs in rheumatoid arthritis. J. Rheumatol. 48, 648–655. https://doi.org/10.3899/jrheum.200265 (2021).
Greenberg, J. D. et al. Tumour necrosis factor antagonist use and associated risk reduction of cardiovascular events among patients with rheumatoid arthritis. Ann. Rheum. Dis. 70, 576–582. https://doi.org/10.1136/ard.2010.129916 (2011).
Smolen, J. S. et al. Clinical trials of new drugs for the treatment of rheumatoid arthritis: Focus on early disease. Ann. Rheum. Dis. 75, 1268–1271. https://doi.org/10.1136/annrheumdis-2016-209429 (2016).
van Gestel, A. M. et al. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheumatism. 39, 34–40. https://doi.org/10.1002/art.1780390105 (1996).
Rupp, I., Boshuizen, H. C., Dinant, H. J., Jacobi, C. E. & van den Bos, G. A. Disability and health-related quality of life among patients with rheumatoid arthritis: Association with radiographic joint damage, disease activity, pain, and depressive symptoms. Scand. J. Rheumatol. 35, 175–181. https://doi.org/10.1080/03009740500343260 (2006).
Keystone, E. C. et al. Longterm effect of delaying combination therapy with tumor necrosis factor inhibitor in patients with aggressive early rheumatoid arthritis: 10-year efficacy and safety of adalimumab from the randomized controlled PREMIER trial with open-label extension. J. Rheumatol. 41, 5–14. https://doi.org/10.3899/jrheum.130543 (2014).
Smolen, J. S. et al. Pooled analysis of TNF inhibitor biosimilar studies comparing radiographic progression by disease activity states in rheumatoid arthritis. RMD Open https://doi.org/10.1136/rmdopen-2019-001096 (2020).
Nazha, B., Yang, J. C. & Owonikoko, T. K. Benefits and limitations of real-world evidence: Lessons from EGFR mutation-positive non-small-cell lung cancer. Future Oncol. (Lond. Engl.) 17, 965–977. https://doi.org/10.2217/fon-2020-0951 (2021).
Sung, Y. K. et al. Korean Observational Study Network for Arthritis (KORONA): Establishment of a prospective multicenter cohort for rheumatoid arthritis in South Korea. Semin. Arthritis Rheum. 41, 745–751. https://doi.org/10.1016/j.semarthrit.2011.09.007 (2012).
Min, H. K. et al. Time-averaged DAS28 and HAQ predict cardiovascular disease in patients with rheumatoid arthritis: Data from KORONA registry. Joint Bone Spine https://doi.org/10.1016/j.jbspin.2022.105401 (2022).
Arnett, F. C. et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 31, 315–324. https://doi.org/10.1002/art.1780310302 (1988).
von Elm, E. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 147, 573–577. https://doi.org/10.7326/0003-4819-147-8-200710160-00010 (2007).
van der Heijde, D. M. et al. Judging disease activity in clinical practice in rheumatoid arthritis: First step in the development of a disease activity score. Ann. Rheum. Dis. 49, 916–920. https://doi.org/10.1136/ard.49.11.916 (1990).
Arts, E. E., Fransen, J., den Broeder, A. A., Popa, C. D. & van Riel, P. L. The effect of disease duration and disease activity on the risk of cardiovascular disease in rheumatoid arthritis patients. Ann. Rheum. Dis. 74, 998–1003. https://doi.org/10.1136/annrheumdis-2013-204531 (2015).
Kamarudin, A. N., Cox, T. & Kolamunnage-Dona, R. Time-dependent ROC curve analysis in medical research: Current methods and applications. BMC Med. Res. Methodol. 17, 53. https://doi.org/10.1186/s12874-017-0332-6 (2017).
Neovius, M. et al. Drug survival on TNF inhibitors in patients with rheumatoid arthritis comparison of adalimumab, etanercept and infliximab. Ann. Rheum. Dis. 74, 354–360. https://doi.org/10.1136/annrheumdis-2013-204128 (2015).
Souto, A., Maneiro, J. R. & Gómez-Reino, J. J. Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: A systematic review and meta-analysis of drug registries and health care databases. Rheumatology (Oxford) 55, 523–534. https://doi.org/10.1093/rheumatology/kev374 (2016).
Lin, C. T. et al. Predictors of drug survival for biologic and targeted synthetic DMARDs in rheumatoid arthritis: Analysis from the TRA Clinical Electronic Registry. PLoS ONE 16, e0250877. https://doi.org/10.1371/journal.pone.0250877 (2021).
Cho, S. K. et al. Drug retention and safety of TNF inhibitors in elderly patients with rheumatoid arthritis. BMC Musculoskelet. Disord. 17, 333. https://doi.org/10.1186/s12891-016-1185-6 (2016).
Iannone, F. et al. Longterm retention of tumor necrosis factor-α inhibitor therapy in a large italian cohort of patients with rheumatoid arthritis from the GISEA registry: An appraisal of predictors. J. Rheumatol. 39, 1179–1184. https://doi.org/10.3899/jrheum.111125 (2012).
Favalli, E. G. et al. Twelve-year retention rate of first-line tumor necrosis factor inhibitors in rheumatoid arthritis: Real-life data from a local registry. Arthritis Care Res. 68, 432–439. https://doi.org/10.1002/acr.22788 (2016).
Hamann, P. D. H., Pauling, J. D., McHugh, N., Hyrich, K. & Shaddick, G. Early response to anti-TNF predicts long-term outcomes including sustained remission: An analysis of the BSRBR-RA. Rheumatology (Oxford) 59, 1709–1714. https://doi.org/10.1093/rheumatology/kez518 (2020).
Novella-Navarro, M. et al. Clinical predictors of multiple failure to biological therapy in patients with rheumatoid arthritis. Arthritis Res. Ther. 22, 284. https://doi.org/10.1186/s13075-020-02354-1 (2020).
de la Vega, M. et al. Predictors of response to etanercept-methotrexate treatment: a post hoc logistic regression analysis of a randomized, open-label study in Latin American patients with rheumatoid arthritis. Adv. Rheumatol. (Lond. Engl). 61, 56. https://doi.org/10.1186/s42358-021-00213-4 (2021).
Atzeni, F. et al. Predictors of response to anti-TNF therapy in RA patients with moderate or high DAS28 scores. Joint Bone Spine 81, 37–40. https://doi.org/10.1016/j.jbspin.2013.04.005 (2014).
Law-Wan, J. et al. Predictors of response to TNF inhibitors in rheumatoid arthritis: an individual patient data pooled analysis of randomised controlled trials. RMD Open https://doi.org/10.1136/rmdopen-2021-001882 (2021).
Atzeni, F. et al. Predicting response to anti-TNF treatment in rheumatoid arthritis patients. Autoimmun. Rev. 8, 431–437. https://doi.org/10.1016/j.autrev.2009.01.005 (2009).
Ory, P. A. Interpreting radiographic data in rheumatoid arthritis. Ann. Rheum. Dis. 62, 597–604. https://doi.org/10.1136/ard.62.7.597 (2003).
Acknowledgements
The authors thank all Korean Observational Study Network for Arthritis (KORONA) investigators for their invaluable contributions.
Funding
This research was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology, Republic of Korea (NRF-2021R1A2C1010075, Recipient: H.R. Kim).
Author information
Authors and Affiliations
Contributions
H.K.M. performed study design, data collection, analysis, and manuscript writing & revision. H.R.K. supervised the study, and gained the fund. S.H.L. and S.H.K. aid on data analysis. All authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Min, H.K., Kim, S.H., Lee, SH. et al. Baseline bony erosions and time-averaged DAS28 predict discontinuation of TNF inhibitors in rheumatoid arthritis. Sci Rep 12, 19951 (2022). https://doi.org/10.1038/s41598-022-24027-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-24027-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.