Abstract
To evaluate the recurrence rate and risk factors of recurrence after robot-assisted laparoscopic partial nephrectomy for solitary renal cell carcinoma (RCC). A total of 1265 cases of initial solitary localized RCC were analyzed. The baseline characteristics, complexity (REANL nephrometry score), intra- and peri-operative outcomes, and recurrence were evaluated. Logistic regression was performed to evaluate the factors affecting recurrence after RAPN for solitary localized RCC. Recurrence after robot-assisted partial nephrectomy (RAPN) occurred in 29 patients (2.29%). The median follow-up was 36.0 months. The N domain (nearness to collecting system/sinus) (odd ratio (OR) 3.517, 95% confidence interval (CI) 1.557–7.945, p = 0.002), operation time (OR 1.005, 95% CI 1.001–1.010, p = 0.013), and perioperative transfusion (OR 5.450, 95% CI 1.197–24.816, p = 0.028) affected recurrence. Distant metastasis among patients with recurrence was significantly associated with nearness to the collecting system/sinus (OR 2.982, 95% CI 1.162–7.656, p = 0.023) and distance between the mass and collecting system/sinus (OR 0.758, 95% CI 0.594–0.967, p = 0.026). Nearness to the collecting system/sinus, operation time, and perioperative transfusion affect recurrence after RAPN for solitary localized RCC. Moreover, the proximity to the collecting system/sinus and distance between the mass and collecting system/sinus were significantly related to distant metastasis after RAPN.
Similar content being viewed by others
Introduction
With the recent increase in health evaluations using ultrasound or computed tomography (CT), renal cell carcinoma (RCC) is mostly diagnosed at an early stage1. Partial nephrectomy (PN) has become the standard treatment for localized RCC over radical nephrectomy due to the equivalent oncological outcomes and advantages of nephron sparing2. The decision whether to perform radical nephrectomy or PN as a treatment for localized RCC is determined by the experience of the operator, T stage, and renal mass complexity3. In the past, as technical hurdles existed for PN, resulting in a high incidence of adverse sequelae such as chronic renal disease, radical nephrectomy was overused4,5. However, due to recent advances in surgeon proficiency and robotic surgery, PN is performed not only for small RCCs less than 2 cm but also for large RCCs of T1b/T26. Even in the case of large RCCs, PN showed similar oncological outcomes and better functional preservation than radical nephrectomy; therefore, PN should be recommended7. Moreover, robot-assisted partial nephrectomy (RAPN) has now become the first choice of treatment for RCC below T2 due to the popularization of robotic surgery.
In RCC, there are only some reports on local recurrence or distant metastasis rates after PN, with recurrence rates varying from 1 to 40%8. Moreover, the focus has been on the feasibility and oncological outcomes of PN compared with radical nephrectomy9,10. However, the recurrence rate and risk factors after RAPN as a treatment for solitary RCC have not been reported. Recently, a systematic review by Henderickx et al. reported that a positive surgical margin (PSM) in pT1 RCC could increase the risk of recurrence after partial nephrectomy11. However, the risk of bias of the analyzed previous studies was high, and the high heterogeneity of this study made it difficult to evaluate as an optimistic systematic review.
In the present study, we assessed the recurrence rate and risk factors after RAPN as a treatment for solitary RCC through a single-surgeon large-scale observational study.
Results
Demographics
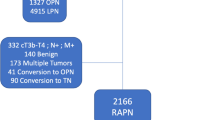
Among the 1465 patients who underwent RAPN, 1265 met the inclusion/exclusion criteria, and recurrence occurred in 29 (2.29%) of them (Fig. 1). The median follow-up period of the 1265 patients was 36.0 months. The mean ages of patients in the no recurrence and recurrence groups were 52.87 ± 12.06 and 52.10 ± 12.32, respectively (p = 0.742). Hypertension was present in 33.25% (411/1236) and 51.72% (15/29) of the no recurrence and recurrence group, respectively (p = 0.037). A Family history of RCC was present in 3.24% (24/1236) of the no recurrence group, and no patients in the recurrence group had a familial history (p = 0.005). There was no significant difference in renal function between the two groups. Regarding clinical stage, 34.48% (10/29) of the recurrence group were T1b, 13.51% (167/1236) were T1b, and 0.49% (6/1236) were T2a in the no recurrence group (p = 0.005). According to the nephrometry score, 45.95% (568/1236) were at intermediate risk, 8.50% (105/1236) were at high risk in the recurrence group, 55.17% (16/29) were at intermediate risk, and 24.14% (7/29) were at high risk in the no-recurrence group (p = 0.003) (Table 1).
The operation times were 261.14 ± 61.66 and 195.15 ± 87.03 min in the recurrence and no recurrence groups, respectively (p < 0.001). Perioperative transfusion was performed in 10.34% (3/29) and 1.46% (18/1236) of the recurrence and no recurrence groups, respectively (p < 0.001). As for the pathologic stage, 20.69% (6/29) were T1b and 6.90% (2/29) were T3a in the recurrence group. In the no recurrence group, 15.05% (186/1236) were T1b, 7.28% (9/1236) were T2a, and 1.46% (18/1236) were T3a (p = 0.005). In terms of Fuhrmann grade, 60.03% (742/1236) of the patients in the no recurrence group and 41.38% (12/29) of the patients in the recurrence group were low-grade (p = 0.035) (Table 2).
Recurrence after RAPN
On multivariate analysis, N domain (nearness to collecting system/sinus) (OR 3.517, 95% CI 1.557–7.945, p = 0.002), operation time (OR 1.005, 95% CI 1.001–1.010, p = 0.013), and perioperative transfusion (OR 5.450, 95% CI 1.197–24.816, p = 0.028) were significantly related to recurrence (Table 3). According to N domain classification, the mean recurrence free survival period was 155.04 ± 1.22 months (≥ 7 mm), 135.74 ± 1.98 months (> 4 mm but < 7 mm), and 129.90 ± 4.14 months (≤ 4 mm) (p < 0.001). The hazard ratio of recurrence is 2.833 (95% CI 0.708–11.344, p = 0.141) in > 4 mm but < 7 mm group and 8.584 (95% CI 2.545–28.959, p = 0.001) (Fig. 2).
Among the 29 patients with recurrence, 34.48% (10/29) had local recurrence in the ipsilateral kidney and 65.52% (19/29) had distant metastasis. In addition, metastasis to multiple sites was observed in 13.79% (4/29) of the patients (Fig. 3). Distant metastasis among patients with recurrence was significantly associated with nearness to the collecting system/sinus (OR 2.982, 95% CI 1.162–7.656, p = 0.023) and distance between the mass and the collecting system/sinus (OR 0.758, 95% CI 0.594–0.967, p = 0.026) (Table 4) (Supplementary 3).
Discussion
Nearness to the collecting system/sinus, operation time, and perioperative transfusion affect recurrence after RAPN. In particular, proximity to the collecting system/sinus and the distance between the mass and collecting system/sinus are significantly related to distant metastasis.
As a treatment for clinically localized small renal masses, PN showed similar surgery-related mortality, cancer-specific survival, and time-to-recurrence with superiority in decreased time-to-death from any cause compared to radical nephrectomy12. Moreover, compared to radical nephrectomy, PN as a treatment for large renal masses (T1b or T2) also has equivalent cancer control and better preservation of renal function with the potential for better long-term survival13.
However, there are few studies on the risk factors for recurrence after PN for RCC. Recently, through a systematic review, Henderickx et al. reported that a PSM was a risk factor for local recurrence; the percentage of cases with a PSM ranged from 0 to 34.4% and local recurrence varied from 0 to 9.1%11. In the present study, PSM was defined as the presence of malignant cells at the surgical margin in the pathologic report, and only six RAPN patients (0.41%) were evaluated as having a PSM. All six patients had no recurrence during follow-up. Khalifey et al. reported that no factor, including tumor size, pathological stage, tumor grade, multiple tumors, or surgeon learning curve, could predict a PSM after partial nephrectomy14. However, in RAPN, the surgeon factor cannot be avoided in PSM. Although the association between recurrence and PSM could not be clearly confirmed due to the very low incidence of PSMs in this study, reducing PSMs is needed for better oncological outcomes.
Tumor incision is a known risk factor for tumor recurrence and metastasis15. However, in our results, there was no significant relationship between capsular incision and tumor recurrence. Moreover, a retrospective study by Ito et al. reported that capsular incision during PN was not associated with poor oncological outcomes16. However, in both results, the number of capsular incisions was too small to draw definitive conclusions. Yoshino et al. reported that RCC cells remained on the surface of the scissors after capsular incision, and elimination of the tumor cell using monopolar electrical treatment is needed17. In addition, Li et al. suggested that capsular incision would affect tumor recurrence and recommended that scissors be treated with povidone-iodine after capsular incision18. Although there is no definite conclusion regarding the effect of capsular incision on recurrence, if capsular incision occurs, it is necessary to secure a safe surgical margin through additional resection and treatment of the scissor surface. In this study, the incidence of capsular incision and PSM was low, and appropriate evaluations could not be assessed. However, avoiding obvious risk factors such as tumor violation or margin status could improve oncological outcomes.
In our results, the N domain (nearness to collecting system/sinus) in the RENAL nephrometry score was significantly related to recurrence. Maxwell et al. also reported that the N domain had a significant effect on recurrence after thermal ablation (hazard ratio 3.15, 95% confidence intervals 1.31–7.62, p < 0.0001)19. Unlike the present study, they reported that the R domain (the diameter of the mass) was associated with recurrence. This may be due to the difference in the treatment characteristics of ablation and surgical resection. Additionally, propensity score matching was performed for familial history, hypertension, and clinical T stage to further assess the risk of N domain recurrence. After matching, there was no statistical difference in the baseline characteristics (Supplementary 1), and through logistic regression, the N domain was the only factor that significantly affected recurrence (Supplementary 2).
In the present study, operation time also affected recurrence. This may be due to the longer tumor manipulation time than the surgical time itself. Wan et al. reported that high plasma cell-free DNA levels were associated with a significantly higher recurrence rate in clear cell RCC20. Although it has not been reported that tumor manipulation increases circulating tumor cells during PN, it is known that surgical management causes dissemination of circulating tumor cells21. Even in the case of RCC, if the tumor manipulation time is prolonged, the level of circulating tumor cells may increase, which could affect recurrence. Moreover, nearness to the collecting system/sinus and the distance between the mass and collecting system/sinus were associated with distant metastasis in our results. It is possible that the level of circulating tumor cells during surgery might increase more when the sinus and tumor are closer. Further evaluation using a prospective study is required.
Abu-Ghanem et al. reported that perioperative blood transfusion was associated with reduced recurrence-free, cancer-specific, and overall survival in patients undergoing nephrectomy for RCC22. Our results also showed that perioperative blood transfusion was associated with tumor recurrence after RAPN. The mechanism of the adverse oncological effects in transfusion may be related to the suppressive effects on the immune system23,24. Moreover, transient immune impairment, which comes from transfusion, may enhance a condition favorable to cancer cells25. However, there is still controversy regarding transfusion and oncological outcomes in RCC.
This was a retrospective study, and its limitation was the relatively short follow-up period. Moreover, whether the recurrence in ipsilateral RCC was an incidental lesion or a metastatic lesion was not clearly determined. However, a pathological review was performed in all 10 cases, and the histology was confirmed to be the same as that of previous RCC on biopsy. The bias may have been reduced with the data of a single expert surgeon, which can reduce the surgeon factor. In addition, this study is significant in that it is the first large-scale study to evaluate risk factors for recurrence after RAPN as a treatment for solitary RCC.
Methods
Patients
A total of 1465 patients who underwent RAPN between 2008 and 2022 were retrospectively analyzed. RAPN was performed by a single expert surgeon. RAPN was performed when it was determined that nephron-sparing surgery was possible for a localized renal mass of T1 or T2 stage. We excluded patients with multiple renal masses, non-RCC, lymph node invasion, and recurrent masses from this study.
Among these patients, 1265 cases of initial solitary localized RCC were selected based on pathological reports (Fig. 1). To evaluate the recurrence risk factors after RAPN as a treatment for solitary RCC, the recurrence and no recurrence groups were compared.
Clinicopathologic assessment
The baseline characteristics of the patients, including age at RAPN, underlying disease, familial history, routine laboratory test, and diethylenetriamine pentaacetic acid renal scan were evaluated. Abdominal computed tomography (CT) with/without magnetic resonance imaging (MRI) was performed on the patient, and complexity was evaluated using the RENAL nephrometry score26. Estimated blood loss, warm ischemic time, capsular incision, and intraoperative complications, including transfusion, were evaluated. In addition, the length of hospital stay and perioperative complications were evaluated. The histopathology was evaluated by experienced uropathologists.
Follow up
After RAPN, the patients were generally followed up every 3–6 months in the first year. Thereafter, follow-up was performed at intervals of 6–12 months. Patients underwent abdominal CT or MRI, chest radiography, and routine laboratory tests at each visit. Recurrence-free survival was defined as the interval between the date of surgery and the time of the first tumor recurrence. The cause of death was determined by the physicians responsible and death certificates.
Surgical approach
The operation was performed using a four-arm da Vinci Robotic System (Intuitive Surgical, Seoul, Korea), using five ports. Based on the location of the renal mass, a retroperitoneal or transperitoneal approach was performed according to the surgeon's decision. The operation was performed through main artery clamping without selective ischemia, and PN was performed under warm ischemia in all patients. In most cases, tumor excision uses a modified tumor enucleation method with a safety margin of less than 1 cm through blunt dissection and incision using monopolar scissors. A capsular incision was defined as a case where tumor violation occurred due to an unintentional incision in the mass during tumor excision. When a capsular incision was made, the safety margin was secured by additional resection. For the tumor bed, continuous running suture was performed through barbed suture, and renorrhaphy was also performed using barbed suture. Warm ischemia was maintained from mass excision to the completion of renorrhaphy.
Statistical analysis
The baseline characteristics were compared between the patients with and without recurrence after RAPN using the chi-square test for categorical variables and the independent t-test for continuous variables. Kaplan–Meier survival analysis was used to calculate the estimates for recurrence-free survival. Logistic regression analysis was performed to evaluate the factors affecting recurrence after RAPN for solitary localized RCC.
Ethics statement
This study was conducted in accordance with the Declaration of Helsinki and current ethical guidelines. The Institutional Review Board of Samsung Medical Center approved the current study (approval number: 2023-03-053). All methods were conducted in accordance with relevant guidelines and regulations. The requirement for written informed patient consent was waived by the Institutional Review Board of the Samsung Medical Center due to the retrospective nature of the study. Personal identifiers were completely deleted to ensure that the data were analyzed anonymously.
Conclusions
Nearness to the collecting system/sinus, operation time, and perioperative transfusion affect recurrence after RAPN for solitary localized RCC. Moreover, the proximity to the collecting system/sinus and distance between the mass and collecting system/sinus were significantly related to distant metastasis after RAPN.
Data availability
The raw data for this study are available upon reasonable request from the corresponding author.
References
Kume, H. et al. Distant metastasis of renal cell carcinoma with a diameter of 3 cm or less-which is aggressive cancer?. J. Urol. 184, 64–68. https://doi.org/10.1016/j.juro.2010.03.019 (2010).
Ristau, B. T. et al. Partial nephrectomy is not associated with an overall survival advantage over radical nephrectomy in elderly patients with stage Ib-II renal masses: An analysis of the national cancer data base. Cancer 124, 3839–3848. https://doi.org/10.1002/cncr.31582 (2018).
Joshi, S. S. & Uzzo, R. G. Renal tumor anatomic complexity: Clinical implications for urologists. Urol. Clin. N. Am. 44, 179–187. https://doi.org/10.1016/j.ucl.2016.12.004 (2017).
Miller, D. C., Hollingsworth, J. M., Hafez, K. S., Daignault, S. & Hollenbeck, B. K. Partial nephrectomy for small renal masses: An emerging quality of care concern?. J. Urol. 175, 853–857. https://doi.org/10.1016/s0022-5347(05)00422-2 (2006) (discussion 858).
Huang, W. C. et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: A retrospective cohort study. Lancet Oncol. 7, 735–740. https://doi.org/10.1016/s1470-2045(06)70803-8 (2006).
Sharafeldeen, M. et al. Partial nephrectomy for T1b/T2 renal mass: An added shift from radical nephrectomy. J. Kidney Cancer VHL 9, 1–5. https://doi.org/10.15586/jkcvhl.v9i4.255 (2022).
Klett, D. E. et al. Partial versus radical nephrectomy in clinical T2 renal masses. Int. J. Urol. 28, 1149–1154. https://doi.org/10.1111/iju.14664 (2021).
Speed, J. M., Trinh, Q. D., Choueiri, T. K. & Sun, M. Recurrence in localized renal cell carcinoma: A systematic review of contemporary data. Curr. Urol. Rep. 18, 15. https://doi.org/10.1007/s11934-017-0661-3 (2017).
Huang, R., Zhang, C., Wang, X. & Hu, H. Partial nephrectomy versus radical nephrectomy for clinical T2 or higher stage renal tumors: A systematic review and meta-analysis. Front. Oncol. 11, 680842. https://doi.org/10.3389/fonc.2021.680842 (2021).
Tang, A. B. et al. Perioperative and long-term outcomes of robot-assisted partial nephrectomy: A systematic review. Am. Surg. 87, 21–29. https://doi.org/10.1177/0003134820948912 (2021).
Henderickx, M. et al. Surgical margins after partial nephrectomy as prognostic factor for the risk of local recurrence in pT1 RCC: A systematic review and narrative synthesis. World J. Urol. 40, 2169–2179. https://doi.org/10.1007/s00345-022-04016-0 (2022).
Kunath, F. et al. Partial nephrectomy versus radical nephrectomy for clinical localised renal masses. Cochrane Database Syst. Rev. 5, Cd012045. https://doi.org/10.1002/14651858.CD012045.pub2 (2017).
Mir, M. C. et al. Partial nephrectomy versus radical nephrectomy for clinical T1b and T2 renal tumors: A systematic review and meta-analysis of comparative studies. Eur. Urol. 71, 606–617. https://doi.org/10.1016/j.eururo.2016.08.060 (2017).
Khalifeh, A. et al. Positive surgical margins in robot-assisted partial nephrectomy: A multi-institutional analysis of oncologic outcomes (leave no tumor behind). J. Urol. 190, 1674–1679. https://doi.org/10.1016/j.juro.2013.05.110 (2013).
Curet, M. J. Port site metastases. Am. J. Surg. 187, 705–712. https://doi.org/10.1016/j.amjsurg.2003.10.015 (2004).
Ito, H. et al. Impact of accidental tumor incision during laparoscopic partial nephrectomy on the oncologic and clinical outcomes. Clin. Genitourin. Cancer 14, e291-297. https://doi.org/10.1016/j.clgc.2015.11.013 (2016).
Yoshino, H. et al. Is it safe to use the same scissors after accidental tumor incision during partial nephrectomy? Results of in vitro and in vivo experiments. J. Endourol. 31, 391–395. https://doi.org/10.1089/end.2016.0848 (2017).
Li, G., Zhi, C., Zhu, D., Liu, Z. & Niu, Y. Efficacy of povidone-iodine against accidental tumor incision during nephron-sparing surgery: Experimental study in patients with renal cell carcinoma. J. Int. Med. Res. 47, 4993–5002. https://doi.org/10.1177/0300060519874155 (2019).
Maxwell, A. W. P., Baird, G. L., Iannuccilli, J. D., Mayo-Smith, W. W. & Dupuy, D. E. Renal cell carcinoma: Comparison of RENAL nephrometry and PADUA scores with maximum tumor diameter for prediction of local recurrence after thermal ablation. Radiology 283, 590–597. https://doi.org/10.1148/radiol.2016161225 (2017).
Wan, J., Zhu, L., Jiang, Z. & Cheng, K. Monitoring of plasma cell-free DNA in predicting postoperative recurrence of clear cell renal cell carcinoma. Urologia internationalis 91, 273–278. https://doi.org/10.1159/000351409 (2013).
Cheng, X., Zhang, H., Hamad, A., Huang, H. & Tsung, A. Surgery-mediated tumor-promoting effects on the immune microenvironment. Semin. Cancer Biol. 86, 408–419. https://doi.org/10.1016/j.semcancer.2022.01.006 (2022).
Abu-Ghanem, Y., Zilberman, D. E., Dotan, Z., Kaver, I. & Ramon, J. Perioperative blood transfusion adversely affects prognosis after nephrectomy for renal cell carcinoma. Urol. Oncol. 36(12), e15-12.e20. https://doi.org/10.1016/j.urolonc.2017.09.006 (2018).
Linder, B. J. et al. The impact of perioperative blood transfusion on cancer recurrence and survival following radical cystectomy. Eur. Urol. 63, 839–845. https://doi.org/10.1016/j.eururo.2013.01.004 (2013).
Cata, J. P., Wang, H., Gottumukkala, V., Reuben, J. & Sessler, D. I. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br. J. Anaesth. 110, 690–701. https://doi.org/10.1093/bja/aet068 (2013).
Abel, E. J. et al. Perioperative blood transfusion and radical cystectomy: Does timing of transfusion affect bladder cancer mortality?. Eur. Urol. 66, 1139–1147. https://doi.org/10.1016/j.eururo.2014.08.051 (2014).
Kutikov, A. & Uzzo, R. G. The R.E.N.A.L. nephrometry score: A comprehensive standardized system for quantitating renal tumor size, location and depth. J. Urol. 182, 844–853. https://doi.org/10.1016/j.juro.2009.05.035 (2009).
Funding
This study was supported by a research grant from the National Research Foundation of Korea, funded by the Ministry of Science and ICT (2022R1A2C1005496).
Author information
Authors and Affiliations
Contributions
J.H.C.: Protocol/project development, Data collection, Data analysis, manuscript writing. W.S., M.K., H.H.S., and H.G.J.: Data collection, Literature research. B.C.J., S.S.J., and H.M.L.: Interpretation, Critical revision. S.I.S.: Protocol/project development, Data collection, supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chung, J.H., Song, W., Kang, M. et al. Risk factors of recurrence after robot-assisted laparoscopic partial nephrectomy for solitary localized renal cell carcinoma. Sci Rep 14, 4481 (2024). https://doi.org/10.1038/s41598-023-51070-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-51070-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.