Abstract
Biomarker in metastatic castration resistant prostate cancer (mCRPC) treatment are rare. We aimed to compare the clinical value of circulating tumor cells (CTCs) and androgen receptor splice variant 7 (AR-V7) as biomarker in mCRPC patients undergoing androgen receptor-targeted agent (ARTA) treatment. Overall cohort (65 patients) was stratified regarding either CTC or AR-V7 status followed by further sub-stratification of the respective other marker. Subsequently, prostate specific antigen (PSA) response, progression free survival (PFS) and overall survival (OS)) of subgroups was compared. CTCs and AR-V7 were detected in 54 (83%) and 33 (61%) patients, respectively. All AR-V7 + were CTC +. We detected PSA response in all subgroups. For PFS and OS, biomarker stratification revealed differences between all subgroups. Interestingly, no significant differences of AR-V7 transcript copy numbers were detected between responding and non-responding patients. Additionally, multivariable analysis revealed no independent prognostic value of AR-V7 positivity. Both biomarkers show clinical value in prognosticating clinical outcome. Nonetheless, AR-V7 stratification underestimates the heterogenous subgroup of CTC − and CTC + patient, the latter requiring more intense clinical surveillance. Additionally, AR-V7 level does not correlate with clinical response. Thus, the value of AR-V7 as a clinical biomarker must be considered skeptically.
Similar content being viewed by others
Introduction
Studies on circulating tumor cells (CTCs) have significantly increased knowledge of dissemination of tumor cells from primary and metastatic tumor sites1. Large patient analyses of CTCs revealed a prognostic threshold of CTCs in prostate cancer2,3.
Besides analysis of CTCs, expression of androgen receptor splice variant 7 (AR-V7) in CTCs of prostate cancer patients has gained clinical interest for prediction of non-response to androgen receptor-targeted agents (ARTAs), i.e., abiraterone or enzalutamide4,5,6,7. However, there are inconsistencies among study designs analyzing the correlation between AR-V7 and treatment response. Initially, correlation analysis was performed solely in CTC + patients, further subdivided into AR-V7- and AR-V7 + subgroups4. A follow-up study then included CTC − patients, resulting in three groups (CTC −, CTC +/AR-V7- and CTC +/AR-V7 + patients)7. Further studies stratified AR-V7- and AR-V7 + only, without determination of the CTC status6,8,9,10,11,12. Of note, all studies showed a subset of AR-V7- patients not responding to ARTA treatment, demonstrating additional resistance mechanisms other than AR-V7.
Thus, both, CTCs as well as AR-V7, have been demonstrated to be potential clinical biomarkers. Nonetheless, both stratification approaches lack the integration of the other marker. Therefore, we aimed to comprehensively compare the risk assessment of CTC and AR-V7 stratification on clinical outcome in a defined cohort of mCRPC patients undergoing ARTA treatment.
Material and methods
CTC enrichment and determination
Blood sample preparation and CTC analysis have been described previously13,14. Briefly, blood samples of mCRPC patients (5 ml of each patient) were processed using the Dynabeads™ Epithelial Enrich Kit followed by mRNA isolation using the Dynabeads™ mRNA DIRECT™ Purification Kit (both ThermoFisher Scientific, Waltham, MA, USA). Complementary DNA (cDNA) synthesis was performed using the SuperScript™ IV VILO™ Master Mix (ThermoFisher Scientific, Waltham, MA, USA) according to manufacturer’s recommendation, except for using a temperature of 55 °C.
For determination of CTCs, patient samples were processed followed by qPCR using a KLK3-PSA TaqMan assay (Hs03063374_m1) (ThermoFisher Scientific, Waltham, MA, USA). A patient sample was determined CTC + when displaying a qPCR signal for KLK3-PSA. Given the nature of our CTC detection system, we are not able to determine the actual number of CTCs. However, patients display similar expression levels of KLK3-PSA in single CTCs15. Thus, determination of KLK3-PSA transcript copy numbers can be used as a semi-quantitative tool to dissect CTC distribution. AR-V7 status was analyzed using a previously described custom-made AR-V7 TaqMan PCR assay, demonstrating identical performance compared to the Johns Hopkins University (JHU) AR-V7 assay14. All qPCR runs were performed along with TaqMan qPCR assays for housekeeping genes RPL37A (Hs01102345_m1) and HPRT1 (Hs99999909_m1) (ThermoFisher Scientific, Waltham, MA, USA).
Patient subgrouping
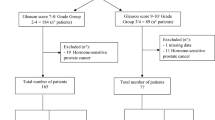
For comparative biomarker stratification and clinical outcome analyses the overall cohort was sub-grouped based on either KLK3-PSA mRNA detection as a surrogate for CTCs or AR-V7 mRNA expression into CTC − and CTC + as well as AR-V7- and AR-V7 + groups, respectively. The CTC + subgroup was further subdivided into CTC +/AR-V7- and CTC +/AR-V7 + patients. Also, the AR-V7- subgroup was further subdivided into CTC −/AR-V7- and CTC +/AR-V7- patients (Fig. 1).
Patient subgrouping according to biomarker status. For comparative analysis of either CTC (A) or AR-V7 (B) as clinical biomarker, the overall cohort was stratified according to the respective biomarker status. Subsequently, subgroups were further subdivivded according to the respective other biomarker status. Colored boxes indicate identical subgroups.
Outcome measures
The primary endpoint was clinical or radiographic PFS. The assessment of response status took place at each routinely planned visit and was independently performed by experienced experts in the field of mCRPC. For the classification of response status, general condition (ECOG), presence of pain, laboratory constellations (mainly PSA) as well as imaging were considered. Clinical progression was defined as symptomatic progression (worsening or new prostate cancer-related symptoms). PSA progression was defined as confirmed increase of 25% or greater from baseline in case of no prior PSA decline and an absolute increase of 2 ng/ml or more from nadir (Prostate Cancer Working Group 3 criteria). During clinically and biochemically stable disease, imaging consisting of CT- or MRI-scans of thorax, abdomen, and pelvis as well as bone scans, was not performed routinely. When biochemical progression occurred or PD was otherwise presumed, soft tissue metastases were evaluated by CT- and MRI-scans and bone metastases were assessed by bone scans. For all patients, PD was defined according to RECIST 1.1 criteria for cross-sectional imaging and by PCWG3 criteria for bone scans.
Secondary endpoints included PSA response and OS. PSA response was defined as a PSA decline of 50% or more from baseline at any time under therapy; best PSA response (maximum percentage decrease from baseline) was also determined. OS was defined as the interval from start of ARTA to death from any cause.
Statistical analyses
Statistical analyses were performed using SPSS-Statistics V28.0 (IBM Inc., Armonk, NY, USA) and Prism 8 V8.4.3 (GraphPad Software, LLC., San Diego, CA, USA). The descriptive statistics are reported as medians with interquartile ranges (IQR). Uni- and multivariate analyses were done by Cox-regression-models. Hazard ratios (HR) are given with 95% confidence intervals (CI). All reported p values are two-sided and statistical relevance was assumed with a p value > 0.05.
Further detailed information can be found in the Supplementary Material.
Ethics approval and consent to participate
The study was approved by the local Ethics committee (Ethik-Kommission der Ärztekammer Westfalen-Lippe und der Westfälischen Wilhelms-Universität; 2007-467-f-S and 2016–585-f-S) and all patients provided written informed consent. The study was conducted with provisions of the Declaration of Helsinki.
Consent for publication
The authors agree the terms of conditions for publication.
Results
Patients
Between June 2016 and December 2020, we prospectively enrolled 65 patients who started on either abiraterone (n = 46) or enzalutamide (n = 19). At the time of study closure in March 2021, the median follow-up was 14.0 (IQR 8–31) months. 33 (50.8%) patients started as first-line treatment, 15 (23.1%) patients as a second-line treatment and 17 (26.2%) patients were treated in a third- or higher line of treatment (Table 1). All baseline characteristics are displayed in Table 1.
Eleven (17.0%) patients of the overall cohort were found to be negative for KLK3-PSA expression, i.e., absence of CTCs, whereas 54 (83.0%) patients were found to be positive for CTCs. We did not notice AR-V7 expression in any CTC − patient. Within the subgroup of CTC + patients, we detected AR-V7 in 33 (61.1%) patients (CTC +/AR-V7 +) whereas 21 (38.9%) patients did not display AR-V7 expression (CTC +/AR-V7-). In the AR-V7- subgroup, 11 patients (33.3%) displayed absence of CTCs, 21 patients were found to be positive for CTCs (66.7%).
PSA response
We observed a PSA decline ≥ 50% (PSA response) in 31 (47.7%) patients of the overall cohort. Compared to the CTC + group (23/54 (42.6%)), more CTC − patients showed a PSA response (8/11 (72.7%; p = 0.10) (Fig. 2A). When comparing the AR-V7 + versus AR-V7- group, we detected a PSA response in 11 (33.3%) of the AR-V7 + patients and 20 (62.5%) of the AR-V7- patients (p = 0.03) (Fig. 2B).
PSA response. Waterfall plots depicting best PSA responses in patients starting ARTA treatment according to CTC status, (A, left panel) CTC − patients, (A, right panel) CTC + patients, and AR-V7 status, (B, left panel) AR-V7- patients, (B, right panel) AR-V7 + patients. The dotted line illustrates the threshold of PSA 50% decline defining a PSA response. When comparing patients concerning CTC status, PSA response rates were 8/11 (72.7%) and 23/54 (42.6%) in CTC − and CTC + patients, respectively, without statistical significance (p = 0.10; Fisher’s exact test). PSA response rates were 20/32 (62.5%) and 11/33 (33.3%) in AR-V7- and AR-V7 + patients, respectively (p = 0.03; Fisher’s exact test).
Progression free survival (PFS)
For the overall cohort, median PFS was 9 months (CI 7.1–10.9). By comparing CTC + versus CTC − patients, PFS was significantly longer for CTC − patients with 22 months (CI not estimable) versus 8 months (CI 5.5–10.5) for CTC + patients (p < 0.01) (Fig. 3A, left panel). Within the CTC + subgroup, AR-V7- patients showed prolonged PFS (10 months (CI 8.9–11.1)) compared to AR-V7 + patients (6 months (CI 3.6–8.4)), however, without statistical significance (p = 0.07) (Fig. 3A, right panel).
Progression free survival and overall survival. Kaplan–Meier curves including patient numbers at risk indicating PFS according to CTC status (A) and AR-V7 status (B) of patients undergoing ARTA treatment. (A, left panel) For the overall cohort, median PFS was 22 months (CI not estimable) and 8 months (CI 5.5–10.5) for CTC − patients and CTC + patients, respectively (p < 0.01). (A, right panel) Within the CTC + cohort, median PFS was 10 months (CI 8.9–11.1) and 6 months (CI 3.6–8.4) for AR-V7- patients and AR-V7 + patients, respectively, without statistical significance (p = 0.07). (B, left panel) For the overall cohort, median PFS was 16 months (CI 7.0–25.0) and 6 months (CI 3.6–8.4) for AR-V7- patients and AR-V7 + patients, respectively (p < 0.01). (B, right panel) Within the AR-V7- subgroup, median PFS was 22 months (CI not estimable) and 10 months (CI 8.9–11.1) for CTC − patients and CTC + patients, respectively (p = 0.03). Kaplan–Meier curves including patient numbers at risk indicating OS according to CTC status (C) and AR-V7 status (D) of patients undergoing ARTA treatment. (C, left panel) For the overall cohort, median OS was not reached for CTC − patients and 15 months (CI 8.7–21.3) for CTC + patients (p < 0.01). (C, right panel) Within the CTC + cohort, median OS was 27 months (CI 25.1–28.9) and 13 months (CI 9.2–16.8) for AR-V7- patients and AR-V7 + patients, respectively (p > 0.01), without statistical significance (p = 0.07). (D, left panel) For the overall cohort, median OS was 31 months (CI 25.2–36.8) and 13 months (CI 9.2–16.8) for AR-V7- patients and AR-V7 + patients, respectively (p < 0.01). (D, right panel) Within the AR-V7- subgroup, median OS was not reached and 27 months (CI 25.1–28.9) for CTC − patients and CTC + patients, respectively, (p = 0.02).
By comparing AR-V7- and AR-V7 + patients, PFS was significantly longer for AR-V7- (16 months (CI 7.0–25.0) versus AR-V + patients (6 months (CI 3.6–8.4)) (p < 0.01) (Fig. 3B, left panel). Within the AR-V7- subgroup, CTC − patients displayed improved PFS (22 months (CI not estimable)) versus (10 months (CI 8.9–11.1)) in CTC + patients (p = 0.03) (Fig. 3B, right panel).
Performing univariate analysis for the overall cohort, prior abiraterone or enzalutamide (p < 0.01), Hb ≤ 12 g/dl at baseline (p = 0.01), absence of PSA decline ≥ 50% (p < 0.01), CTC positivity (p < 0.01) and AR-V7 positivity (p < 0.01) were statistically prognostic for PFS (Table S1). Within the CTC + subgroup, prior abiraterone or enzalutamide (p < 0.01), PSA doubling time < 3 months (p = 0.04) and absence of PSA decline ≥ 50% (p < 0.01) were significantly associated with worse PFS, whereas AR-V7 positivity did not show association with an inferior PFS (p = 0.09) (Table S2). Within the AR-V7- subgroup, prior abiraterone or enzalutamide (p < 0.01), absence of PSA decline ≥ 50% (p < 0.01) and CTC positivity (p < 0.05) were significantly associated with worse PFS (Table S3).
In multivariate cox regression analysis for the overall cohort, only prior abiraterone or enzalutamide (p = 0.01) was independently prognostic (Table S4). In CTC + patients, none of the parameters was independently prognostic (Table S5). Within the AR-V7- subgroup, prior abiraterone or enzalutamide (p = 0.02) was independently prognostic (Table S6).
Overall survival (OS)
For the overall cohort, median OS was 20 months (CI 10.5–29.5). By comparing CTC + versus CTC − patients, median OS was not reached for CTC − patients compared to 15 months (CI 8.7–21.3) for CTC + patients (p < 0.01) (Fig. 3C, left panel). By comparing AR-V7- and AR-V7 + patients within the subgroup of CTC + patients, median OS was 27 months (CI 25.1–28.9) for AR-V7- patients and 13 months (CI 9.2–16.8) for AR-V + patients, displaying statistical significance (p = 0.03) (Fig. 3C, right panel).
By comparing AR-V7- and AR-V7 + patients, OS was significantly longer for AR-V7- patients (31 months (CI 25.2–36.8)) compared to AR-V7 + patients (13 months (CI 9.2–16.8)) (p < 0.01) (Fig. 3D, left panel). Within the AR-V7- subgroup, CTC − patients displayed improved OS (not reached) versus 27 months (CI 25.1–28.9) in CTC + patients (p = 0.02) (Fig. 3D, right panel).
Univariate analysis for the whole cohort showed that prior abiraterone or enzalutamide (p < 0.01), bone metastases (p = 0.03), ALP elevated at baseline (p < 0.01), Hb ≤ 12 g/dl at baseline (p < 0.01), absence of PSA decline ≥ 50% (p < 0.01), CTC positivity (p < 0.01), AR-V7 positivity (p < 0.01) were prognosticators of worse OS (Table S1).
Within the CTC + group of patients, prior docetaxel (p < 0.01), prior abiraterone or enzalutamide (p < 0.01), Hb ≤ 12 g/dl at baseline (p < 0.01), PSA doubling time < 3 months (p = 0.03) and AR-V7 positivity (p = 0.04) were prognosticators of inferior OS (Table S2). Within the AR-V7- subgroup, Hb ≤ 12 g/dl at baseline (p < 0.01) and CTC positivity (p = 0.03) were significantly associated with worse OS (Table S3).
In multivariable cox-regression-analysis for the overall cohort, CTC positivity (p = 0.04), prior abiraterone or enzalutamide (p = 0.02) and Hb ≤ 12 g/dl at baseline (p < 0.01) were independent prognosticators for worse OS (Table S4). For CTC + patients, in multivariate analysis, only Hb ≤ 12 g/dl at baseline (p < 0.01) was independently prognostic for worse OS (Table S5). Within the AR-V7- subgroup, CTC positivity (p = 0.04) and Hb ≤ 12 g/dl at baseline (p < 0.01) were independent prognosticators for worse OS (Table S6).
Quantification of biomarker transcripts and correlation to clinical outcome
For correlation analyses we determined KLK3-PSA and AR-V7 transcript copy numbers in CTC + and AR-V7 + patients, respectively, and stratified patients into responding (R) and non-responding (N) subgroups.
For PSA response, no significant difference of KLK3-PSA transcript copy number was detected between responders and non-responders in the CTC + subgroup, whereas AR-V7 transcript levels were significantly lower in responding patients compared to non-responding patients (Fig. 4A). By applying the median PFS to differentiate between responders and non-responders, we detected a significantly higher number of KLK3-PSA transcript copy numbers within the subgroup of non-responders compared to responders, suggesting a worse clinical outcome in patients displaying a higher number of CTCs. However, no difference of KLK3-PSA transcript copy numbers between responders and non-responders was observed with respect to OS. No significant differences in PFS and OS were detected within the AR-V7 + subgroup (Fig. 4B).
Correlation analysis of responding (R) and non-responding (N) patients and copy number distribution/biomarker appearance and levels. Comparison of mRNA copy numbers KLK3-PSA (upper panel) and AR-V7 (lower panel) per blood sample (5 ml) in biomarker positive patients (upper panel: CTC +; lower panel: AR-V7 +) regarding (A) PSA response, (B) PFS and OS categorized into responders ((R); PFS and OS duration > median PFS and OS) and non-responders ((N); PFS and OS duration < median PFS and OS) and (C) PFS and OS categorized into responders ((R); PFS and OS duration > CI upper limit for PFS and OS) and non-responders ((N); PFS and OS duration < CI upper limit for PFS and OS). P values (Mann–Whitney test) are as follows. CTC +: PSA50: p = 0.11; Median, PFS: p < 0.02, OS: p = 0.11; upper limit: PFS: p = 16, OS: p < 0.01; AR-V7 +: PSA50: p = 0.03; Median, PFS: p = 0.30, OS: p = 0.42; upper limit: PFS: p = 0.15, OS: p = 0.63. (D) Biomarker appearance among different lines of treatment. P values (Fisher’s exact test) are as follows. CTC +: p > 0.99; AR-V7 +: p = 0.03. (E) Biomarker levels among different lines of treatment. mRNA copy numbers of KLK3-PSA (as a surrogate for actual number of CTCs) and AR-V7 in different lines of treatment. P values (Mann–Whitney test) are as follows. KLK3-PSA: p > 0.01, AR-V7: p = 0.73.
We next aimed to perform a more conservative estimation by using the upper confidence interval as threshold to stratify patients into responders (above upper limit) and non-responders (below upper limit). By this, significant differences in KLK3-PSA transcript copy numbers were observed for both PFS and OS. However, for AR-V7, we did not detect any significant differences (Fig. 4C). This validates a correlation between a high number of CTCs and worse clinical outcome. Contrary, the expression level of AR-V7 does not influence clinical outcome.
Association of CTC and AR-V7 status and advanced stage of disease
By dissecting distribution of biomarker positive patients (CTC + and AR-V7 +) among distinct lines of treatment, we detected a significant increase of AR-V7 + patients during disease progression, while the number of CTC + patients tend to stay similar (Fig. 4D). Contrary, the actual numbers of KLK3-PSA mRNA transcripts increase over different lines of treatment, whereas the number of AR-V7 mRNA transcripts did not show significant differences (Fig. 4E). This validates an increase in CTC numbers and AR-V7 appearance, yet no increase in transcriptional levels, over time.
We next intended to correlate biomarker subgroups and criteria of an advanced stage of disease (i.e., Hb, AP, LDH, presence of metastases, PSA doubling time). We detected a significantly higher number of criteria representing a more advanced stage of disease within both, CTC + compared to CTC − and AR-V7 + compared to AR-V7- subgroups (Fig. S1A). Whereas the count of criteria was similar in both, CTC + and AR-V7 + subgroups, a lower number of criteria was detected in the CTC − compared to the AR-V7- subgroup, yet without statistical significance (Fig. S1B).
Discussion
In this study, we comprehensively compare the correlation of both, CTC and AR-V7 status, with clinical parameters in a cohort of mCRPC patients undergoing ARTA treatment. The results of our study indicate that both, CTC + versus CTC − and AR-V7 + versus AR-V7- stratification, can effectively prognosticate clinical outcome. Nonetheless, we demonstrate potential flaws by focusing on AR-V7 as a sole biomarker.
The presence of CTCs vividly holds the potential of a prognostic biomarker in mCRPC16,17,18,19,20. The number of more than 5 CTCs per 7.5 ml blood sample highly correlates with a worse prognosis21. Furthermore, CTC analyses not only determine CTC counts at baseline but might also be applicable during treatment. A decrease of CTC count of more than 30% during the first 12 weeks of treatment is associated with a good prognosis19. Also, PCWG3 guidelines recommend CTC counts as an endpoint in patients with unfavorable counts at baseline in clinical trials22. Our results validate the prognostic power of CTC determination by demonstrating a worse clinical outcome in CTC + versus CTC − patients with respect to PSA response, PFS and OS. When semi-quantifying the number of KLK3-PSA transcripts as a potential surrogate for actual number of CTCs, we detected a significant increase in transcript copy numbers in non-responding patients. This demonstrates the value of CTC number determination to prognosticate clinical outcome. Additionally, CTC positivity is associated with a more advanced stage of disease. Our results therefore underscore the validity of CTC detection approaches for clinical applications.
Besides, AR-V7 has been discussed as both, a prognostic and predictive biomarker in mCRPC. Initially, expression of AR-V7 has been correlated to non-response to ARTA in CTC + patients4. Although clinical outcome was worse in AR-V7 + patients, results might have been overestimated as AR-V7 + patients tend to suffer from an advanced stage of disease, e.g., higher number of previous treatments, higher serum PSA levels, and alkaline phosphatase levels. An extended follow-up study thus included CTC − patients and stratified patients into three categories: CTC −, CTC +/AR-V7- and CTC +/AR-V7 + 7. Within this study, significant differences were observed among the three subgroups, demonstrating the worst clinical outcome in CTC +/AR-V7 + patients. Consequently, the authors concluded, that AR-V7 analysis might be applied for prediction of ARTA treatment response. A biomarker-based application would imply clinical testing of AR-V7 status of all patients. Most recent studies then integrated dichotomous stratification of AR-V7 + or AR-V7- patients, without previous determination of CTC status6,8,9,10,11,12. In case of a strong predictive biomarker this stratification might be adequate. However, sole AR-V7 discrimination might be at risk of mis-consideration of severe clinical factors.
First, although PSA response is lower in AR-V7 + compared to CTC + patients, we show a substantial PSA response even in AR-V7 + patients. This is in line with previous reports demonstrating PSA response to ARTA even in AR-V7 + patients11,23. When focusing on the CTC + cohort, no significant differences in PFS were observed between CTC +/AR-V7- and CTC +/AR-V7 + patients. Additionally, we did not notice differences in AR-V7 transcript levels between patients responding and non-responding to ARTA treatment. Thus, the actual levels of AR-V7 seem not to correlate with either response or non-response. Hence, our results challenge the clinical value of AR-V7 as a predictive biomarker for ARTA treatment.
Second, in case of OS, we detected a significant difference between CTC +/AR-V7- and CTC +/AR-V7 + patients, demonstrating a prognostic value of AR-V7. However, for neither PFS nor OS, AR-V7 positivity was found to be an independent prognostic factor for both the overall cohort as well as the CTC + subgroup. Appearance of AR-V7 increases over different lines of treatment. Furthermore, AR-V7 positivity is associated with presence of criteria related to a later stage of disease as well as a more aggressive subtype of prostate cancer. These results are in line with a recent study by Sharp et al., demonstrating a correlation of AR-V7 and a more advanced stage of disease24. Given the lack of independent prognostic validity along with the fact, that a set of distinct criteria to stage patients into a high-risk subgroup, are already used in clinical routine, it remains unclear whether AR-V7 detection can add further information to improve patient treatment.
Third—and presumably of highest clinical impact, focusing on AR-V7 neglects the heterogeneity of the AR-V7- cohort consisting of both CTC − and CTC + patients. A sole focus on AR-V7 would thus overlook the heterogeneity of this subgroup of CTC + and CTC − patients. As discussed earlier, CTC + patients display a higher probability of worse prognosis compared to CTC − patients and thus, need accurate and frequent clinical surveillance. Indeed, we detected significant differences between AR-V7-/CTC − and AR-V7-/CTC + patients with respect to both PFS and OS. Additionally, in terms of OS, presence of CTCs is an independent prognostic factor for both the overall cohort and the AR-V7 + subgroup. Therefore, sole AR-V7 stratification is of risk of underestimating the risk of rapid disease progression in a subset of AR-V7- yet CTC + patients.
A limitation of this study is the relatively small number of included patients. Nonetheless, differences within the two biomarkers are obvious and significant.
Conclusively, our results demonstrate a prognostic value of mCRPC patient stratification regarding both, CTC and AR-V7 status. However, CTC status is clinically more meaningful in prognostication, whereas AR-V7 status is of risk of underestimating the clinical differences in AR-V7- patients by not discriminating between AR-V7-/CTC − and AR-V7-/CTC + patients. Ultimately, similar PFS outcomes of AR-V7 + and AR-V7- patients within the subgroup of CTC + patients strongly question the power of AR-V7 as a predictive biomarker for non-response to ARTAs. Thereby, our study underscores recent expert opinion neglecting the usage of AR-V7 for clinical decision making25.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AR:
-
Androgen receptor
- AR-V7:
-
Androgen receptor splice variant 7
- ARTA:
-
Androgen receptor-targeted agent
- CTC:
-
Circulating tumor cells
- cDNA:
-
Complementary DNA
- CI:
-
Confidence interval
- HR:
-
Hazard ratio
- IQR:
-
Interquartile range
- JHU:
-
Johns Hopkins University
- mCRPC:
-
Metastatic castration resistant prostate cancer
- OS:
-
Overall survival
- PFS:
-
Progression free survival
- PSA:
-
Prostate specific antigen
References
Heidrich, I., Ackar, L., Mossahebi Mohammadi, P. & Pantel, K. Liquid biopsies: Potential and challenges. Int. J. Cancer 148, 528–545. https://doi.org/10.1002/ijc.33217 (2021).
de Bono, J. S. et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 14, 6302–6309. https://doi.org/10.1158/1078-0432.CCR-08-0872 (2008).
Allard, W. J. & Terstappen, L. W. CCR 20th anniversary commentary: Paving the way for circulating tumor cells. Clin. Cancer Res. 21, 2883–2885. https://doi.org/10.1158/1078-0432.CCR-14-2559 (2015).
Antonarakis, E. S. et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 371, 1028–1038. https://doi.org/10.1056/NEJMoa1315815 (2014).
Maillet, D. et al. Improved androgen receptor splice variant 7 detection using a highly sensitive assay to predict resistance to abiraterone or enzalutamide in metastatic prostate cancer patients. Eur. Urol. Oncol. 4, 609–617. https://doi.org/10.1016/j.euo.2019.08.010 (2021).
Armstrong, A. J. et al. Prospective multicenter validation of androgen receptor splice variant 7 and hormone therapy resistance in high-risk castration-resistant prostate cancer: The PROPHECY study. J. Clin. Oncol. 37, 1120–1129. https://doi.org/10.1200/JCO.18.01731 (2019).
Antonarakis, E. S. et al. Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J. Clin. Oncol. 35, 2149–2156. https://doi.org/10.1200/JCO.2016.70.1961 (2017).
Armstrong, A. J. et al. Prospective multicenter study of circulating tumor cell AR-V7 and taxane versus hormonal treatment outcomes in metastatic castration-resistant prostate cancer. JCO Precis. Oncol. https://doi.org/10.1200/PO.20.00200 (2020).
Scher, H. I. et al. Assessment of the validity of nuclear-localized androgen receptor splice variant 7 in circulating tumor cells as a predictive biomarker for castration-resistant prostate cancer. JAMA Oncol. 4, 1179–1186. https://doi.org/10.1001/jamaoncol.2018.1621 (2018).
Scher, H. I. et al. Association of AR-V7 on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate cancer. JAMA Oncol. 2, 1441–1449. https://doi.org/10.1001/jamaoncol.2016.1828 (2016).
To, S. Q. et al. Expression of androgen receptor splice variant 7 or 9 in whole blood does not predict response to androgen-axis-targeting agents in metastatic castration-resistant prostate cancer. Eur. Urol. 73, 818–821. https://doi.org/10.1016/j.eururo.2018.01.007 (2018).
Seitz, A. K. et al. AR-V7 in peripheral whole blood of patients with castration-resistant prostate cancer: Association with treatment-specific outcome under abiraterone and enzalutamide. Eur. Urol. 72, 828–834. https://doi.org/10.1016/j.eururo.2017.07.024 (2017).
Bernemann, C. et al. Comparative analysis of AR variant AR-V567es mRNA detection systems reveals eminent variability and questions the role as a clinical biomarker in prostate cancer. Clin. Cancer Res. 25, 3856–3864. https://doi.org/10.1158/1078-0432.CCR-18-4276 (2019).
Bernemann, C. et al. Performance comparison of two androgen receptor splice variant 7 (AR-V7) detection methods. BJU Int. 122, 219–226. https://doi.org/10.1111/bju.14146 (2018).
Miyamoto, D. T. et al. RNA-Seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandrogen resistance. Science 349, 1351–1356. https://doi.org/10.1126/science.aab0917 (2015).
Belderbos, B. P. S. et al. Associations between AR-V7 status in circulating tumour cells, circulating tumour cell count and survival in men with metastatic castration-resistant prostate cancer. Eur. J. Cancer 121, 48–54. https://doi.org/10.1016/j.ejca.2019.08.005 (2019).
Scher, H. I. et al. Circulating tumor cell biomarker panel as an individual-level surrogate for survival in metastatic castration-resistant prostate cancer. J. Clin. Oncol. 33, 1348–1355. https://doi.org/10.1200/JCO.2014.55.3487 (2015).
Scher, H. I. et al. Trial design and objectives for castration-resistant prostate cancer: Updated recommendations from the prostate cancer clinical trials working group 3. J. Clin. Oncol. 34, 1402–1418. https://doi.org/10.1200/JCO.2015.64.2702 (2016).
Lorente, D. et al. Decline in circulating tumor cell count and treatment outcome in advanced prostate cancer. Eur. Urol. 70, 985–992. https://doi.org/10.1016/j.eururo.2016.05.023 (2016).
Heller, G. et al. Circulating tumor cell number as a response measure of prolonged survival for metastatic castration-resistant prostate cancer: A comparison with prostate-specific antigen across five randomized phase III clinical trials. J. Clin. Oncol. 36, 572–580. https://doi.org/10.1200/JCO.2017.75.2998 (2018).
Miller, M. C., Doyle, G. V. & Terstappen, L. W. Significance of circulating tumor cells detected by the cell search system in patients with metastatic breast colorectal and prostate cancer. J. Oncol. 2010, 617421. https://doi.org/10.1155/2010/617421 (2010).
Lorente, D. et al. Circulating tumour cell increase as a biomarker of disease progression in metastatic castration-resistant prostate cancer patients with low baseline CTC counts. Ann. Oncol. 29, 1554–1560. https://doi.org/10.1093/annonc/mdy172 (2018).
Taplin, M. E. et al. Androgen receptor modulation optimized for response-splice variant: A phase 3, randomized trial of galeterone versus enzalutamide in androgen receptor splice variant-7-expressing metastatic castration-resistant prostate cancer. Eur. Urol. 76, 843–851. https://doi.org/10.1016/j.eururo.2019.08.034 (2019).
Sharp, A. et al. Clinical utility of circulating tumour cell androgen receptor splice variant-7 status in metastatic castration-resistant prostate cancer. Eur. Urol. 76, 676–685. https://doi.org/10.1016/j.eururo.2019.04.006 (2019).
Gillessen, S. et al. Management of patients with advanced prostate cancer: Report of the advanced prostate cancer consensus conference 2019. Eur. Urol. 77, 508–547. https://doi.org/10.1016/j.eururo.2020.01.012 (2020).
Acknowledgements
The authors thank all patients and their families for supporting this study.
Funding
Open Access funding enabled and organized by Projekt DEAL. We acknowledge support from the Open Access Publication Fund of the University of Muenster. This study was supported by the Deutsche Forschungsgemeinschaft ((DFG), BE5347/4-1).
Author information
Authors and Affiliations
Contributions
Conception and design K.S., K.S., N.W., M.B., A.S., C.B. Acquisition of data K.S., K.S., N.W., V.H., N.G., D.T., M.B., C.B. Analysis and interpretation of data K.S., K.S., N.W., J.S., K.R., L.M.K., M.B., A.S., C.B. Drafting of the manuscript K.S., K.S., N.W., C.B. Critical revision of the manuscript for important intellectual content All authors Statistical analysis K.S., K.S., N.W., N.G., C.B. Obtaining funding C.B. Supervision A.S., C.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schlack, K., Seitzer, K., Wüstmann, N. et al. Comparison of circulating tumor cells and AR-V7 as clinical biomarker in metastatic castration-resistant prostate cancer patients. Sci Rep 12, 11846 (2022). https://doi.org/10.1038/s41598-022-16094-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-16094-6
This article is cited by
-
New advances of the androgen receptor in prostate cancer: report from the 1st International Androgen Receptor Symposium
Journal of Translational Medicine (2024)
-
Co-expression and clinical utility of AR-FL and AR splice variants AR-V3, AR-V7 and AR-V9 in prostate cancer
Biomarker Research (2023)
-
Circulating tumor cell quantification during abiraterone plus prednisone therapy may estimate survival in metastatic castration-resistant prostate cancer patients
International Urology and Nephrology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.