Abstract
Androgen receptor splice variant-7 (AR-V7) expression in circulating tumor cells (CTCs) in metastatic castration-resistant prostate cancer (mCRPC) is associated with abiraterone and enzalutamide resistance. We determine whether cabazitaxel (CBZ) is equally effective in AR-V7-positive and -negative CRPC and whether AR-V7-positive patients retain CBZ sensitivity. This is the first prospective, open-label, Asian validation study of CBZ in Japanese patients with mCRPC after docetaxel (n = 48; four CBZ cycles; 2017–2020, Juntendo University Hospitals). Primary endpoint was prostate-specific antigen response rate (PSA-RR); secondary endpoints included overall survival (OS), bone scan index (BSI) PSA-RR (≥ 50% decline from baseline) for CTC−/ARV7−, CTC+ /ARV7−, and CTC +/ARV7+ groups. PSA-RR ≥ − 30% was 38% (18/48) and ≥ − 50% was 26% (12/48). BSI-change rate ≥ − 30% was 19% (9/41) and ≥ − 50% was 17% (8/41). Median OS was 13.7(12.2–18.9) months. PSA decline in early CBZ treatment associated with OS (p = 0.00173). BSI decline associated with OS (p = 0.0194). PSA-RR(≥ 50%) was 43%(6/14) in CTC−/ARV7−, 19%(5/26) in CTC+ ARV7−, and 12%(1/8) in CTC+/ARV7+ ( p > 0.05). AR-V7 in CTCs at baseline not associated with OS. AR-V7 was not associated with CBZ resistance in CTCs. Reductions in BSI and PSA in early stages of CBZ treatment may predict OS.
Similar content being viewed by others
Introduction
Androgen receptor (AR) signaling is an important survival pathway for castration-resistant prostate cancer (CRPC) cells. In most CRPC cells, the AR maintains its function as a nuclear receptor in a ligand-independent manner, allowing it to evade androgen deprivation therapy1. Approximately 20–40% of the patients with CRPC have primary resistance to new anti-androgen agents such as enzalutamide and abiraterone2,3. Moreover, virtually all patients who initially respond to these agents develop secondary resistance over time. One plausible explanation for this resistance could be the presence of AR splice variants4,5. Although truncated AR in these splice variants is incapable of binding ligand, it is constitutively active as a transcription factor. One of the variants by which the AR can become ligand independent is the androgen receptor splice variant-7 (AR-V7). AR-V7 lacks a ligand-binding domain and remains functionally active even in the absence of androgen, which can lead to a poor prognosis6,7. AR-V7 expression in circulating tumor cells (CTCs) from patients with CRPC has recently been linked to resistance to abiraterone and enzalutamide4,5.
Theoretically, taxane-based chemotherapy should be effective against CRPC with constitutively active AR because its anti-tumor effect is exerted through a different mechanism, as a tubulin-stabilizing agent. Antonarakis et al. reported that the detection of AR-V7 in CTCs from men with metastatic CRPC (mCRPC) was not associated with primary resistance to taxane chemotherapy8. In addition, Onstenk et al. reported that cabazitaxel (CBZ) response may be independent of the AR-V7 status of CTCs from patients with mCRPC, and that CBZ could be a viable treatment option for patients with AR-V7-positive CTCs9. However, there are limited data on the efficacy of taxane-based chemotherapy for AR-V7-positive CRPC.
This prospective, real-world study aims to investigate if CBZ is equally effective in AR-V7-positive and AR-V7-negative CRPC, and if AR-V7-positive patients retain CBZ sensitivity. This first-of-its-kind Asian validation study is an open-label, single-arm, single-center (Juntendo University hospital) cohort study to assess the efficacy of CBZ in Japanese patients with mCRPC with or without AR-V7-positive CTCs who have demonstrated disease progression on docetaxel treatment.
Results
A total of 55 patients with mCRPC were enrolled and received CBZ, with the majority of patients (n = 48) receiving more than four cycles of CBZ. Table 1 shows patient characteristics at baseline; the median (range) age was 71 (51–82) years, and the median time to CRPC diagnosis was 10 (3–78) months. At the time of diagnosis, 83% (40/48) of the patients had locally advanced prostate cancer, and 79% (38/48) of the patients had high-grade prostate cancer (Gleason score was ≥ 8). As local treatment, 13% (6/48) of the patients had surgery (radical prostatectomy or robotic laparoscopic prostatectomy), and 27% (13/48) of the patients had radiation therapy (intensity modulated radiation therapy [IMRT] for primary cancer or irradiation to bone metastasis). Prior hormonal or chemotherapy treatment lines for CRPC prior to CBZ treatment initiation included: 19% of the patients (n = 9) received one line of treatment, 39% (n = 17) received two lines of treatment, 26% (n = 13) received three lines of treatment, and 19% (n = 9) received ≥ 4 lines of treatment.
Initial treatments for mCRPC included: enzalutamide, abiraterone, radium-223 chloride, flutamide, ethinylestradiol, estramustine phosphate, and docetaxel. Prior to CBZ treatment, all patients had received docetaxel; the median (range) was 6 (1–39). Prior to enrollment, 17 patients (35%) had received abiraterone, 30 patients (62%) had received enzalutamide, and 10 patients (21%) had received both enzalutamide and abiraterone (Table 2).
The median observation period for all 48 patients treated with CBZ was 13.7 (7.3–34.6) months. The median OS for all patients was 13.7 months (95% CI, 12.2–18.9) (Fig. 1). There was no significant difference in the number of treatment lines before CBZ treatment and the OS rate (HR, 0.98 [95% CI, 0.41–2.34]; p = 0.19) and no significant difference in time to CRPC and the OS (HR, 0.84 [95% CI, 0.37–1.93]; p = 0.85).
Kaplan–Meier curve of overall survival (OS) rate of all patients with CRPC after four or more cycles of cabazitaxel treatment. Survival rate at median observation period [95% CI]; 0.686 [0.534–0.798]. Median overall survival (days) [95% CI]; 418 [372–575], N = 48. CBZ, cabazitaxel; CI, confidence interval; CRPC, castration-resistant prostate cancer; OS, overall survival.
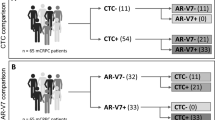
At baseline, CTCs were detected in 34 of 48 blood samples (70.8%), and eight of these 34 samples (16%) had detectable AR-V7-positive cells (Table 3). Seven of the eight (88%) AR-V7 positive patients had previously received either enzalutamide or abiraterone, and two of these seven (29%) AR-V7-positive patients had previously received both enzalutamide and abiraterone.
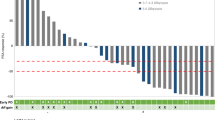
After a minimum of four cycles of CBZ treatment, the best PSA reduction rate was ≥ 30% in 18 patients (38%) and ≥ 50% in 12 patients (25%) (Fig. 2). There was no significant difference in PSA best change rate between the CTC-negative/AR-V7-positive, CTC-positive/AR-V7-negative, and CTC-positive/AR-V7-positive groups. PSA-RR (≥ 50%) was observed in 43% (6/14) of the patients in the CTC-negative/AR-V7-negative group, 29% (5/26) of patients in the CTC-positive/AR-V7-negative group, and 13% (1/8) of the patients in the CTC-positive/AR-V7-positive group. There was no significant difference between the AR-V7-positive and AR-V7-negative groups (p = 0.662) (Fig. 2).
Waterfall plot of the best PSA change rate after four or more cycles of cabazitaxel. Group A, CTC (−) / AR-V7 (−); Group B, CTC ( +) / AR-V7 (−); Group C, CTC ( +) / AR-V7 ( +). A vs. B, p = 0.318; B vs. C, p = 0.391; A vs. C, p = 0.685. AR-V7, androgen splicing variant-7; CBZ, cabazitaxel; CRPC, castration-resistant prostate cancer; CTC, circulating tumor cell; PSA, prostate specific antigen; (−), negative; ( +), positive.
Despite receiving more than three cycles of CBZ, 42% (20/48) of the patients did not show a PSA-lowering response to chemotherapy. Overall survival was significantly worse in patients who did not have a PSA-lowering response in the early stages of CBZ treatment (HR, 2.84 [95% CI, 1.22–6.57]; p = 0.00173) (Supplemental Figure). Therefore, the PSA decline in the early stages of CBZ treatment could predict better survival with CBZ treatment.
Findings from the CTC analyses showed that median OS was 13.9 months (95% CI, 9.5–22.0) in the AR-V7-positive group, 13.4 months (95% CI, 9.5–20.4) in the CTC-positive/AR-V7-negative group, and 16.7 months (95% CI, 7.8–NA) in the CTC-negative/AR-V7-negative group (Fig. 3). The presence of AR-V7 in CTCs at baseline was not associated with OS (CTC-positive/AR-V7-positive versus CTC-positive/AR-V7-negative, HR: 1.02 [95% CI, 0.28–3.71]; p = 0.97; CTC-positive/AR-V7-positive versus CTC-negative/AR-V7-negative, HR: 0.89 [95% CI 0.34–2.27]; p = 0.80).
Kaplan–Meier curve of overall survival (OS) rate of groups with or without CTC and AR-V7 status at baseline. Group A, CTC (−) / AR-V7 (−); Group B, CTC ( +) / AR-V7 (−); Group C, CTC ( +) / AR-V7 ( +), analyzed by log-rank test (N = 48). Median OS was 13.9 months (95% CI, 9.5–22.0) in the group C, 13.4 months (95% CI, 9.5–20.4) in the group B, and 16.7 months (95% CI, 7.8–NA) in the group A. HR (95% CI): A vs. B, 0.85 (0.21–3.37), p = 0.82; B vs. C, 1.02 (0.28–3.71), p = 0.97; A vs. C, 0.89 (0.34–2.27) p = 0.80. AR-V7, androgen splicing variant-7; CI, confidence interval; HR, hazard ratio; CTC, circulating tumor cell; OS, overall survival; (−), negative; ( +), positive.
During CBZ treatment, BSI change rates were available for 41 patients; nine patients (19%) achieved a ≥ 30% reduction, and eight patients (17%) achieved a ≥ 50% reduction after 12 weeks of CBZ treatment (Fig. 4a). CTC analysis revealed that there was no significant difference in the BSI change rate between the CTC-negative, CTC-positive/AR-V7-negative, and CTC-positive /AR-V7-positive groups. However, OS was significantly worse in patients who did not have a BSI decreasing response after CBZ treatment (HR, 1.75 [95% CI, 0.66–4.67]; p = 0.0194). (Fig. 4b). Therefore, the effect of CBZ treatment on bone metastases, as objectively evaluated by bone scintigraphy BSI, could predict favorable survival during CBZ treatment.
a Waterfall plots of percent change in bone scan index (BSI) after cabazitaxel treatment. Group A, CTC (−) / AR-V7 (−); Group B, CTC ( +) / AR-V7 (−); Group C, CTC ( +) / AR-V7 ( +) (N = 41). A vs. B, p = 0.697; B vs. C, p = 0.560; A vs. C, p = 0.433. b Kaplan–Meier curve of overall survival (OS) rate of patients with CRPC after cabazitaxel treatment, with or without bone scan index (BSI) reduction response. Group A, patients with BSI decline; Group B, patients without BSI decline (log-rank test) (N = 42). Survival rate at median observation period [95% CI]: Group A (n = 17), 0.76 [0.480–0.903]; Group B (n = 25), 0.68 [0.461–0.825]. Median overall survival (days) [95% CI]: Group A (n = 17), 575 [357–NA]; Group B (n = 25), 418 [341–533]. HR 1.75 [95% CI, 0.66–4.67], p = 0.0194. AR-V7, androgen splicing variant-7; BSI, Bone Scan Index; CI, confidence interval; CRPC, castration-resistant prostate cancer; CTC, circulating tumor cell; HR, hazard ratio; OS, overall survival; (−), negative; ( +), positive.
For adverse events of grade 2–3 and above, CBZ administration was continued with appropriate dose reductions. Although febrile neutropenia (FN) of G3 or higher developed and the dose of CBZ was reduced in six cases, pegfilgrastim prevented severe FN and treatment-related death in the majority of patients. Although CBZ administration was not discontinued, blood transfusions were required in four patients with G3 anemia and one patient with G3 thrombocytopenia (Supplemental Table). Four patients withdrew in less than three cycles due to factors such as distance to the hospital, pain from bone metastases, and a desire to transfer to a local referral hospital, and for three patients, CBZ was discontinued before the completion of four cycles due to other disease causes (cerebral hemorrhage, myocardial infarction, and other advanced cancers). As a result, seven of the 55 patients who entered the study withdrew after receiving less than three cycles of CBZ.
Discussion
This study was conducted in Japanese patients with mCRPC in a setting similar to the CABARESC study by Onstenk et al., i.e., all patients had previously received docetaxel chemotherapy, and the effect of CBZ treatment was evaluated based on CTC status10. The median OS rate in this real-world study, which included patients with poor performance status or severe comorbidities (Table 2), was 13.7 months, which was comparable to the median OS of 15.1 months in the multinational phase III TROPIC trial11. The median OS in this study was also comparable to that of the other real-world clinical trials of CBZ in Japanese patients with mCRPC (10.6–16.3 months)12,13,14.
This study is the first to prospectively investigate the correlation between the presence or absence of CTCs and AR-V7 status to determine the effect of CBZ treatment in Japanese patients with mCRPC. Recent studies have reported that the presence of AR-V7 in CTC is associated with resistance to the novel AR-targeted therapies, such as abiraterone or enzalutamide, but not to taxane-based chemotherapy4,8. In this study, we found that the efficacy of CBZ was independent of AR-V7 status in both patients who were CTC-positive and -negative (Fig. 2). These findings are comparable to those from the PROPHECY and CABARESC studies, where the response to CBZ appeared to be independent of the AR-V7 status of CTCs from patients with mCRPC10,15. Moreover, there were no significant differences in OS rates from the start of CBZ chemotherapy for the three groups: AR-V7-positive, AR-V7-negative/CTC-positive, and CTC-negative (Fig. 3). This suggests that CBZ should be used preferentially in patients with mCRPC with AR-V7-positive CTCs after docetaxel treatment. Markowski et al. reported a similar viewpoint for AR-V7-positive patients, where treatment was frequently changed from AR targeted therapy to taxane-based chemotherapy (43%), or inclusion into clinical trials (43%)16. Zhang et al. reported that AR-V7-positive patients with mCRPC benefit from taxane chemotherapy regimens rather than additional AR targeted therapies17. However, Belderbos et al. found that AR-V7 status in CTCs had no prognostic value in patients with mCRPC, who progressed after docetaxel and/or enzalutamide or abiraterone and were started on CBZ as the next line of treatment18. However, the authors noted that further prospective trials are needed18, and this current study adds to that evidence base.
Since AR-V7 is most sensitively detected in CTC samples19, we collected and analyzed CTCs using AdnaTest kit. According to recent findings, the number of CTCs must be considered when describing the relationship between AR-V7 status and prognosis in CTCs20. Even though AdnaTest measured CTC to be negative, CellSearch detected 69.5% of CTCs20, implying that AdnaTest was less sentitive than CellSearch. However, in this study, we used AdnaTest as a “Point Of Care Test,” focusing on the simplicity and feasibility of the CTC analysis. In this study, we demonstrated that AR-V7 in CTCs could be detected in any laboratory without the use of expensive equipment, and the results can be applied in the context of precision medicine. It was also considered that AdnaTest would be useful in clinical practice as a POCT tool that could easily evaluate AR-V7 status even if the CTC detection sensitivity was slightly inferior. Thus, we used the AdnaTest to perform the following three-group analysis: CTC-negative/AR-V7-negative (29%), CTC-positive/AR-V7-negative (54%), and CTC-positive/AR-V7-positive (17%) (Table 3). Antonarakis et al. Used the same three prognostic categories (26%, 56%, and 18%, respectively) after treatment with abiraterone or enzalutamide9. Although several studies investigated two groups, AR-V7-positive and AR-V7-negative, in which CTC could be detected, it was also interesting to see if there was any difference in efficacy from the CTC negative group, as reported by Antonarakis et al. and Cattrini et al.9,21. CTC detection is the most important prognostic factor for drug efficacy in mCRPC22. In our study, we found no significant difference in survival between the presence and absence of CTC detection (Fig. 3). One reason might be the insufficient number of cases and the lack of test power. Secondly, AdnaTest is considered to be less sensitive than CellSearch, so using CellSearch might have resulted in a significant reduction in the number of CTC negatives. Third, Sharp et al. reported that 63% (10/16) of the AdnaTest-detected patients who were CTC-negative had detectable AR-V7 protein expression in their matched mCRPC biopsy20. Thus, it is possible that the CRPC tissue itself is AR-V7 positive below the CTC detection limit. Furthermore, such cases may have a poor prognosis.
We propose that the effect of CBZ on bone metastases is also independent of CTCs and AR-V7 status (Fig. 4a). Furthermore, the effect of CBZ on bone metastases—a decrease in BSI as measured by bone scintigraphy, could be associated with prolongation of survival (Fig. 4b). The use of bone scintigraphy to measure BSI is an objective parameter to evaluate bone metastases and is a useful tool for the diagnosis and evaluation of metastatic prostate cancer in clinical practice.
In a retrospective study, Reza et al. reported that reduction of BSI (ΔBSI) after abiraterone administration correlated with survival time in patients with mCRPC23. A retrospective study by Anand et al. also showed that ΔBSI after enzalutamide administration was correlated with survival in patients with mCRPC and bone metastases24. However, a subsequent retrospective study by Miyoshi et al. reported no such correlation between ΔBSI and OS after 16 weeks of CBZ administration25. Although the findings of Miyoshi et al. differ from our findings, there are differences between the studies: prospective versus retrospective study design; slightly larger patient population (48 versus 32 patients); early versus late treatment line with CBZ in mCRPC; and a higher rate of visceral metastases. While there were not many cases of visceral metastases in this study, over 90% of patients had bone metastases; it is, therefore, theoretically convincing that the effect of CBZ on bone metastases contributed to the prolongation of survival.
There were no new safety concerns identified in this cohort of Japanese patients with mCRPC receiving prophylactic pegfilgrastim, suggesting that tolerability to CBZ in Japanese patients is comparable to that of mCRPC patients in Western countries.
This study has a number of limitations as well. First, because CBZ was most frequently administered in patients with relatively advanced stages of mCRPC, it was challenging to evaluate all patients until the end of the study period (seven patients withdrew prematurely). Second, the false positive rate due to benign bone degeneration and medication-related osteonecrosis of the jaw might have affected the analyses in patients with a low percentage of bone metastases (BSI < 1%). Third, and most important, we used AdnaTest exclusively for CTC collection and analysis procedures. If we had used a highly sensitive device capable of counting CTCs, such as CellSearch, the number of CTC-negative groups would have significantly decreased. It may have also contributed to a higher survival rate in the CTC-negative group. Lastly, the full-length andogen receptor (AR-FL) could have been analyzed alongside AR-V7 as an additional associated marker in mCRPC21. Additionally, other androgen receptor variants, such as AR-V1, AR-V3, and AR-V9, could have been analyzed for completeness26.
Conclusions
The findings of this Japanese cohort study confirm previously reported outcomes for CBZ in patients with mCRPC from Western countries who were previously treated with docetaxel. The detection of AR-V7 in CTCs was not associated with CBZ resistance. Findings also suggest that the BSI-reducing response using bone scintigraphy and the PSA-reducing response during the initial stages of CBZ administration could be important predictors of CBZ efficacy on OS rate. CTC-based AR-V7 detection has the potential to be used as a treatment selection biomarker in patients with mCRPC. AdnaTest would be useful in clinical practice as a POCT tool for evaluating AR-V7 status.
Materials and methods
Patients
Patients with mCRPC who had progressed on docetaxel were recruited from Juntendo University Hospitals (between February 2017 and August 2020) for phase 2, a non-randomized, open-label, single-arm, estimation-based study. The baseline AR-V7 status of CTCs was determined, and all patients received CBZ irrespective of AR-V7 status. Patients received 20 mg/m2 of CBZ every 3–4 weeks until disease progression or the occurrence of unacceptable toxicity. To reduce the incidence of severe febrile neutropenia, Granulocyte Colony Stimulating Factor (G-CSF; longer-acting pegylated GCSF [pegfilgrastim]) was administered prophylactically to all patients beginning with the first cycle of CBZ.
This study was approved by the Institutional Review Board of Juntendo University Hospital (admission number: 14–052), and all experiments were carried out in accordance with approved guidelines. All participants provided written informed consent.
CTC analyses
The AdnaTest (QIAGEN, Germany) was used to detect CTCs in accordance with the manufacturer’s protocol: 5 mL of blood was collected in EDTA-3 K tubes, RNA was extracted using antibody-conjugated magnetic beads (AdnaTest ProstateCancerSelect), and mRNA was then extracted using AdnaTest ProstateCancerDetect4,9,27. The extracted mRNA was subjected to reverse transcription using the Sensiscript Reverse Transcriptase Kit (QIAGEN). Reverse transcription polymerase chain reaction (RT-PCR) was used to detect PSA, AR-V7, and AR in CTCs. PSA and AR were amplified using the AdnaTest PrimerMix ProstateDetect (PCR conditions for PSA: 95 °C for 15 min, 42 cycles of 30 s at 94 °C, 61 °C for 30 s, 72 °C for 30 s, followed by 10 min of extension, and PCR conditions for AR: 95 °C for 15 min, 35 cycles of 30 s at 94 °C, 60 °C for 30 s, 72 °C for 60 s, followed by 10 min of extension). These experiments confirmed that all samples were positive for PSA. Thus, we concluded that PSA positivity is a common denominator in this study and defined successful CTC detection as positive PSA expression. The primer set and PCR conditions for AR-V7 RT-PCR are as follows: AR-V7 primer set designed to yield 125-bp AR-V7-specific band—5′-CCATCTTGTCGTCTTCGGAAATGTTA-3′ and 5′-TTTGAATGAGGCAAGTCAGCCTTTCT-3′ (PCR conditions for AR-V7: 95 °C for 5 min, 39 cycles at 95 °C for 10 s, 58 °C for 30 s, 72 °C for 30 s, followed by 10 min of extension). Amplified PCR products were electrophoresed and visualized by the DNA 1 K Experion™ automated electrophoresis system (Bio-Rad, CA, USA). To evaluate gene expression, the fluorescence intensity scale was set to “scale to local” (default setting), and any visible bands with detectable peaks under these conditions were considered positive.
Study endpoints
The primary end point was PSA response rate (PSA-RR), and the primary objective was to compare PSA-RR between CTC-negative, AR-V7-positive, and AR-V7-negative (CTC-positive) cohorts. PSA-RR was defined as a ≥ 30% and ≥ 50% decline in PSA level from baseline to 12 weeks of CBZ treatment, or earlier if treatment was discontinued. Secondary end points included overall survival in patients with or without PSA decline 3 months following CBZ treatments, with or without CTCs and AR-V7 status at baseline, and the relationship with or without decline of Bone Scan Index (BSI) by bone scintigraphy. The safety of CBZ in Japanese patients with mCRPC was assessed using CTCAE 4.0 protocol.
Statistical analysis
The Kruskal–Wallis test was used for statistical analysis of patient background. Survival was analyzed using Cox regression models and visualized in Kaplan–Meier plots, and OS curves were generated using the Kaplan–Meier method. The PSA-RR (≥ 50% and ≥ 30% decline in PSA from baseline PSA following chemotherapy) was compared for three groups: CTC-negative/AR-V7-negative; CTC-positive/AR-V7-negative; and CTC-positive/AR-positive; and analyzed using the chi-square or Fisher’s exact tests. Statistical differences between the three groups were compared using the log-rank test.
Data availability
The datasets generated and analyzed during this study are available in the NBDC (National Bioscience Database Center) Human Database repository, [https://humandbs.biosciencedbc.jp/en/ Research ID : hum0354].
References
Chandrasekar, T., Yang, J. C., Gao, A. C. & Evans, C. P. Mechanisms of resistance in castration-resistant prostate cancer (CRPC). Transl. Androl. Urol. 4, 365–380 (2015).
Ryan, C. J. et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 368, 138–148 (2013).
Beer, T. M. et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 371, 424–433 (2014).
Antonarakis, E. S. et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 371, 1028–1038 (2014).
Todenhöfer, T. et al. AR-V7 transcripts in whole blood RNA of patients with metastatic castration resistant prostate cancer correlate with response to abiraterone acetate. J. Urol. 197, 135–142 (2017).
Tagawa, S. T. et al. Expression of AR-V7 and ARv567es in circulating tumor cells correlates with outcomes to taxane therapy in men with metastatic prostate cancer treated in TAXYNERGY. Clin. Cancer Res. 25, 1880–1888 (2019).
Bastos, D. A. & Antonarakis, E. S. CTC-derived AR-V7 detection as a prognostic and predictive biomarker in advanced prostate cancer. Expert Rev. Mol. Diagn. 18, 155–163 (2018).
Antonarakis, E. S. et al. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol. 1, 582–591 (2015).
Antonarakis, E. S. et al. Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men With metastatic castration-resistant prostate cancer treated With first- and Second-Line abiraterone and enzalutamide. J. Clin. Oncol. 35, 2149–2156 (2017).
Onstenk, W. et al. Efficacy of cabazitaxel in castration-resistant prostate cancer is independent of the presence of AR-V7 in circulating tumor cells. Eur. Urol. 68, 939–945 (2015).
de Bono, J. S. et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 376, 1147–1154 (2010).
Suzuki, K. et al. Safety and efficacy of cabazitaxel in 660 patients with metastatic castration-resistant prostate cancer in real-world settings: Results of a Japanese post-marketing surveillance study. Jpn. J. Clin. Oncol. 49, 1157–1163 (2019).
Terada, N. et al. The efficacy and toxicity of cabazitaxel for treatment of docetaxel-resistant prostate cancer correlating with the initial doses in Japanese patients. BMC Cancer 19, 156 (2019).
Yamamoto, T. et al. Safety and efficacy of cabazitaxel in Japanese patients with castration-resistant prostate cancer. Prostate Int. 8, 27–33 (2020).
Armstrong, A. J. et al. Prospective multicenter study of circulating tumor cell AR-V7 and taxane versus hormonal treatment outcomes in metastatic castration-resistant prostate cancer. JCO Precis. Oncol. 4 (2020).
Markowski, M. C. et al. Clinical utility of CLIA-grade AR-V7 testing in patients with metastatic castration-resistant prostate cancer. JCO Precis. Oncol. 1, 1–9 (2017).
Zhang, T., Karsh, L. I., Nissenblatt, M. J. & Canfield, S. E. Androgen receptor splice variant, AR-V7, as a biomarker of resistance to androgen axis-targeted therapies in advanced prostate cancer. Clin. Genitourin. Cancer 18, 1–10 (2020).
Belderbos, B. P. S. et al. Associations between AR-V7 status in circulating tumour cells, circulating tumour cell count and survival in men with metastatic castration-resistant prostate cancer. Eur. J. Cancer 121, 48–54 (2019).
Nimir, M. et al. Detection of AR-V7 in liquid biopsies of castrate resistant prostate cancer patients: A comparison of AR-V7 analysis in circulating tumor cells circulating tumor RNA and exosomes. Cells 8, 688 (2019).
Sharp, A. et al. Clinical utility of circulating tumour cell androgen receptor splice variant-7 status in metastatic castration-resistant prostate cancer. Eur. Urol. 76, 676–685 (2019).
Cattrini, C. et al. Role of circulating tumor cells (CTC), androgen receptor full length (AR-FL) and androgen receptor splice variant 7 (AR-V7) in a prospective cohort of castration-resistant metastatic prostate cancer patients. Cancers 11, 1365 (2019).
Heller, G. et al. circulating tumor cell number as a response measure of prolonged survival for metastatic castration-resistant prostate cancer: A comparison with prostate-specific antigen across five randomized phase III clinical trials. J. Clin. Oncol. 36, 572–580 (2018).
Reza, M. et al. Bone scan index as an imaging biomarker in metastatic castration-resistant prostate cancer: A multicentre study based on patients treated with abiraterone acetate (zytiga) in clinical practice. Eur. Urol. Focus 2, 540–546 (2016).
Anand, A. et al. Automated bone scan index as a quantitative imaging biomarker in metastatic castration-resistant prostate cancer patients being treated with enzalutamide. EJNMMI Res. 6, 23. https://doi.org/10.1186/s13550-016-0173-z (2016).
Miyoshi, Y. et al. Correlation between automated bone scan index change after cabazitaxel and survival among men with castration-resistant prostate cancer. Urol. Int. 103, 279–284 (2019).
Sieuwerts, A. M. et al. AR splice variants in circulating tumor cells of patients with castration-resistant prostate cancer: Relation with outcome to cabazitaxel. Mol. Oncol. 13, 1795–1807 (2019).
Nagaya, N. et al. Prostate-specific membrane antigen in circulating tumor cells is a new poor prognostic marker for castration-resistant prostate cancer. PLoS ONE 15, e0226219 (2020).
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Funding
This clinical research received funding from Sanofi Inc. (Paris, France).
Author information
Authors and Affiliations
Contributions
All authors have significantly contributed to the study, and are in agreement with the content of the manuscript. Each author’s contribution is as follows: T.A. and M.N. were responsible for patient recruitment, treatments and follow-up for the patients. S.N., H,H., N.N. and Y.L. performed the experiments and analyzed data. M.N. and S.H designed the study. T.A. and M.N. wrote the manuscript, and S.H. revised it. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
Regarding the Competing Interests statement to be declared, this clinical research was funded by Sanofi Inc.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ashizawa, T., Nagata, M., Nakamura, S. et al. Efficacy of cabazitaxel and androgen splicing variant-7 status in circulating tumor cells in Asian patients with metastatic castration-resistant prostate cancer. Sci Rep 12, 18016 (2022). https://doi.org/10.1038/s41598-022-22854-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-22854-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.