Abstract
Spermidine (SPD), a polyamine naturally present in living organisms, is known to prolong the lifespan of animals. In this study, the role of SPD in melanogenesis was investigated, showing potential as a pigmenting agent. SPD treatment increased melanin production in melanocytes in a dose dependent manner. Computational analysis with RNA-sequencing data revealed the alteration of protein degradation by SPD treatment without changes in the expressions of melanogenesis-related genes. Indeed, SPD treatment significantly increased the stabilities of tyrosinase-related protein (TRP)-1 and -2 while inhibiting ubiquitination, which was confirmed by treatment of proteasome inhibitor MG132. Inhibition of protein synthesis by cycloheximide (CHX) showed that SPD treatment increased the resistance of TRP-1 and TRP-2 to protein degradation. To identify the proteins involved in SPD transportation in melanocytes, the expression of several solute carrier (SLC) membrane transporters was assessed and, among 27 transporter genes, SLC3A2, SLC7A1, SLC18B1, and SLC22A18 were highly expressed, implying they are putative SPD transporters in melanocytes. Furthermore, SLC7A1 and SLC22A18 were downregulated by SPD treatment, indicating their active involvement in polyamine homeostasis. Finally, we applied SPD to a human skin equivalent and observed elevated melanin production. Our results identify SPD as a potential natural product to alleviate hypopigmentation.
Similar content being viewed by others
Introduction
Hypopigmentation disorders result in the appearance of white macules in the skin. These anomalies may occur due to a decrease in the number of melanocytes in the body, their inability to produce melanin, or abnormal transfer of mature melanosomes to neighboring keratinocytes1. Hypopigmentation may be diffused or localized, acquired or congenital, and is associated with a specific distribution pattern2. Acquired disorders include vitiligo, pityriasis alba, tinea versicolor and postinflamatory hypopigmentation, while congenital disorders include albinism, piebaldism, tuberous sclerosis and hypomelanosis of Ito. In addition, premature hair graying is a condition characterized by the early appearance of gray hair, generally before the age of 20 years in Caucasians and before 30 years in African Americans3. Though benign, these conditions can cause discomfort and affect the psychological state of the individual, and also increase UV sensitivity. In the cosmeceutical sector, melanogenesis modulators derived from natural sources are typically more appealing to customers. Several treatments for hypopigmentation include steroids, oral drugs, phototherapy and transplants, raising concerns for the safety and commodity of these methods4. For example, topical corticosteroids used to treat vitiligo can cause skin atrophy5,6. Selenium sulfide 2.5%, used to treat pityriasis versicolor can cause contact dermatitis7,8. Also, imidazoles and triazoles can commonly cause gastrointestinal disturbances, such as nausea and vomiting, when treated orally9,10. Therefore, it is important to develop new hypopigmentation treatments that are more natural and human friendly.
Polyamines are ubiquitous positively charged amines found in all living organisms11. These molecules are formed by the reaction of two or more amino groups, and can easily bind with various negatively charged macromolecules, including DNA, RNA, proteins, and acidic phospholipids12,13. As a result, they play essential roles in cell growth, proliferation, differentiation, gene regulation, immunity, as well as in the synthesis of proteins and nucleic acids. Spermidine (SPD) is one of the most prevalent natural mammalian polyamines. This molecule is synthesized from putrescine (PUT) or by oxidative degradation of spermine (SPM). In the polyamine pathway, the precursor ornithine initially undergoes decarboxylation by ornithine decarboxylase to generate the diamine PUT. Afterwards, the triamine SPD and the tetraamine SPM are formed by spermidine synthase and spermine synthase reactions, respectively. Moreover, intracellular SPD levels are dependent on polyamine uptake from the extracellular space, endogenous biosynthesis, catabolism, and excretion14. SPD plays important roles in cellular biochemical functions, including nucleic acid and protein synthesis, structure maintenance, and stability. It is estimated that approximately 13% of SPD is bound to DNA, and approximately 57% is bound to RNA to stimulate and improve protein synthesis15. Despite the capacity of an organism to produce SPD by de novo biosynthesis, external supplementation is essential to maintain a balanced polyamine pool16. Plant-derived foods, such as whole grains, vegetables, and legumes contain high levels of SPD, as do aged cheese and raw animal tissues17,18,19. Moreover, tissue SPD concentrations typically diminish with aging20. As a result, SPD supplementation exerts anti-aging effects and increases the overall lifespan in several animal models, including nematodes, flies, rodents, and humans21. More specifically, SPD can reduce cancer-associated mortality, enhance autophagy, ameliorate mitochondrial biogenesis, induce intestinal maturation, and improve function of the cardiovascular system, skeletal muscle, the immune system, liver, kidneys, and nervous system.
Melanogenesis is a complex process that regulates pigmentation. Melanin biosynthesis is tightly regulated by melanocyte-specific enzymes: TYR, tyrosinase-related protein 1 (TRP-1) and TRP-222,23. TYR is a bifunctional enzyme that catalyzes the hydroxylation of l-tyrosine to l-3,4-dihydroxyphenylalanine (l-DOPA), followed by the oxidation of l-DOPA to l-dopaquinone. Moreover, TRP-2 functions as a dopachrome tautomerase that converts dopachrome to its carboxylate derivative, 5,6-dihydroxyindole-2-carboxylic acid (DHICA), whereas TRP-1 functions as a DHICA oxidase that converts DHICA to a carboxylated indole-quinone that forms eumelanin (a brown-black pigment)24,25. Following synthesis and subsequent processing in the endoplasmic reticulum and Golgi apparatus, melanogenesis proteins are trafficked to melanosomes for pigment formation26. Subsequently, tyrosinase degradation occurs spontaneously, which is dependent on the ubiquitin proteasome and on the endosomal/lysosomal systems26,27. While practically all proteins are affected by such mechanisms, diminished stability and function of tyrosinase has severe implications on pigmentation. Therefore, improving the stability of tyrosinase and its associated proteins constitutes a promising strategy to overcome pigmentation defects. Since SPD has suggested to interact with proteins and improve their stability28,29,30, we aimed to find whether this polyamine could be a potential natural compound to target hypopigmentation.
In this study, we analyzed the potential of SPD for hypopigmentation treatment through a systematic exploration approach which revealed changes in protein degradation. Subsequently, we analyzed the role of SPD in protein stability and, as polyamine transporter expression can vary according to cell type, we also aimed to identify the main SPD transporter present in melanocytes. Lastly, we investigated the potential of SPD application in vivo through a human skin equivalent model.
Results
SPD treatment increases melanin production
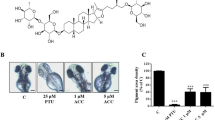
SPD is a polyamine compound (C7H19N3) that exerts various metabolic functions in living organisms (Fig. 1a). To investigate the effect of SPD on melanogenesis, normal human primary melanocytes and a human melanoma MNT-1 cell line, with the capacity to produce all stages of melanosomes, were obtained. The cytotoxic effects of SPD were evaluated by treating MNT-1 cells with various concentrations of SPD (Fig. 1b). It was confirmed that cell viability was affected only at concentrations of 50 and 100 μM. Therefore, in this study, SPD was administered in concentrations below these values. Quantification of melanin content in MNT-1 cells treated with increasing concentrations of SPD revealed increased melanin production in these cells (Fig. 1c). Moreover, based on a previous report indicating that SPD exerts distinct effects in young vs. aged tissues31, young and aged primary human melanocytes were treated with SPD. SPD increased melanin production in both cell types (increments of 15 ± 5% and 12 ± 2% in young and aged cells, respectively) (Fig. 1d,e).
Spermidine (SPD) treatment increases melanin production. (a) Scheme of the chemical structure of SPD. (b) Cell viability of MNT-1 cells was evaluated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays after 24 h of SPD treatment. (c) Melanin levels in MNT-1 cells treated with 0, 0.25, 0.5, 1, and 2 μM of SPD for 10 days were measured at 450 nm. The data are representative of two independent experiments. (d,e) Melanin levels in young and aged primary human melanocyte cells treated with 1 μM of SPD for 10 days were measured at 450 nm. The data are representative of three independent experiments. CTL: control, *P < 0.05, **P < 0.01, ***P < 0.005.
SPD treatment modulates genes involved protein degradation
To further understand the molecular implications of SPD treatment, the effects of this polyamine were analyzed through a systematic approach. Firstly, RNA sequencing performed in SPD-treated cells revealed the modulation of only 181 downregulated and 82 upregulated genes (Fig. 2a), of which melanogenesis-related genes were not included. To further confirm this, we next analyzed the effects of SPD on the expression levels of tyrosinase family genes TYR, TRP-1 and TRP-2, which tightly regulate melanogenesis (Fig. 2b). mRNA expression levels confirmed that this polyamine did not alter melanogenesis-associated gene expression. However, we found that several of the genes whose activity was altered by SPD were related to protein degradation (Fig. 2c). Several of the altered genes are involved in ubiquitination, a system for degradation of proteins that is also associated with the degradation of melanogenesis proteins. Altogether, these data infer that SPD does not increase melanogenesis by upregulating its associated genes' expression levels, but suggests it might influence melanogenesis by modulating protein stability.
A systematic exploration reveals that SPD treatment modulates genes involved protein degradation. (a) RNA-sequencing analysis of 1 µg of total RNA of primary melanocytes after treatment with 1 μM of SPD for 7 days revealing the upregulation (red) and downregulation (green) in gene expression levels. (b) mRNA expression levels of melanogenesis-related genes of primary melanocytes after treatment with 1 μM of SPD for 10 days. The data represent the results of three independent experiments. CTL: control, NS: non-significant. (c) RNA-sequencing analysis of 1 µg of total RNA of primary melanocytes after treatment with 1 μM of SPD for 7 days revealing the upregulation (red) and downregulation (green) in expression levels of genes involved in protein degradation.
SPD treatment improves the stabilities of TRP-1 and TRP-2
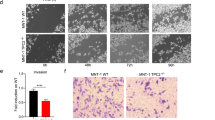
Since melanin production is regulated by a balance of synthesis and degradation of melanogenesis-associated proteins, we next aimed to better understand the effects of SPD at the protein level. SPD treatment resulted in a significant increase in TRP-1 and TRP-2 protein levels but not TYR (Fig. 3a). Moreover, since the proteasome system is involved in the degradation of melanogenesis-related proteins32, the expression of ubiquitin under SPD treatment was analyzed and revealed an altered ubiquitin expression pattern. This result, which is consistent with the RNA-seq data (Fig. 2c), may indicate that SPD increases melanogenesis by decreasing protein degradation via ubiquitination. Moreover, after treatment with SPD and subsequent application of the proteasome inhibitor MG132, expression levels of TRP-1 and TRP-2 were also significantly increased, unlike TYR, and ubiquitin expression decreased. These data further demonstrate that SPD modulates ubiquitination in addition to confirming that it has no impact on TYR expression. In addition, confocal microscopy revealed accumulation of the late-stage melanosome marker TA99 in cells treated with SPD (Fig. 3b), indicating the accumulation of highly pigmented melanosomes in these cells. Finally, to block the transcription of melanogenesis-related genes, cycloheximide (CHX) was applied (Fig. 3c). Under these conditions, SPD treatment improved the stabilities of TRP-1 and TRP-2, further demonstrating that SPD increases melanogenesis by improving protein stability which, in turn, increases TRP-1 and TRP-2 expression levels.
SPD treatment improves the stabilities of tyrosinase-related protein (TRP)-1 and TRP-2. (a) Western blots of B16F10 cells treated with 5 μM of SPD for 2 days and subsequent treatment with 10 μM of MG132 for 2 h. Samples were derived from the same experiment and blots were processed in parallel. Original blots are presented in Fig. S1. The data represent the results of three independent experiments. (b) TA99 protein levels examined by confocal microscopy at a magnification of 400× in MNT-1 cells treated with 2 μM of SPD for 10 days. Cell nuclei (blue) were stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI). (c) Western blotting of MNT-1 cells with treated with 10 μM of CHX for the indicated time periods and subsequent treatment with 2 μM of SPD. Samples were derived from the same experiment and blots were processed in parallel. Original blots are presented in Fig. S2. The data represent the results of three independent experiments. CTL: control, NS; non-significant, *P < 0.05, **P < 0.01, ***P < 0.005.
Identification of putative SPD transporters in primary human melanocytes
Intracellular polyamine levels are regulated by a combination of their synthesis, catabolism, and transport33. In order to identify the proteins involved in SPD transportation in melanocytes, the expression of several solute carrier (SLC) membrane transporters was assessed since they are reportedly involved in polyamine transportation12,34,35,36,37,38. Firstly, transcriptome data (Fig. 4a) revealed that, among the 27 transporter genes, SLC3A2, SLC7A1, SLC18B1, and SLC22A18 were highly expressed in primary human melanocytes. Since the balance of a substrate is maintained due to the selective action of transporters, and transporter protein expression levels decrease under high substrate concentration as a strategy to maintain ideal cytosolic levels and avoid cytotoxic effects39, we next aimed to observe the mRNA expression levels of these three genes under SPD treatment (Fig. 4b). Interestingly, SLC7A1 and SLC22A18 were the only genes responsive to the treatment, indicating their active involvement in polyamine homeostasis. Hence, SPD supplementation leads to an increase of the cellular polyamine pool, mediated by the actions of SLC3A2, SLC7A1, SLC18B1, and SLC22A18, thus increasing the stability of TRP-1 and -2 (Fig. 4c). These data demonstrate the potential role these transporters play in polyamine homeostasis to support protein stability for melanogenesis.
Identification of putative SPD transporters in primary human melanocytes. (a) RNA-sequencing analysis of 1 µg of total RNA of primary melanocytes representing the expression levels of solute carrier (SLC) family transporters. (b) mRNA expression levels of primary melanocytes after treatment with 1 μM of SPD for 10 days. The data represent the results of three independent experiments. CTL: control, NS: non-significant, *P < 0.05, **P < 0.01, ***P < 0.005. (c) Schematic representation of the effects of SPD treatment in the improvement of the stabilities of TRP-1 and TRP-2 through the increase of the cellular polyamine pool.
SPD increases melanogenesis in vivo
Finally, we aimed to analyze the potential of applying SPD as a treatment for hypopigmentation. We confirmed the effects of SPD in vivo by applying this polyamine to a human skin equivalent containing melanocytes. SPD supplementation resulted in a significant increase in melanin levels (increment of 10 ± 5%), confirming its potential application for treatment of hypopigmentation disorders (Fig. 5).
Discussion
In the present study, a systematic exploration approach revealed that SPD treatment modulates genes involved in protein degradation. Furthermore, protein analysis demonstrated that the melanogenesis-related proteins TRP-1 and TRP-2 were stabilized following SPD treatment. Finally, a human skin equivalent model revealed that SPD increased melanin production in vivo. Therefore, our findings demonstrate the potential of SPD for ameliorating hypopigmentation.
The polyamine pool gradually declines with age33. A previous study analyzed polyamine levels in 14 different tissues of an aging mouse model and revealed that SPD levels dropped in 11 of the 14 tissues40. SPM decreased only in the skin, heart, and muscles, while PUT levels were remarkably low in all tissues and at all ages. Indeed, SPD plays important roles in many tissues, and SPD supplementation leads to the relief of several age-related conditions. Moreover, hypopigmented macules, such as stellate pseudoscars and idiopathic guttate hypomelanosis, are frequently observed in photodamaged skin of aged individuals, and hair graying is a common feature of aging41. With this in consideration, since aged cells have a deficient polyamine pool, it seems reasonable that SPD treatment can restore it to more optimal levels thus increasing melanogenesis. However, our results also demonstrate that SPD treatment led to melanogenesis improvement in young cells. The beneficial effects of SPD supplementation in young mouse models have also been previously reported42. Thus, this infers that the benefits of SPD treatment are not limited to aged individuals, despite the polyamine pool knowingly decreasing during aging, and may also target non-age-related hypopigmentation, or premature hair graying. Hence, we state that SPD is a promising treatment to alleviate hypopigmentation, in which therapy doses should be customized to each patient for maximum efficacy.
Melanogenesis in human skin is tightly regulated by the expression of melanin-associated genes and the subsequent enzymatic reactions caused by these genes. Our results revealed that SPD treatment improved melanogenesis without altering the expressions of melanogenesis genes. Instead, SPD altered the expression of genes related with protein degradation, including proteasome-associated genes. Simultaneously, we observed an improvement of the stability of the melanogenesis proteins TRP-1 and -2 and decreased ubiquitination after SPD application. Indeed, melanogenesis proteins are digested by the proteasome system27,43,44. Ubiquitination is a system that targets eukaryotic proteins for breakdown and is dependent on a cascade of E1 ubiquitin-activating enzymes, E2 ubiquitin-conjugating enzymes and E3 ubiquitin-protein ligases that subsequently bind the target protein for degradation in the proteasome15. SPD has been implied in the impairment of ubiquitination by inhibition of ubiquitin ligases45, thus this is a plausible explanation for why SPD stabilizes TRP-1 and TRP-2. Interestingly, SPD did not significantly affect TYR expression. A previous study that observed the degradation rates of TYR, TRP-1, and TRP-2 revealed that TYR has a greater half-life than TRP-1 and TRP-246. Therefore, TYR may have a more rigid protein structure that confers it better stability. Another factor to take into consideration is that protein stability is also influenced by differences in the degradation machinery. Humans are estimated to possess 500–1000 E3 ubiquitin ligases, which are thought to play a key role in protein identification47. In this regard, it is also possible that TYR might be tagged by different ubiquitin ligases from those binding TRP-1 and TRP-2. Further research about the distinct structures of these proteins and their ubiquitin binding sites is necessary to answer these questions. Furthermore, fatty acids have been shown to regulate melanogenesis48. Similar to our findings, palmitic acid treatment of melanocytic cells led to decrease in ubiquitination, which increased the stability of melanogenesis proteins without alterations of their mRNA levels. However, in contrast with our work, palmitic acid treatment increased TYR expression, but not TRP-1 and -2. It is interesting to note that one of the molecular targets of SPD is lipid metabolism, which is regulated through autophagy49. Indeed, activation of autophagy has been linked with induction of melanogenesis and regulation of melanosome biogenesis in melanocytes50. Therefore, we cannot rule out the possibility that SPD might also alter the stability of melanogenesis proteins through modulation of autophagy. However, since our bioinformatics data did not identify changes in autophagy after SPD supplementation, we did not focus on that mechanism in this investigation. This may be due, in part, to the fact that we used a lower dose of SPD than other researches, which was based on the results of the cell viability assay. The modification of lipid metabolism following SPD treatment needs to be further investigated in order to fully comprehend these events. Nonetheless, our results infer that targeting protein stability could constitute a novel therapeutic strategy to overcome hypopigmentation.
Since polyamines are positively charged at physiological pH, they require a transport system to take up exogenous polyamines and/or eliminate excess polyamines from the cell51,52. Moreover, while the mechanisms involved in polyamine biosynthesis and catabolism have been thoroughly studied, the mammalian polyamine transport system is still poorly known. The SLC superfamily, which has 384 members, is the second-largest group of membrane transporter proteins in the human genome53. SLC transporters are primarily involved in the transportation of small molecules into cells by moving substrates via ion or electrochemical gradients like sodium or proton gradients54. Several SLC transporters have been implied in polyamine transportation36. Despite that, the SPD transporters in melanocytes had not been defined yet. We observed that SLC3A2, SLC7A1, SLC18B1, and SLC22A18 transporters genes were highly expressed in melanocytes. Moreover, since substrate balance is maintained by the selective action of transporters, we hypothesized that SPD supplementation would downregulate their expression levels to maintain cytosolic levels. However, only SLC7A1 and SLC22A18 were responsive to SPD treatment. As a result, we can imply that these two proteins may be actively involved in SPD-dependent polyamine homeostasis in melanocytes. In future works, a more thorough analysis will be required to better define the SPD transporters in melanocytes. Cell-based transport assays are limited since various endogenous transporters can disturb the transportation of a target molecule. Instead, proteoliposome-based transport assay performed with a purified pure transporter offers an effective model system for investigating the transportation of a target molecule55. Prior to reconstructing a proteoliposome, expression and purification investigations should be addressed. These methodologies present the potential to complement our investigation and precisely identify the SPD transporters in melanocytes.
In conclusion, this study indicates that SPD is a promising compound for the treatment of hypopigmentation disorders through improved protein stability, thus demonstrating great economical value given the current market demand for natural and human-friendly cosmetic products.
Materials and methods
Cell culture and materials
Moderately pigmented normal human primary melanocytes (Cascade Biologics, Portland, OR, USA) were cultured in M-254 medium (Cascade Biologics) containing human melanocyte growth supplement (HMGS; Cascade Biologics) and antibiotics (100 μg/mL penicillin, 100 μg/mL streptomycin; Thermo Fisher Inc., Waltham, MA, USA). MNT-1, a malignant melanoma cell line, was kindly provided by Dr. Ai-Young Lee at Dongguk University, who originally received it as a gift from Dr. Vincent J. Hearing at the National Institutes of Health (Bethesda, Maryland, USA). B16F10 cells were obtained from the Korean Cell Line Bank (Seoul, Republic of Korea). MNT-1 and B16F10 cells were cultured in minimum essential medium (Thermo Fisher Inc.) containing 20% fetal bovine serum (Gibco, Carlsbad, CA, USA), antibiotics (100 μg/mL penicillin, 100 μg/mL streptomycin), 20 mM 4-(2-hydroxyethyl)-1-piperazineëthanesulfonic acid, and 10% Dulbecco's modified Eagle's medium/high glucose (Lonza, Basel, Switzerland). All cells were maintained at 37 °C and 5% CO2. SPD (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in distilled water, and new stocks were prepared monthly. CHX and MG132 were purchased in Sigma-Aldrich and kept at − 30 °C until use.
Cell viability assay
Cells were seeded at a density of 1 × 104 cells/well in a 96-well plate (Corning, New York, USA). After overnight incubation, cells were treated with 0, 2, 5, 10, 20, 50, and 100 μM of SPD for 24 h. Subsequently, 100 μL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide reagent was added to the medium in each well and incubated for 3 h at 37 °C. The reduced formazan crystals were solubilized in 50 μL of dimethyl sulfoxide (Sigma-Aldrich) and absorbances were measured at 570 nm in a microplate reader (SpectraMax 190 Absorbance Plate Reader; Molecular Devices, Sunnyvale, CA, USA).
Melanin assay
Melanocytes were seeded at a density of 1 × 105 cells/dish. After overnight incubation, cells were treated with SPD for 7 days. Cells were lysed in 0.5 M Tris–HCl (pH 7.5) containing 1% NP-40, 0.15 M NaCl, and 5 mM MgCl2, as previously described56. Cell lysates were centrifuged at 15,000 rpm and 4 °C for 20 min. The protein content of each supernatant was measured using a bicinchoninic acid (BCA) assay, and 100 μL of NaOH solution was added to dissolve each pellet and heated at 80 °C for 1 h. Melanin levels in the cells were determined by measuring absorbance at 450 nm. Absorbances were divided by the amount of protein used to determine each total melanin content.
Western blotting analysis
Cells were lysed in a solution of 0.05 M Tris–HCl, pH 7.5, 0.15 M NaCl, and 0.01 M MgCl2. Cell lysates were centrifuged at 15,000 rpm and 4 °C for 20 min, and protein contents were quantified using a BCA assay. Aliquots of 20 μg of protein per well were separated in a sodium dodecyl sulfate–polyacrylamide gel, and electro-transferred from the gel to a polyvinylidene fluoride membrane (Sigma-Aldrich). After blocking with 2.5% bovine serum albumin for 1 h, membranes were incubated with primary antibodies targeting TYR (1:300; Thermo Fisher Inc.), ubiquitin (1:500; Santa Cruz Biotechnology, Dallas, TX, USA), TRP-1 (1:4000), TRP-2 (1:4000), and GAPDH (1:4000) (Abcam, Cambridge, UK).
Immunofluorescence
Cells were cultured on Lab-Tek chamber slides (Nunc, NY, USA), as described previously56. Cells were stained with TA99 (Thermo Fisher Inc.) and 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich). Secondary antibody staining was performed using Alexa Fluor 488–conjugated F(ab′)2 fragment of goat anti-mouse IgG antibody (Invitrogen, Waltham, Massachusetts, USA). Images were obtained using an LSM 800 confocal microscope (Carl Zeiss, Jena, Germany).
RNA-sequencing analysis
Total RNA was isolated from saline- and SPD-treated melanocytes using RNeasy Mini Kit columns (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. RNA quality was assessed using an Agilent 2100 bioanalyzer with an RNA 6000 Nano Chip (Agilent Technologies, Amstelveen, Netherlands), and RNA quantity was determined using an ND-1000 Spectrophotometer (NanoDrop Technologies, Inc., DE, USA). The RNA integrity number (RIN) values for all of the samples were larger than 9. Poly(A) mRNA isolation from the total RNA and subsequent fragmentation were performed the TruSeq Stranded mRNA LT Sample Prep Kit (Illumina, San Diego, CA, USA), according to the manufacturer’s instructions. The adaptor ligated libraries were sequenced using an Illumina NovaSeq 6000 (Macrogen, Inc., Seoul, South Korea). mRNA sequencing was performed for three biological replicates of each condition. From the resulting read sequences for each sample, adapter sequences (TruSeq universal and indexed adapters) were removed using the cutadapt software (version 2.7)57. The remaining reads were then aligned to the Homo sapiens reference genome (GRCh38) using TopHat2 software (version 2.1.1) with default parameters58. After the alignment, we counted the numbers of reads mapped to the gene features (GTF file of GRCh38.89) using HTSeq59. Read counts for the samples in each condition were then normalized using TMM (trimmed mean of M-values) normalization of the edgeR package60.
Identification of differentially expressed genes (DEGs)
To identify DEGs, a statistical hypothesis test was performed57. For each gene, a T-statistic value was calculated using the Student’s t-test for comparison of saline- and SPD-treated melanocytes. An empirical distribution of the T-statistic value for null hypothesis was estimated by performing all possible combinations of random permutations of the samples. Adjusted P-values were obtained for each gene using a two-tailed Student’s t-test with an empirical null distribution. The DEGs were identified as genes with adjusted P-values ≤ 0.05, and absolute log2-fold changes > 0.428 (1.35-fold; the mean of the 0.5th and 99.5th percentiles of the null distribution of log2-fold changes).
qPCR
Melanocytes treated with 1 μM SPD for 7 days were subjected to RNA extraction using a RNeasy Plus Mini Kit (Qiagen, Hilden, Germany). RNA concentrations were measured using a Nano-400A Micro Spectrophotometer (Allsheng, Zheijang, China). cDNA synthesis was performed using the AccuPower RT PreMix (Bioneer, Daejeon, Korea). cDNA, primer (F/R), and CYBR Q GreenBlue qPCR Master Mix (Cellsafe, Yongin, Republic of Korea) were dispensed into MicroAmp Fast Reaction Tubes (Applied Biosystems, Überlingen, Germany), and mRNA expression levels were determined using an AriaMx real-time PCR system (Agilent Technologies, Palo Alto, CA, USA).
Human skin equivalent
A human skin equivalent model composed of primary human keratinocytes and melanocytes (KeraSkin™, Biosolution, Korea) was used. The basal layer of this human skin equivalent model is composed of dendritic cells that produce melanin granules. These cells produced pigmentation and became fully differentiated when cultured in an air–liquid interface culture for 2 weeks. Cells were then treated with 2 and 4 µM SPD for 2 weeks.
Statistical analysis
Two-tailed Student’s t-test was used to analyze the differences between two groups.
Data availability
The raw and processed RNA-seq data were deposited into the Gene Expression Omnibus (GEO) database with accession ID GSE209538.
References
Plensdorf, S. & Martinez, J. Common pigmentation disorders. Am. Fam. Phys. 79, 109–116 (2009).
Fistarol, S. K. & Itin, P. H. Disorders of pigmentation. J. German Soc. Dermatol. 8, 187–201. https://doi.org/10.1111/j.1610-0387.2009.07137.x (2010) (quiz 201–182).
Tobin, D. J. & Paus, R. Graying: Gerontobiology of the hair follicle pigmentary unit. Exp. Gerontol. 36, 29–54 (2001).
Schaffer, J. V. & Bolognia, J. L. The treatment of hypopigmentation in children. Clin. Dermatol. 21, 296–310. https://doi.org/10.1016/s0738-081x(03)00045-2 (2003).
Bleehen, S. S. The treatment of vitiligo with topical corticosteroids. Light and electronmicroscopic studies. Br. J. Dermatol. 94, 43–50 (1976).
Coondoo, A., Phiske, M., Verma, S. & Lahiri, K. Side-effects of topical steroids: A long overdue revisit. Indian Dermatol. Online J. 5, 416 (2014).
Sánchez, J. L. & Torres, V. M. Double-blind efficacy study of selenium sulfide in tinea versicolor. J. Am. Acad. Dermatol. 11, 235–238 (1984).
Hull, C. A. & Johnson, S. M. A double-blind comparative study of sodium sulfacetamide lotion 10% versus selenium sulfide lotion 2.5% in the treatment of pityriasis (tinea) versicolor. Cutis 73, 425–429 (2004).
Renati, S., Cukras, A. & Bigby, M. Pityriasis versicolor. BMJ 350, h1394. https://doi.org/10.1136/bmj.h1394 (2015).
Kyle, J. A. The fungus among us: An antifungal review. US Pharm. 35, 44–56 (2010).
Bae, D.-H., Lane, D. J., Jansson, P. J. & Richardson, D. R. The old and new biochemistry of polyamines. Biochim. Biophys. Acta Gen. Subjects 1862, 2053–2068 (2018).
Li, J., Meng, Y., Wu, X. & Sun, Y. Polyamines and related signaling pathways in cancer. Cancer Cell Int. 20, 1–16 (2020).
Park, M. H. & Igarashi, K. Polyamines and their metabolites as diagnostic markers of human diseases. Biomol. Ther. 21, 1 (2013).
Madeo, F., Eisenberg, T., Pietrocola, F. & Kroemer, G. Spermidine in health and disease. Science https://doi.org/10.1126/science.aan2788 (2018).
Igarashi, K. & Kashiwagi, K. Modulation of cellular function by polyamines. Int. J. Biochem. Cell Biol. 42, 39–51 (2010).
Bardócz, S. et al. The importance of dietary polyamines in cell regeneration and growth. Br. J. Nutr. 73, 819–828 (1995).
Atiya Ali, M., Poortvliet, E., Stromberg, R. & Yngve, A. Polyamines in foods: Development of a food database. Food Nutr. Res. https://doi.org/10.3402/fnr.v55i0.5572 (2011).
Muñoz-Esparza, N. C. et al. Polyamines in food. Front. Nutr. https://doi.org/10.3389/fnut.2019.00108 (2019).
Madeo, F. et al. Nutritional aspects of spermidine. Annu. Rev. Nutr. 40, 135–159 (2020).
Madeo, F., Bauer, M. A., Carmona-Gutierrez, D. & Kroemer, G. Spermidine: A physiological autophagy inducer acting as an anti-aging vitamin in humans?. Autophagy 15, 165–168 (2019).
Madeo, F., Carmona-Gutierrez, D., Kepp, O. & Kroemer, G. Spermidine delays aging in humans. Aging 10, 2209 (2018).
Chang, T.-S. Natural melanogenesis inhibitors acting through the down-regulation of tyrosinase activity. Materials 5, 1661–1685 (2012).
Ando, H. et al. Possible involvement of proteolytic degradation of tyrosinase in the regulatory effect of fatty acids on melanogenesis. J. Lipid Res. 40, 1312–1316 (1999).
Kobayashi, T. et al. Tyrosinase related protein 1 (TRP1) functions as a DHICA oxidase in melanin biosynthesis. EMBO J. 13, 5818–5825 (1994).
Tsukamoto, K., Jackson, I. J., Urabe, K., Montague, P. M. & Hearing, V. A second tyrosinase-related protein, TRP-2, is a melanogenic enzyme termed DOPAchrome tautomerase. EMBO J. 11, 519–526 (1992).
Ando, H., Kondoh, H., Ichihashi, M. & Hearing, V. J. Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J. Investig. Dermatol. 127, 751–761 (2007).
Ando, H., Ichihashi, M. & Hearing, V. J. Role of the ubiquitin proteasome system in regulating skin pigmentation. Int. J. Mol. Sci. 10, 4428–4434 (2009).
Momeni, L., Shareghi, B., Saboury, A. A. & Farhadian, S. Comparative studies on the interaction of spermidine with bovine trypsin by multispectroscopic and docking methods. J. Phys. Chem. B 120, 9632–9641 (2016).
Sadeghi-Kaji, S., Shareghi, B., Saboury, A. A. & Farhadian, S. Spectroscopic and molecular docking studies on the interaction between spermidine and pancreatic elastase. Int. J. Biol. Macromol. 131, 473–483 (2019).
Hosseini-Koupaei, M., Shareghi, B., Saboury, A. A., Davar, F. & Raisi, F. The effect of spermidine on the structure, kinetics and stability of proteinase K: Spectroscopic and computational approaches. RSC Adv. 6, 105476–105486 (2016).
Sacitharan, P. K., Lwin, S., Gharios, G. B. & Edwards, J. R. Spermidine restores dysregulated autophagy and polyamine synthesis in aged and osteoarthritic chondrocytes via EP300. Exp. Mol. Med. 50, 1–10 (2018).
Halaban, R. et al. Aberrant retention of tyrosinase in the endoplasmic reticulum mediates accelerated degradation of the enzyme and contributes to the dedifferentiated phenotype of amelanotic melanoma cells. Proc. Natl. Acad. Sci. 94, 6210–6215 (1997).
Minois, N., Carmona-Gutierrez, D. & Madeo, F. Polyamines in aging and disease. Aging 3, 716 (2011).
Uemura, T., Stringer, D. E., Blohm-Mangone, K. A. & Gerner, E. W. Polyamine transport is mediated by both endocytic and solute carrier transport mechanisms in the gastrointestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G517–G522 (2010).
Soulet, D., Gagnon, B., Rivest, S., Audette, M. & Poulin, R. A fluorescent probe of polyamine transport accumulates into intracellular acidic vesicles via a two-step mechanism. J. Biol. Chem. 279, 49355–49366. https://doi.org/10.1074/jbc.M401287200 (2004).
Abdulhussein, A. A. & Wallace, H. M. Polyamines and membrane transporters. Amino Acids 46, 655–660. https://doi.org/10.1007/s00726-013-1553-6 (2014).
Takeuchi, T. et al. Vesicular polyamine transporter mediates vesicular storage and release of polyamine from mast cells. J. Biol. Chem. 292, 3909–3918. https://doi.org/10.1074/jbc.M116.756197 (2017).
Poulin, R., Casero, R. A. & Soulet, D. Recent advances in the molecular biology of metazoan polyamine transport. Amino Acids 42, 711–723. https://doi.org/10.1007/s00726-011-0987-y (2012).
Hou, W.-K. et al. Influence of blood glucose on the expression of glucose transporter proteins 1 and 3 in the brain of diabetic rats. Chin. Med. J. 120, 1704–1709 (2007).
Nishimura, K., Shiina, R., Kashiwagi, K. & Igarashi, K. Decrease in polyamines with aging and their ingestion from food and drink. J. Biochem. 139, 81–90. https://doi.org/10.1093/jb/mvj003 (2006).
Ortonne, J. P. Pigmentary changes of the ageing skin. Br. J. Dermatol. 122, 21–28 (1990).
Jeevanandam, M., Holaday, N. J., Begay, C. K. & Petersen, S. R. Nutritional efficacy of a spermidine supplemented diet. Nutrition 13, 788–794 (1997).
Seo, E. Y. et al. UCHL1 regulates melanogenesis through controlling MITF stability in human melanocytes. J. Investig. Dermatol. 137, 1757–1765 (2017).
Kageyama, A. et al. Down-regulation of melanogenesis by phospholipase D2 through ubiquitin proteasome-mediated degradation of tyrosinase. J. Biol. Chem. 279, 27774–27780 (2004).
Sayas, E. et al. Polyamines interfere with protein ubiquitylation and cause depletion of intracellular amino acids: A possible mechanism for cell growth inhibition. FEBS Lett. 593, 209–218 (2019).
Kobayashi, T., Imokawa, G., Bennett, D. C. & Hearing, V. J. Tyrosinase stabilization by Tyrp1 (the brown locus protein). J. Biol. Chem. 273, 31801–31805 (1998).
Nakayama, K. I. & Nakayama, K. Ubiquitin ligases: Cell-cycle control and cancer. Nat. Rev. Cancer 6, 369–381 (2006).
Ando, H. et al. Fatty acids regulate pigmentation via proteasomal degradation of tyrosinase: A new aspect of ubiquitin-proteasome function. J. Biol. Chem. 279, 15427–15433 (2004).
Minois, N., Rockenfeller, P., Smith, T. K. & Carmona-Gutierrez, D. Spermidine feeding decreases age-related locomotor activity loss and induces changes in lipid composition. PLoS ONE 9, e102435 (2014).
Kim, J. Y. et al. Autophagy induction can regulate skin pigmentation by causing melanosome degradation in keratinocytes and melanocytes. Pigment Cell Melanoma Res. 33, 403–415 (2020).
Palmer, A. J. & Wallace, H. M. The polyamine transport system as a target for anticancer drug development. Amino Acids 38, 415–422 (2010).
Corral, M. & Wallace, H. M. Upregulation of polyamine transport in human colorectal cancer cells. Biomolecules 10, 499 (2020).
Höglund, P. J., Nordström, K. J., Schiöth, H. B. & Fredriksson, R. The solute carrier families have a remarkably long evolutionary history with the majority of the human families present before divergence of Bilaterian species. Mol. Biol. Evol. 28, 1531–1541 (2011).
Fujiwara, R., Takenaka, S., Hashimoto, M., Narawa, T. & Itoh, T. Expression of human solute carrier family transporters in skin: Possible contributor to drug-induced skin disorders. Sci. Rep. 4, 1–8 (2014).
Sejwal, K. et al. Proteoliposomes—A system to study membrane proteins under buffer gradients by cryo-EM. Nanotechnol. Rev. 6, 57–74 (2017).
Bin, B. H. et al. Hyperosmotic stress reduces melanin production by altering melanosome formation. PLoS ONE 9, e105965. https://doi.org/10.1371/journal.pone.0105965 (2014).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Trapnell, C., Pachter, L. & Salzberg, S. L. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–1111 (2009).
Anders, S., Pyl, P. T. & Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Robinson, M. D. & Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 11, 1–9 (2010).
Funding
This research was supported by a grant from the National Research Foundation of Korea (NRF) funded by the Korean government (MSIT) (No. 2019005607 to B.-H.B.), the Ajou University Research Fund (to B.-H.B.), the Gyeonggido Business & Science Accelerator (GBSA) grant (to B.-H.B. and M.-G.L.), a grant provided by the Basic Research Program through the Korea Brain Research Institute (22-BR-01-02) funded by the Korean Ministry of Science and ICT (to S.C.), a grant provided by the Korea Initiative for fostering University of Research and Innovation Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (No. NRF2021M3H1A104892211; to B.-H.B. and B.-M.K.), and a grant provided by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1A6A1A10044950; to B.-H.B and B.-M.K).
Author information
Authors and Affiliations
Contributions
S.B. performed the experiments, analyzed the results, and wrote the manuscript. H.H. performed the experiments, analyzed the results, and wrote the manuscript. B.C. performed the experiments and analyzed the results. S.-H.L. performed experiments and analyzed the results. S.C. designed the experiments, performed experiments, analyzed the results, and supervised the project. M.-G.L. designed the experiments, performed experiments, analyzed the results, and supervised the project. B.-M.K. planned the study, revised the manuscript, and supervised the project. B.-H.B. conceived the idea for the study, designed the experiments, revised the manuscript, and supervised the project. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brito, S., Heo, H., Cha, B. et al. A systematic exploration reveals the potential of spermidine for hypopigmentation treatment through the stabilization of melanogenesis-associated proteins. Sci Rep 12, 14478 (2022). https://doi.org/10.1038/s41598-022-18629-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-18629-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.