Abstract
The oncological impact of positive surgical margins (PSM) after robot-assisted partial nephrectomy (RAPN) is still under debate. We compared PSM and Negative Surgical Margins (NSM) in terms of recurrence-free survival (RFS), metastasis-free survival (MFS) and overall survival (OS) after RAPN, and we identified predictive factors of PSM. Multi-institutional study using the UroCCR database, which prospectively included 2166 RAPN between April 2010 and February 2021 (CNIL DR 2013-206; NCT03293563). Two groups were retrospectively compared: PSM versus NSM. Prognostic factors were assessed using Kaplan–Meyer curves with log-Rank test, cox hazard proportional risk model and logistic regression after univariate comparison. 136 patients had PSM (6.3%) and 2030 (93.7%) had NSM. During a median follow-up of 19 (9–36) months after RAPN, 160 (7.4%) recurrences were reported. Kaplan–Meier curves and analysis suggested that RFS, MFS and OS were not affected by a PSM (p = 0.68; 0.71; 0.88, respectively). In multivariate analysis predictors of PSM were a lower RENAL score (p = 0.001), longer warm ischemia time (WIT) (p = 0.003) and Chromophobe Renal Cell Carcinoma (chrRCC) (p = 0.043). This study found no impact of PSM on RFS, MFS or OS, and predictors of PSM were the RENAL score, WIT and chrRCC.
Similar content being viewed by others
Introduction
Surgically resected tumors show a lower TNM stage than was the case 20 years ago, thanks to routine cross-section imaging1,2. Partial nephrectomy (PN), whenever possible, is the reference treatment since 2010 to date3,4. The proliferation of robotic surgical platforms has resulted in an exponential increase in the number of patients undergoing robotic‐assisted partial nephrectomy (RAPN)5.
The aim of this nephron-sparing surgery (NSS) is an optimal oncological control while preserving overall renal function, without any post-operative complication. However, its main pitfall remains the possibility of positive surgical margins, occurring in 0.1–10.7% of cases6.
European guidelines recommend intensified follow-up after PSM encounter but there is no consensus for a particular strategy and the association between PSM and recurrence is still under debate3,7. The majority of studies reported so far have indicated that positive surgical margins do not correlate with a higher risk of metastases or decreased cancer specific survival (CSS)8,9. On the other hand, large retrospective studies showed that PSM, are an independent predictor of recurrence10,11. Absence of PSM, such as in Trifecta12 achievement, has also shown a predictive role on long term outcomes after RAPN13.
The objective of this study was to compare the oncological outcomes of patients undergoing RAPN for RCC according to the surgical margin status, and to identify factors associated with PSM.
Patients and methods
Study design
After institutional review board approval, the MARGINS study was conducted in the framework of the UroCCR project (NCT03293563). All patients were given oral and written information about the objectives and methodology of the UroCCR project, and data of those who provided written and informed consent were prospectively included in the UroCCR database. All experimental protocols were approved by the ethics committee of Commission Nationale de l'Informatique et des Libertés (CNIL authorization number DR-2013-206). We reviewed the medical records of all patients in this database, between April 2010 and February 2021 in 20 French centers.
Given the retrospective multicenter study design, the surgical techniques and preoperative work-up were not standardized across centers, but European Guidelines3 were followed. The following preoperative data were collected for each patient: age at RAPN, sex, BMI, ECOG and ASA score, Tumor characteristics, including the clinical TNM stage, clinical tumor size, tumor side and radiological RENAL nephrometry score. All patients had pre-operative CT scan including abdominal and pelvic sequences for evaluation of the tumor, most of the time with thoracic sequences for assessment of extension.
The following surgical data were recorded: indication of NSS (elective, imperative, relative), surgical approach (transperitoneal (TP), retroperitoneal (RP)), overall operative time, type of clamping, warm ischemia time (WIT), estimated blood loss, peri-operative complications and conversion to open surgery. The surgical approach was considered discordant when the tumor was anterior with an RP approach and inversely for posterior tumors with a TP approach.
Pathology characteristics included histologic subtype, pTNM, diameter and grade and UISS prognostic category. Post-operative complications were recorded according to current guidelines14 and graded according to the Clavien–Dindo classification15.
Follow-up protocols were similar across centers. It involved a clinical interview, a physical examination, serum creatinine, a CT scan to assess local recurrence and metastasis progression. Median follow-up was calculated as the median time between surgery and the last consultation.
Outcomes of interest
The primary endpoint was PSM, which was defined as the presence of cancer cells at the margin of the surgical specimen reported for H&E stained tissues.
Statistical analysis
Means and standard deviations were reported for continuous variables, and proportions for nominal variables. The exact Fisher test was used to compare nominal variables, the Kruskal–Wallis test for ordinal variables and the Student test to compare quantitative continuous variables. Non-parametric tests were used for small sample sizes. The probabilities of OS, RFS and MFS were estimated using the Kaplan–Meier method. Multivariable Cox regression analyses were used to seek predictors of OS, RFS and MFS. Only variables with a p-value < 0.25 in univariate analysis were included in the multivariate model. Collinearities were included in the initial model, and the final model was built with factors restricted to those reported in the existing literature (larger size or RENAL score, pT2, grade III-IV, overall operative time, blood loss). R statistical software was used for the statistical analyses16. All tests were two-sided with a significance level at p < 0.05. All methods were performed in accordance with the relevant guidelines and regulations.
Research involving human participants, their data or biological material and informed consent
All patients were given oral and written information about the objectives and methodology of the UroCCR project, and data of those who provided written consent were prospectively included in the UroCCR database (CNIL authorization number DR-2013-206; NCT03293563). The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Results
Patients’ characteristics
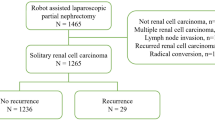
We reviewed the medical records of the 12,936 patients of the UroCCR network, including hereditary syndromes if known at baseline, and excluding patients with previous history of RCC. First, we excluded those who underwent open partial or radical nephrectomy (OPN n = 1327, ORN n = 1345), then those who had laparoscopic partial or radical nephrectomy (LPN n = 4915, LRN n = 2427). Of the 2922 patients who underwent RAPN over the study period, 2166 met the inclusion criteria. Exclusion criteria were cT3b-4, cN + or cM + (n = 332), conversion to OPN (n = 41), conversion to radical nephrectomy (n = 90), benign (n = 140) or multiple (n = 173) tumors. (Fig. 1).
Flowchart. OPN open partial nephrectomy, LPN laparoscopic partial nephrectomy, OTN open total nephrectomy, LTN laparoscopic total nephrectomy, TN total nephrectomy, RAPN robot-assisted partial nephrectomy, cTNM M + , metastasis at diagnosis, PSM Positive surgical margin, NSM negative surgical margin.
Of the 2166 patients included, 136 patients had PSM (6.3%) and 2030 (93.7%) had NSM. Patients’ characteristics are summarized in Table 1. The mean age at surgery (PSM: 60.2 vs. NSM: 59.9, p = 0.094), ASA (p = 0.4), cTNM (p = 0.987) were similar in both groups. The RENAL Score was lower in the PSM group (PSM: 6 vs. NSM: 7, p = 0.05). The overall median follow-up was 199,36 months; 160 (7.4%) recurrences (88 local and 72 metastases) were reported: 9 (6.6%) in the PSM group and 151 (7.4%) in the NSM group.
Perioperative outcomes
PSM was associated with a longer mean WIT (PSM: 20.4 ± 10.3 min vs. NSM: 17.8 ± 8.5 min, p = 0.001). Complications during surgery (PSM: 6.7% vs. NSM: 3.9%, p = 0.168), retroperitoneal approach (n = 168) (PSM: 10.9% vs. NSM: 7.8%, p = 0.279), discordant approaches (PSM: 41.2% vs. NSM: 42.8%, p = 0.845) and on-clamp procedure (PSM: 91% vs. NSM: 86.8%, p = 0.198) were not associated with PSM. Intraoperative data are shown in Table 2.
Pathology findings
chrRCC (PSM: 19.9% vs. NSM: 8.5%, p < 0.001) was significantly associated with PSM, whereas the ccRCC histology type (PSM: 56.6% vs. NSM: 70.3%, p = 0.001) was associated with a lower likelihood of PSM.
Results are shown in Table 3.
The median follow-up was 21.5 mo [9–37] for PSM: versus 19 mo [9–36] for NSM (p = 0.662). Survival analysis did not show any difference between the groups for RFS (Fig. 2a), MFS or OS (Fig. 2b,c) (respectively p = 0.68; 0.71; 0.88).
Multivariate analysis (Fig. 3) showed that a lower RENAL score (p = 0.001), a longer warm ischemia time (WIT) (p = 0.003) and chrRCC histology (p = 0.043) were predictors of PSM.
Discussion
Early-stage diagnosis in RCC and the diagnosis of small renal masses have led to an evolution in surgical approaches. Since 2010 to date, for cT1-T2 renal mass, PN and then RAPN rather than OPN, when feasible, is the recommended surgical approach3,4,17. Therefore, it had no impact on clinical practices over the years for the authors. In order to achieve its nephron-sparing potential, RAPN has to meet three main challenges: WIT < 25 min to prevent renal failure, oncological control, currently evaluated as NSM, and no perioperative complications.
Although a PSM could be regarded as cancer cells remaining in the kidney, hemostasis may induce ischemia and necrosis in these cells. In addition, tumor cells in the surgical specimen may be in tangential contact with the margin, corresponding to a PSM, but with no cancer cells remaining in the resection bed. As for NSM, the analysis of frozen sections during tumor resection led to 5% of false-negative NSM18, and an NSM is no guarantee that a recurrence will not occur7. Given the above, the prognostic value of PSM after RAPN is still a matter of debate.
We found 6.3% of PSM, which is in line with the 0.1–10.7% reported in the literature6, but local recurrence occurred in only 5.9% (N = 8) of our PSM patients. Our median follow-up was 19 months, which is equivalent to the median time to recurrence after PN (19 mo) according to Takagi et al.19. In the literature, there is no consensus on the presence or absence of a statistical correlation between surgical margins and recurrence rates or specific survival. On one hand, Petros et al. showed a close association between PSM and disease progression after PN20 in a large single-center cohort of 2297 PN with 1863 (81%) RCC and 34 (1.48%) PSM. The 34 PSM patients were matched 1:3 with 100 NSM patients for tumor size, RENAL score, grade, and pathologic stage. They found that PSM had an impact on 5-year survival probability for OS (0.99 for NSM vs. 0.97 for PSM), local RFS (0.98 vs. 0.77), and MFS (0.95 vs. 0.84). Moreover, in another analysis, Wood et al.21 demonstrated a strong association between PSM at the time of PN and local tumor bed recurrence after a median time of 23mo: 15.9% of PSM in the recurrence group versus 3% in the control group. Khalifeh et al.22 evaluated the oncological outcomes of 21 PSM among 947 RAPN for malignant tumors with 13 months of median follow-up. Following the 947 procedures, there were nine recurrences and four cases of metastases. PSM were strongly linked to recurrence, with an HR of 18.4 after adjustment. The 3-year recurrence-free rate and metastasis-free survival rate were lower in the PSM group than in the NSM group (respectively 47% vs. 98.3% and 63% vs. 99.5%). In a retrospective study of 1240 PN for RCC with 97 PSM leading to 67 recurrences, Shah et al.23 demonstrated that PSM was an independent risk factor for recurrence. In their subgroup analysis, PSM was found to be a risk factor for recurrence in high-risk tumors (pT2-3a or grades III–IV) but not in low-risk tumors (pT1 or grades I–II). Moreover, Bernhard et al.24 evaluated the predictive factors for ipsilateral recurrence in 809 NSS procedures with 27 months of follow-up. They reported 26 local recurrences and 15.4% of PSM. Multivariate analysis showed a link between PSM and local recurrence with an HR of 11.5 (p < 0.01).
In contrast, Bensallah et al.8 conducted a matched-pair study that included 101 PSM and 102 NSM matched for surgical indication, tumor size, and tumor grade. PSM had no influence on 5-year RFS, 5-year cancer specific survival (CSS) or 5-year OS. Yossepowitch et al.25 studied 77 PSM in 1390 PN with a median follow-up of 40.8 months and found that PSM were not associated with worse RFS or MFS. In addition, Rothberg et al.26 compared 797 NSM with 42 PSM after RAPN and reported that oncological outcomes in PSM patients were no worse than those in NSM patients after a median follow-up of 18.8 months. The outcomes in our study are in line with those of the Rothberg study in that we found no statistical association between PSM and RFS, MFS or OS.
Multivariable analysis showed that higher RENAL scores were associated with NSM. This paradoxical finding could be because smaller masses within the renal parenchyma may be harder to find, and the surgeon might feel overconfident during such a PN procedure, which in theory should be technically easier given the small size of the tumor. This hypothesis is supported by Schiavana et al.27, who founded that smaller tumors cT1a versus cT2, laparoscopic technique rather than open and volume center < 60 RAPN/year are risk factors of PSM. Another hypothesis is that easier procedures may have been left to young surgeons, since most of the centers in the study are academic.
As for more complex tumors, surgeons performing RAPN may cut more deeply into healthy parenchyma to reduce the risk of PSM. Whatever the reason, this result is robust even in multivariable analysis, after the inclusion of tumor size, overall operative time, blood loss and histology.
Like Takagi et al.28, we found that the RP approach (n = 168) was not a risk factor for PSM. They specifically compared RP with TP, though in a smaller sample size (48 RP vs. 290 TP) and found no significant difference between the two groups for PSM. Arora et al.29 also compared 99 RP-RAPN with 394 TP-RAPN and found no difference between the arms for margin status. Both of these studies suffered from the same limitations: retrospective design with a small sample size, and the absence of precise data about the surgeon’s experience and surgical technique for the tumorectomy.
As expected, a longer WIT is also a risk factor for PSM. WIT is a proxy for surgical experience and falls steeply in the early part of the learning curve, as described by Larcher et al.30. A longer WIT also reflects the occurrence of surgical difficulties during the procedure.
The fibrous stromal tumoral reaction and the presence, size and density of the tumor fibrous capsule vary according to histological sub-type. The reduced nature of these elements in chrRCCs may explain the higher frequency of PSMs in this sub-type31.
Finally, we set out to determine the impact of margins on oncological outcomes in this large, prospective, multicenter cohort of patients. To this end, further analyses are ongoing and will be published in due course.
Our study has several limitations that should be acknowledged. First, we deliberately excluded advanced tumors (cT3b-4, cN + , cM +), benign or multiple tumors and metastatic disease in order to establish a homogenous model of RAPN. Secondly, the follow-up was relatively short. Other series had a longer follow-up, especially that of Yossepowitch et al. (40.8mo)25. However, according to part of the literature, the median time to recurrence after RAPN ranges from 13.1 to 20 months19,26,32,33 and median time between partial nephrectomy and detection of local bed tumor recurrence was 23 months21. Experience is also an important factor to include. The number of RAPN already performed at the time of the first procedure recorded in UroCCR was not available for all surgeons, neither was the involvement of trainees. It was therefore impossible to assess the impact of experience on PSM. However, Larcher et al.30 highlighted that although surgeon experience was associated with a shorter warm ischemia time (WIT) and a lower probability of Clavien–Dindo complications (CD) ≥ 2, but it was not associated with a lower rate of PSM. We believe this cohort shows routine practice in university centers, where juniors are operating patients under seniors’ supervision so that EXP is a shallow concept. Finally, like numerous studies about RAPN, we lacked data concerning the length of the margin, the tumorectomy technique and the presence of adherent perinephric fat (APF), but these two last factors do not seem to be predictive of PSM in the literature34,35,36. We also lacked data for the treatment of the recurrence, and this treatment may have generated a selection bias by changing the results for MFS and OS. However, Brassier et al.37 showed that 40% of patients treated for local recurrence after PN by percutaneous ablation or surgical resection experienced disease recurrence within a median follow-up of 23 mo, which also means that only 60% of patients presented controlled disease after retreatment.
Conclusions
Based on this large, French multicenter, retrospective series, PSM rates remained low after RAPN and had no impact on short-term oncological prognosis. PSM was associated with a longer WIT, chrRCC and surprisingly with a lower RENAL score.
References
Ferlay, J. et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 49(6), 1374–1403 (2013).
Kato, M. et al. Natural history of small renal cell carcinoma: Evaluation of growth rate, histological grade, cell proliferation and apoptosis. J. Urol. 172(3), 863–866 (2004).
Ljungberg, B. et al. European association of urology guidelines on renal cell carcinoma: The 2019 update. Eur. Urol. 75(5), 799–810 (2019).
Ljungberg, B. et al. EAU guidelines on renal cell carcinoma: The 2010 update. Eur. Urol. 58(3), 398–406 (2010).
Patel, H. D. et al. Trends in renal surgery: Robotic technology is associated with increased use of partial nephrectomy. J. Urol. 189(4), 1229–1235 (2013).
Laganosky, D. D., Filson, C. P. & Master, V. A. Surgical margins in nephron-sparing surgery for renal cell carcinoma. Curr. Urol. Rep. 18(1), 8 (2017).
Antic, T. & Taxy, J. B. Partial nephrectomy for renal tumors: Lack of correlation between margin status and local recurrence. Am. J. Clin. Pathol. 143(5), 645–651 (2015).
Bensalah, K. et al. Positive surgical margin appears to have negligible impact on survival of renal cell carcinomas treated by nephron-sparing surgery. Eur. Urol. 57(3), 466–473 (2010).
López-Costea, M. Á., Bonet, X., Pérez-Reggeti, J., Etcheverry, B. & Vigués, F. Oncological outcomes and prognostic factors after nephron-sparing surgery in renal cell carcinoma. Int. Urol. Nephrol. 48(5), 681–686 (2016).
Tellini, R. et al. Positive surgical margins predict progression-free survival after nephron-sparing surgery for renal cell carcinoma: Results from a single center cohort of 459 cases with a minimum follow-up of 5 years. Clin. Genitourin. Cancer 17(1), e26-31 (2019).
Carvalho, J. A. M. et al. Impact of positive surgical margins after partial nephrectomy. Eur. Urol. Open Sci. 21, 41–46 (2020).
Brassetti, A., Anceschi, U., Bertolo, R., Ferriero, M., Tuderti, G. & Capitanio, U. et al. Surgical quality, cancer control and functional preservation: introducing a novel trifecta for robot-assisted partial nephrectomy. Minerva Urol. Nefrol [Internet]. [cité 9 oct 2022]; 72(1), (2020) Disponible sur: https://www.minervamedica.it/index2.php?show=R19Y2020N01A0082
Brassetti, A., Anceschi, U., Bertolo, R., Ferriero, M., Tuderti, G. & Costantini, M. et al. Comprehensive long-term assessment of outcomes following robot-assisted partial nephrectomy for renal cell carcinoma: the ROMe’s achievement and its predicting nomogram. Minerva Urol. Nefrol. [Internet]. [cité 9 oct 2022]; 72(4), (2020) Disponible sur: https://www.minervamedica.it/index2.php?show=R19Y2020N04A0482
Mitropoulos, D. et al. Validation of the Clavien–Dindo grading system in urology by the European association of urology guidelines Ad hoc panel. Eur. Urol. Focus 4(4), 608–613 (2018).
Dindo, D., Demartines, N. & Clavien, P. A. Classification of surgical complications. Ann. Surg. 240(2), 205–213 (2004).
R Development Core Team. R Software (R Foundation for Statistical Computing, 2008).
Peyronnet, B. et al. Comparison of 1800 robotic and open partial nephrectomies for renal tumors. Ann. Surg. Oncol. 23(13), 4277–83 (2016).
Gordetsky, J. et al. Frozen section during partial nephrectomy: Does it predict positive margins?. BJU Int. 116(6), 868–872 (2015).
Takagi, T. et al. Predictive factors for recurrence after partial nephrectomy for clinical T1 renal cell carcinoma: A retrospective study of 1227 cases from a single institution. Int. J. Clin. Oncol. 25(5), 892–898 (2020).
Petros, F. G. et al. Oncologic outcomes of patients with positive surgical margin after partial nephrectomy: A 25-year single institution experience. World J. Urol. 36(7), 1093–1101 (2018).
Wood, E. L. et al. Local tumor bed recurrence following partial nephrectomy in patients with small renal masses. J. Urol. 199(2), 393–400 (2018).
Khalifeh, A. et al. Positive surgical margins in robot-assisted partial nephrectomy: A multi-institutional analysis of oncologic outcomes (leave no tumor behind). J. Urol. 190(5), 1674–1679 (2013).
Shah, P. H. et al. Positive surgical margins increase risk of recurrence after partial nephrectomy for high risk renal tumors. J. Urol. 196(2), 327–334 (2016).
Bernhard, J. C. et al. Predictive factors for ipsilateral recurrence after nephron-sparing surgery in renal cell carcinoma. Eur. Urol. 57(6), 1080–1086 (2010).
Yossepowitch, O. et al. Predictors and oncological outcomes following positive surgical margins at partial nephrectomy. J. Urol. 179(6), 2158–2163 (2008).
Rothberg, M. B. et al. A multi-institutional analysis of the effect of positive surgical margins following robot-assisted partial nephrectomy on oncologic outcomes. J. Endourol. 34(3), 304–311 (2020).
Schiavina, R. et al. Predicting positive surgical margins in partial nephrectomy: A prospective multicentre observational study (the RECORd 2 project). Eur. J. Surg. Oncol. 46(7), 1353–1359 (2020).
Takagi, T. et al. Comparisons of surgical outcomes between transperitoneal and retroperitoneal approaches in robot-assisted laparoscopic partial nephrectomy for lateral renal tumors: A propensity score-matched comparative analysis. J. Robot. Surg. 15(1), 99–104 (2021).
Arora, S. et al. Retroperitoneal versus transperitoneal robot-assisted partial nephrectomy: Comparison in a multi-institutional setting. Urology 120, 131–137 (2018).
Larcher, A. et al. The learning curve for robot-assisted partial nephrectomy: Impact of surgical experience on perioperative outcomes. Eur. Urol. 75(2), 253–256 (2019).
Marko, J., Craig, R., Nguyen, A., Udager, A. M. & Wolfman, D. J. Chromophobe renal cell carcinoma with radiologic-pathologic correlation. Radiographics 41(5), 1408–1419 (2021).
Shah, P. H. et al. Partial nephrectomy is associated with higher risk of relapse compared with radical nephrectomy for clinical stage T1 renal cell carcinoma pathologically up staged to T3a. J. Urol. 198(2), 289–296 (2017).
Mouracade, P. et al. Patterns and predictors of recurrence after partial nephrectomy for kidney tumors. J. Urol. 197(6), 1403–1409 (2017).
Minervini, A., Campi, R., Sessa, F., Derweesh, I., Kaouk, J. H. & Mari, A. et al. Positive surgical margins and local recurrence after simple enucleation and standard partial nephrectomy for malignant renal tumors: systematic review of the literature and meta-analysis of prevalence. Minerva Urol. Nephrol. [Internet]. [cité 9 oct 2022];69 (6), (2017) Disponible sur: https://www.minervamedica.it/index2.php?show=R19Y2017N06A0523
Minoda, R., Takagi, T., Yoshida, K., Kondo, T. & Tanabe, K. Comparison of surgical outcomes between enucleation and standard resection in robot-assisted partial nephrectomy for completely endophytic renal tumors through a 1:1 propensity score–matched analysis. J. Endourol. [Internet] https://doi.org/10.1089/end.2021.0213 (2021).
Khene, Z. E. et al. Adherent perinephric fat affects perioperative outcomes after partial nephrectomy: A systematic review and meta-analysis. Int. J. Clin. Oncol. 26(4), 636–646 (2021).
Brassier, M. et al. Percutaneous ablation versus surgical resection for local recurrence following partial nephrectomy for renal cell cancer: A propensity score analysis (REPART study: UroCCR 71). Eur. Urol. Focus 79, 765 (2021).
Author information
Authors and Affiliations
Contributions
A.M.: Project development, Data collection and management, data analysis, manuscript writing I.B.: Project development, data analysis, manuscript editing J.C.B.: Data collection and management K.B.: Data collection and management C.C.: Data collection and management F.B.: Data collection and management N.D.: Data collection and management O.J.: Data collection and management F.A.: Data collection and management B.P.: Data collection and management M.B.: Data collection and management J.A.L.: Data collection and management F.X.N.: Data collection and management N.B.: Data collection and management H.L.: Data collection and management T.C.: Data collection and management E.X.: Data collection and management T.W.: Data collection and management F.G.: Data collection and management R.B.: Data collection and management B.R.: Data collection and management A.S.: Data collection and management D.C.: Project development D.A.: Manuscript editing M.D.: Project development, Data collection and management, Manuscript editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Morrone, A., Bentellis, I., Bernhard, JC. et al. Positive surgical margin’s impact on short-term oncological prognosis after robot-assisted partial nephrectomy (MARGINS study: UroCCR no 96). Sci Rep 12, 18342 (2022). https://doi.org/10.1038/s41598-022-23146-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23146-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.